Dynamic Load Model Systems of Tendon Inflammation and Mechanobiology
- 1School of Chemical, Biological and Environmental Engineering, Oregon State University, Corvallis, OR, United States
- 2School of Mechanical, Industrial and Manufacturing Engineering, Oregon State University, Corvallis, OR, United States
Dynamic loading is a shared feature of tendon tissue homeostasis and pathology. Tendon cells have the inherent ability to sense mechanical loads that initiate molecular-level mechanotransduction pathways. While mature tendons require physiological mechanical loading in order to maintain and fine tune their extracellular matrix architecture, pathological loading initiates an inflammatory-mediated tissue repair pathway that may ultimately result in extracellular matrix dysregulation and tendon degeneration. The exact loading and inflammatory mechanisms involved in tendon healing and pathology is unclear although a precise understanding is imperative to improving therapeutic outcomes of tendon pathologies. Thus, various model systems have been designed to help elucidate the underlying mechanisms of tendon mechanobiology via mimicry of the in vivo tendon architecture and biomechanics. Recent development of model systems has focused on identifying mechanoresponses to various mechanical loading platforms. Less effort has been placed on identifying inflammatory pathways involved in tendon pathology etiology, though inflammation has been implicated in the onset of such chronic injuries. The focus of this work is to highlight the latest discoveries in tendon mechanobiology platforms and specifically identify the gaps for future work. An interdisciplinary approach is necessary to reveal the complex molecular interplay that leads to tendon pathologies and will ultimately identify potential regenerative therapeutic targets.
1 Introduction
Tendon acute injuries and chronic dysfunction (tendinopathy) are a common issue among athletic and occupational settings. Clinical symptoms of tendinopathy include pain, weakness, and the inability to perform activities of daily life. Risk factors for tendinopathy include age, obesity, activity level, and anatomical asymmetries (Riley, 2005; Lorimer and Hume, 2014, 2016; Thampatty and Wang, 2018). In 2015, musculoskeletal disorders accounted for more than half of the disabling health conditions reported by adults in the US (Weinstein et al., 2014), and tendon and ligament injuries have been estimated to account for 20–30% of all musculoskeletal disorders (Fleming et al., 2005). Despite its prevalence, the etiology of tendon pathologies is not well understood. Consequently, treatment options often do not resolve the issue (Riley, 2005).
Tendinopathy is defined by a failure to heal following tissue trauma that leads to a progressively degenerative imbalance in extracellular matrix (ECM) turnover (Burk, 2019). However, the exact mechanisms for the imbalance are not yet well-characterized. Repetitive low-level dynamic loading or underloading of tissues is implicated in tendinopathy etiology though dynamic loading is also necessary for proper maturation and matrix homeostasis. Thus, the boundaries between underloading, homeostatic loading, and overloading may vary across anatomical location (Johnson et al., 1994; Itoi et al., 1995; Maganaris and Paul, 1999), and within species (Goodship et al., 1994; Maganaris and Paul, 1999; Matson et al., 2012; Zitnay and Weiss, 2018), due to variations in mechanical properties. Tendon injuries may be acute and characterized by inflammation, called “tendonitis”, or they are chronic and characterized by a degenerative ECM, called “tendinosis” (Lipman et al., 2018). Both conditions are incorporated into the more generic term “tendinopathy” that highlights tendon pain, dysfunction, and often an inability to specifically diagnose the disorder. Tendinopathy has been adopted in part due to histological findings that suggest noteworthy absence of inflammatory cells in the later stages of the pathological tendon (Riley, 2005; Legerlotz et al., 2013). Instead, histological characteristics most notably include lipid deposition, increased vascularity, increased proteoglycan content, and calcification (Figure 1) (Kannus and Józsa, 1991). However, recent findings suggest low-levels of inflammation persist throughout the course of tendinopathy (Chisari et al., 2019; Arvind and Huang, 2021). Overall, pathological changes in the tendon, including localized regions of decreased collagen fibre thickness, decreased crimp angle, and heterogeneous crimping, have been associated with tendon rupture (Kannus and Józsa, 1991; Järvinen et al., 2004). Conclusively, inflammation may contribute to the onset of tendinopathies, but its temporal role after initiation needs to be characterized.
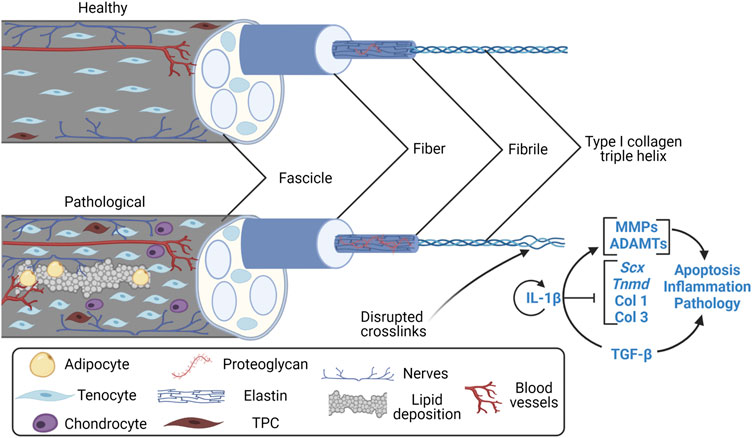
FIGURE 1. Features of the healthy and pathological tendon fascicle. Created with Biorender.com. Features of the clinically pathological tendon fascicle (a hierarchical subunit of whole tendon) include lipid deposition, angiogenesis, nerve ingrowth, adipocyte and chondrocyte-like TPC differentiation (Kannus and Józsa, 1991; Józsa and Kannus, 1997; Zhang and Wang, 2010, 2014; Agarwal et al., 2017), increases in proteoglycan content (Fu, Chan and Rolf, 2007; Samiric et al., 2009; Attia et al., 2014), collagen type III RNA upregulation/deposition (Liu et al., 1995; Samiric et al., 2009), and irregularities in collagen alignment/crosslinking at previous microscopic to macroscopic rupture sites (Kannus and Józsa, 1991; Józsa and Kannus, 1997; Järvinen et al., 2004). Overall, the pathological tendon is mechanically weaker than the healthy tendon. TPC, tendon progenitor cell; Scx, Scleraxis; Tnmd, Tenomodulin; Col 1, collagen type I; Col 3, collagen type III; MMP, matrix metalloproteinases; ADAMT, disintegrin and metalloproteinase with thrombospondin motifs; IL-1β, interleukin-1β; TGF-β, transforming growth factor β.
It is essential to identify the molecular and mechanical mechanisms involved in tendinopathy etiology in order to improve therapeutic outcomes (Screen et al., 2015). Exploration of these mechanisms can be carried out with ex vivo and in vitro models. The core focus of this review is recent advancements in the field of tendon mechanobiology using in vitro and ex vivo platforms and includes several in vivo models of tendinopathy, which were selected based on the described methods below. Also included is primary background on tendon physiology and mechanical properties, which is essential to evaluate these model systems. Future directions for this work are emphasized in the gaps in current understanding and among model system platforms.
2 Methods
Web of Science and PubMed library searches were conducted using key words or MeSH terms such as “Model” AND “Tendinopathy” OR “Tendinosis” OR “Tendonitis” AND “Tendons” AND “Inflammation”. For the section on model systems of tendinopathy, greater focus was placed on the most recent findings in the years 2015–2022. In this year range, all studies relating to dynamic or cyclical loading-induced effects on tendon modulation and inflammation of in vitro and ex vivo systems were included. To highlight the limited research in this field, total publications relating to “tendinopathy models” is about 15-fold lower than cartilage “osteoarthritis models” for the years 2011–2021 in PubMed databases (Supplementary Figure S1). Cartilage is also a hypovascular tissue that shares similar degenerative features to the tendon, yet the role of mechanical loading and inflammation is more heavily researched in osteoarthritis etiology (Robinson et al., 2016; Chisari et al., 2019). Key words and MeSH terms of “Tendinopathy” AND “Inflammation” AND “Model” versus “Osteoarthritis” AND “Inflammation” AND “Model” were used for this comparison of published literature on these topics.
3 Tendon Physiology
Tendon is a densely collagenous, hypocellular tissue, where ECM turnover is a key component of tendon homeostasis and its ability to sustain loading (Nabeshima et al., 1996; Gracey et al., 2020). During embryonic development, tendon tissue shifts from a hypercellular environment dominated by cell-cell interactions, to predominantly cell-matrix interactions within a hypocellular environment (Cosgrove et al., 2016). Tenocytes are the predominant cell-type in the mature tendon, making up 90–95% of cells yet only 5% of tissue volume (Kannus, 2000; Cosgrove et al., 2016; Wu et al., 2018). They are fully differentiated and are primarily responsible for tissue homeostasis via ECM remodeling (Burk, 2019). Meanwhile, the immature tenocytes, or tenoblasts, and tendon progenitor cells (TPCs), make up a large majority of the other cells within the tendon and are necessary for tenocyte proliferation and differentiation, respectively.
Overall, tendon cells are mechanosensing. They are sensitive to various load types (Mendias, Gumucio and Lynch, 2012; Zitnay and Weiss, 2018), frequency (Lavagnino et al., 2003; Kubo et al., 2020), and magnitudes (Screen et al., 2005; Mendias et al., 2012). Neonatal developmental structural and compositional changes of the ECM are associated with increases in load-bearing (Ansorge et al., 2011; Theodossiou et al., 2021). Collagen bundles transfer primarily uniaxial tensile forces to tenocytes while the greater collagen and ECM architecture transfers minor shear and compressive forces during loading (Khan and Scott, 2009). Once a loading threshold has been reached, the cells respond accordingly via a complex interplay of cell signaling with possible activation of inflammatory pathways (Gracey et al., 2020). Ultimately, this results in an anabolic, catabolic, or balanced activity in the extracellular matrix.
3.1 Extracellular Matrix Composition
The main load-bearing component of the tendon ECM is type I collagen (Col1), which composes 80–90% of tendon dry weight (see Supplementary Figure S2) (Kannus, 2000; Wang et al., 2012). Collagen type I is a heterotrimeric molecule composed of two α1 chains and one α2 chain forming a triple helix. Many collagen types (V, IX, X, XI, and XII) are found in smaller amounts within the tendon, though they still provide important functions. For example, collagen fibril diameter is partially regulated by collagen type V (Birk and Mayne, 1997; Wang et al., 2012). Other components involved in ECM modulation include type III collagen (Col3) which is important for quick cross-linking and stability of injury sites (Liu et al., 1995), integrin transmembrane proteins involved in sensing loads, and proteoglycans (decorin, aggrecan, etc., make up 1–5% of tendon dry weight) that hydrate the tendon and contribute to fibrillar slippage and resisting compression, respectively (Fessel and Snedeker, 2009; Wang et al., 2012; Screen et al., 2015). Matrix metalloproteinases (MMPs, mainly 1, 3, and 12) and ADAMTS-1, -4, -5, -8, -9, -15, and -20 (disintegrin and metalloproteinase with thrombospondin motifs, or aggrecanases) enzymatically breakdown various extracellular matrix components (Buono et al., 2013). Glycoproteins such as fibronectin, elastin (1–2% of tendon dry weight) (Kannus, 2000), and tenascin-C contribute to tendon repair and the elastic properties of the tendon (Wang et al., 2012). Intermolecular enzymatic cross-linking is an interaction mainly between thick Col1 fibrils, thinner Col3 fibrils, elastin, tenascin-C (Midwood et al., 2016), and proteoglycan components that occurs during maturation and adaptation to mechanical loads (Eyre et al., 1984; Kubow et al., 2015; Zitnay and Weiss, 2018). Consequently, the architectural arrangement of cross-linked components influences the load-bearing ECM-derived functional properties (Kjaer, 2004).
3.2 Tendon Signaling
Transforming growth factor beta (TGF-β) is highly correlated to mechanical stress intensity and thus the tendon’s mechanoresponse via the Smad2/3 pathway (Figure 2A) (Dahlgren et alk., 2005; Maeda et al., 2011; Munger and Sheppard, 2011; Mendias et al., 2012). Downstream of the Smad2/3 pathway, Scleraxis (Scx), Mohawk (Mhk), and Tenomodulin (Tnmd) are tenocyte-specific markers that regulate proteoglycan production and collagen synthesis (Berthet et al., 2013; Subramanian et al., 2017). In developing mouse tendons, Tnmd expression is downstream of Scx, though they both likely play a role in Col1 transcription and adaptation to mechanical loading as well as tenocyte proliferation (Léjard et al., 2007). These markers have been identified as significant factors involved in tendon homeostasis and degeneration. They are heavily studied in model systems though exact mechanisms have not been clearly identified in all loading scenarios.
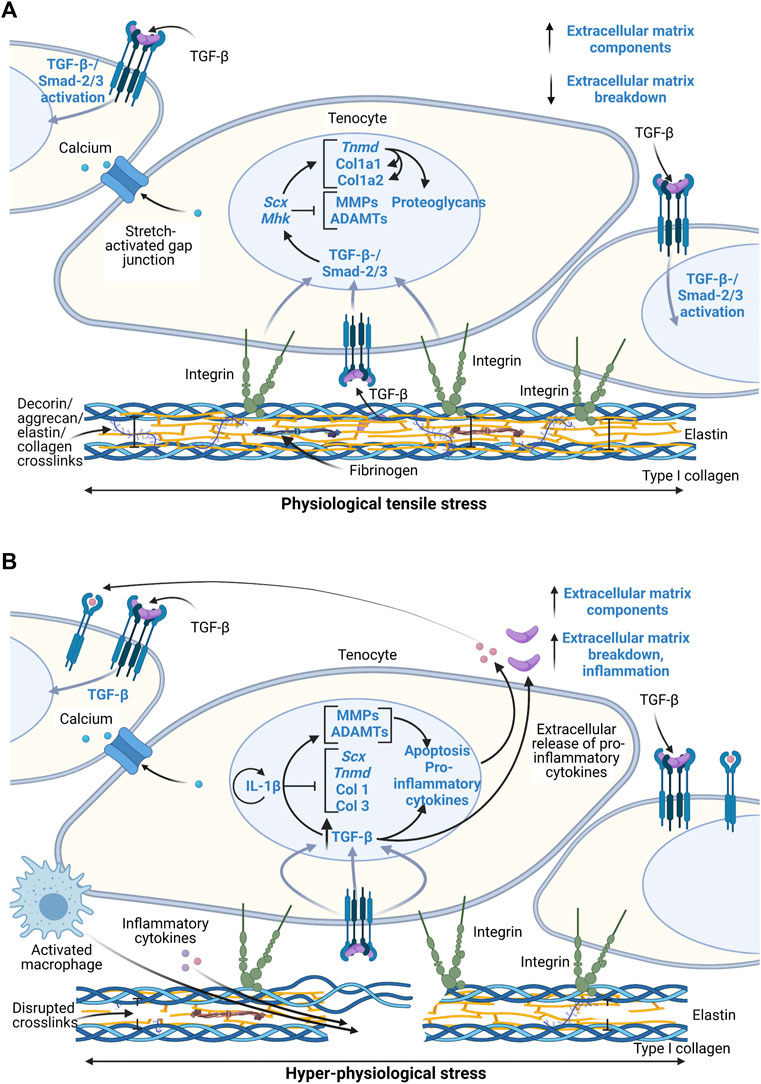
FIGURE 2. Schematic of proposed tenocyte molecular response to physiological and hyper-physiological mechanical loading thresholds. Created with Biorender.com. (A) Molecular cascade following physiological loading resulting in both integrin-mediated pathways and postulated stretch-activated ion channel pathways to induce a TGF-β-/Smad-2/3 activation and thus transcription of various extracellular matrix related genes and regulatory enzymes. (B) Hyper-physiological loading leads to macro-scale ruptures that induce inflammatory cascades through greater activation of TGF-β pathways and IL-1β that lead to paracrine signaling and ultimately matrix degradation and inflammatory cell localization at the injury site. Scx, Scleraxis; Tnmd, Tenomodulin; Col 1, collagen type I; Col 3, collagen type III; MMP, matrix metalloproteinases; ADAMT, disintegrin and metalloproteinase with thrombospondin motifs; IL-1β, interleukin-1β; TGF-β, transforming growth factor β.
Current knowledge of the interaction between integrin proteins in cells and the ECM during loading is limited. It is unclear whether or not the cells respond to loads primarily via an integrin-mediated pathway or via stretch-activated ion channels that are caused by actual deformation of the cytoskeleton and nucleus in the cell. Decoupling of the mechanosensing process during maturation can be achieved using in vitro models of stem cell differentiation and ECM maturation in response to mechanical loads (Cosgrove et al., 2016). In other words, defining the cell-matrix interactions versus cell-cell interactions is necessary using simplified models.
3.3 Inflammation and Matrix Modulation in Tendinopathy
Inflammation is vital to healing of acute injuries, yet the tendon has low regeneration potential once past early post-natal stages (Ansorge et al., 2012; Howell et al., 2017; Stauber et al., 2020). Thus, it is necessary for inflammatory pathways to be resolved to limit tissue damage and prevent continual degradation. The timeline for tendon healing is estimated (Figure 3) where pro-inflammatory signaling resolves between about week 1 and 6 post-injury, yet the underlying mechanisms for resolving inflammation (or not resolving inflammation) is unclear. Endogenous TPCs are capable of regenerating tenocytes, but this ability becomes limited due to aging and their susceptibility to injury (Xu and Liu, 2018). Furthermore, TPCs are implicated in fatty infiltration, fibrosis, and calcification in tendinopathy through TPC differentiation into adipocytes and chondrocytes (Figure 1), signaled by high levels of the pro-inflammatory cytokine prostaglandin E2 (PGE2) (Zhang and Wang, 2010, 2014; Agarwal et al., 2017).
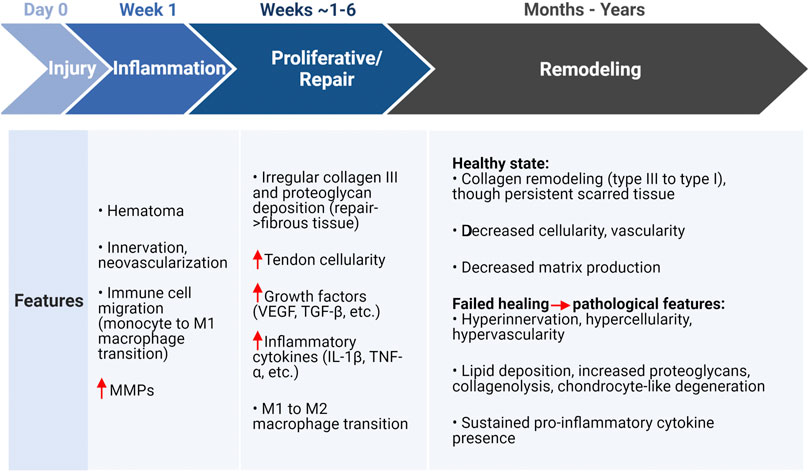
FIGURE 3. Tendon healing estimated timeline and features associated with a healthy or pathological state following injury. Created with Biorender.com. Initial injury due to overloading induces an inflammatory state that is characterized by matrix degradation, cell apoptosis, and immune cell localization (monocytes) that transition to a pro-inflammatory (M1) macrophage for removal of cell and ECM debris. The proliferative or repair phase (estimated 1–6 weeks) is characterized by injury site repair via increased cellularity, growth factors, inflammatory cytokines, and eventually the inflammation resolving M2 macrophage transition. The final remodeling stage can take months to years, where pathological features may persist with sustained inflammatory cytokines (though not immune cells) and degeneration of the ECM. In either the healthy or pathological case, a persistent scar-like tissue remains present (Fu et al., 2010; Lipman et al., 2018; Alim et al., 2020; Gracey et al., 2020). MMPs, matrix metalloproteinases; VEGF, vascular endothelial growth factor; TGF-β, transforming growth factor β; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor α.
Mechanical micro-ruptures and metabolic byproducts during repetitive or hyper-physiological loading stresses the tendon cells. Ultimately, this triggers an inflammatory cascade, leading to ECM degradation and further pathological characteristics (Figure 2B). Other factors highly involved in these pathways include pro-inflammatory cytokines interleukin-1 (IL-1) and tumor necrosis factor (TNF)-α (John et al., 2010), glutamate, vascular endothelial growth factor (VEGF), and substance P (Abate et al., 2009; Schulze-Tanzil et al., 2011). These signals activate angiogenesis and nerve ingrowth, leading to pain. In addition, they contribute to fibrosis formation due to macrophage signaling and localization and thus fibroblast activation (Dakin et al., 2012, 2017). Inflammatory pathways lead to ECM degradation and mechanical weakening of ECM via MMP upregulation and downregulation of tissue inhibitors of MMPs (Abate et al., 2009; Buono et al., 2013). Prolonged presence of macrophages further contributes to degradation of the ECM as they also secrete MMPs. Overall, micro-ruptures may also contribute to the onset of tendinopathy through the inability to properly heal or resolve inflammation before reoccurrence.
4 Tendon Mechanical Properties
Tendon’s passive, viscoelastic properties are dependent on species, age, gender, and anatomical location (Johnson et al., 1994; Itoi et al., 1995; Maganaris and Paul, 1999; Louis-Ugbo et al., 2004; Burgio et al., 2022). They have unique and identifiable material properties, including Young’s Modulus and ultimate failure force. In tendon mechanobiology studies, these properties are often quantified in conjunction with other physiological and structural changes. The two most common mechanical tests include a tensile pull-to-failure, or ultimate stress test (Figure 4A) and a fatigue, or creep test (Figure 4B).
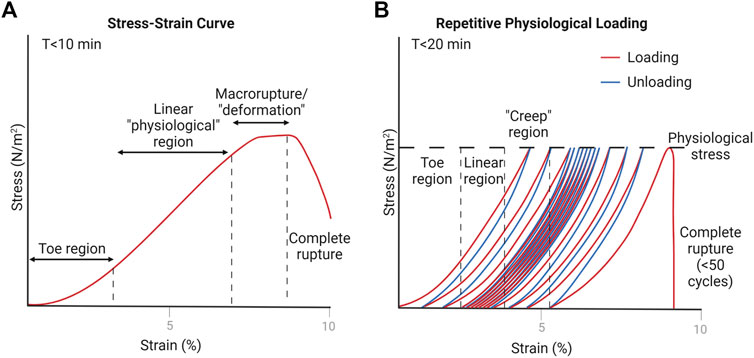
FIGURE 4. Representative estimations of tendon loading schemes. Created with Biorender.com. (A) Pull-to-failure test results in a stress-strain curve when normalized to tendon cross-sectional area (Wang, 2006; Wang et al., 2012). The toe region is due to natural collagen fiber crimping, the linear region is equated to physiological stress values, and the deformation region is where macro-scale ruptures start to occur (Louis-Ugbo et al., 2004). (B) Fatigue test indicates similar regions that occur due to repetitive physiological loading, where “creep” occurs due to macro-scale ruptures eventually leading to complete rupture of the tendon after a number of cycles (usually less than 50) (Thornton et al., 2002).
In ultimate stress tests, the tendon is pulled along the longitudinal axis at a specified rate, and the stress or force values are often measured alongside tissue strain or deformation to form a stress-strain curve. From this curve, Young’s Modulus is determined by the slope of the linear region—representing tendon stiffness. Loading of the in vivo tendon in the linear range is considered physiological loading (generally 2–6% strain) (Wang and Chen, 2018). Various types of loading, even in the physiological range, can cause micro-tearing within the tendon. After the linear range, the tendon starts to experience plastic deformation, or macro-ruptures, and ultimately leads to complete rupture of the tendon. At this point, the ultimate stress value is defined.
Measured values of Young’s Modulus and ultimate stress will vary drastically across species, the specific tendon, and the loading rate used to perform the tensile test (Burgio et al., 2022). For a loading rate of 0.1 mm/s, rat Achilles tendon measurements for Young’s Modulus and ultimate stress range between 179 ± 36 MPa and 45 ± 10 MPa, respectively (Eliasson et al., 2007). For a loading rate of 1 mm/s, however, the Young’s Modulus (405 ± 115 MPa) was much greater for the rat Achilles tendon as well as its ultimate stress values (51.6 ± 10.8 MPa) (Legerlotz et al., 2007). Furthermore, the human Achilles tendon Young’s Modulus of 816 ± 218 MPa and ultimate stress of 7.5 ± 1.1 MPa, measured at a rate of 1 mm/s, vary greatly from the rat (Wren et al., 2001). Burgio et al., 2022, covers the mechanical properties of various tendons in-depth (Burgio et al., 2022).
Fatigue tests provide insight on the tendon’s ability to repeatedly sustain loads, such as in running or walking. The setup is similar to ultimate stress tests, except that the tendon is uniaxially pulled to a specified force under a specific loading rate, then released, and repeated at some frequency. Because of the viscoelastic nature of the tendon, repetitive loading leads to an increase in deformation or strain (“creep”) at the specified force. After a number of cycles (less than 50), the tendon suffers macro-ruptures and eventually ruptures completely (Wang and Chen, 2018).
Heterogeneity in structure and extracellular matrix composition along the length of the tendon affects the biomechanical properties both locally and as a whole. In addition, activity levels can alter the viscoelastic properties of the tendon by increasing or decreasing tendon stiffness and ultimate stress/force (Maeda et al., 2011). In the mature tendon, chronic loading during physical training upregulates collagen turnover, perhaps leading to overall increased collagen synthesis, and improves the tendon’s ability to handle various stresses and loads over time (Kjaer, 2004). As described earlier, what constitutes repetitive overloading in vivo is not consistent as it is both temporally- and architecturally-dependent.
5 Model Systems
5.1 Ex Vivo
Studies in ex vivo models have shown that cell apoptosis and upregulation of inflammatory pathways occurs at 9–20% strain loading values (Maeda et al., 2010; Thorpe et al., 2015; Wang et al., 2015). Tendon rupture also leads to massive tenocyte cell death through high expression of TGF-β (Maeda et al., 2011). Meanwhile, underloading-induced tendon degeneration is driven primarily by enzymatic digestion which affects the tendon mechanics (Maeda et al., 2011; Wang and Chen, 2018). For example, unloaded rabbit patellar tendons in the presence of collagenase have an 80% decrease in elongation to failure, max failure force, and linear stiffness as compared to tendons loaded at 4% static strain (Nabeshima et al., 1996). Only physiological dynamic loading, which is generally between 4–8% strain at 0.25–1 Hz in ex vivo models, leads to increased collagen production and regenerative properties (Legerlotz et al., 2013; Wang et al., 2013, 2015). Highlighted tendon mechanobiology ex vivo work is summarized in Table 1 for recent findings from years 2015–2021. In addition, a general schematic of the combined culture and loading systems (bioreactors) used for ex vivo studies included in this review are illustrated in Figure 5A.
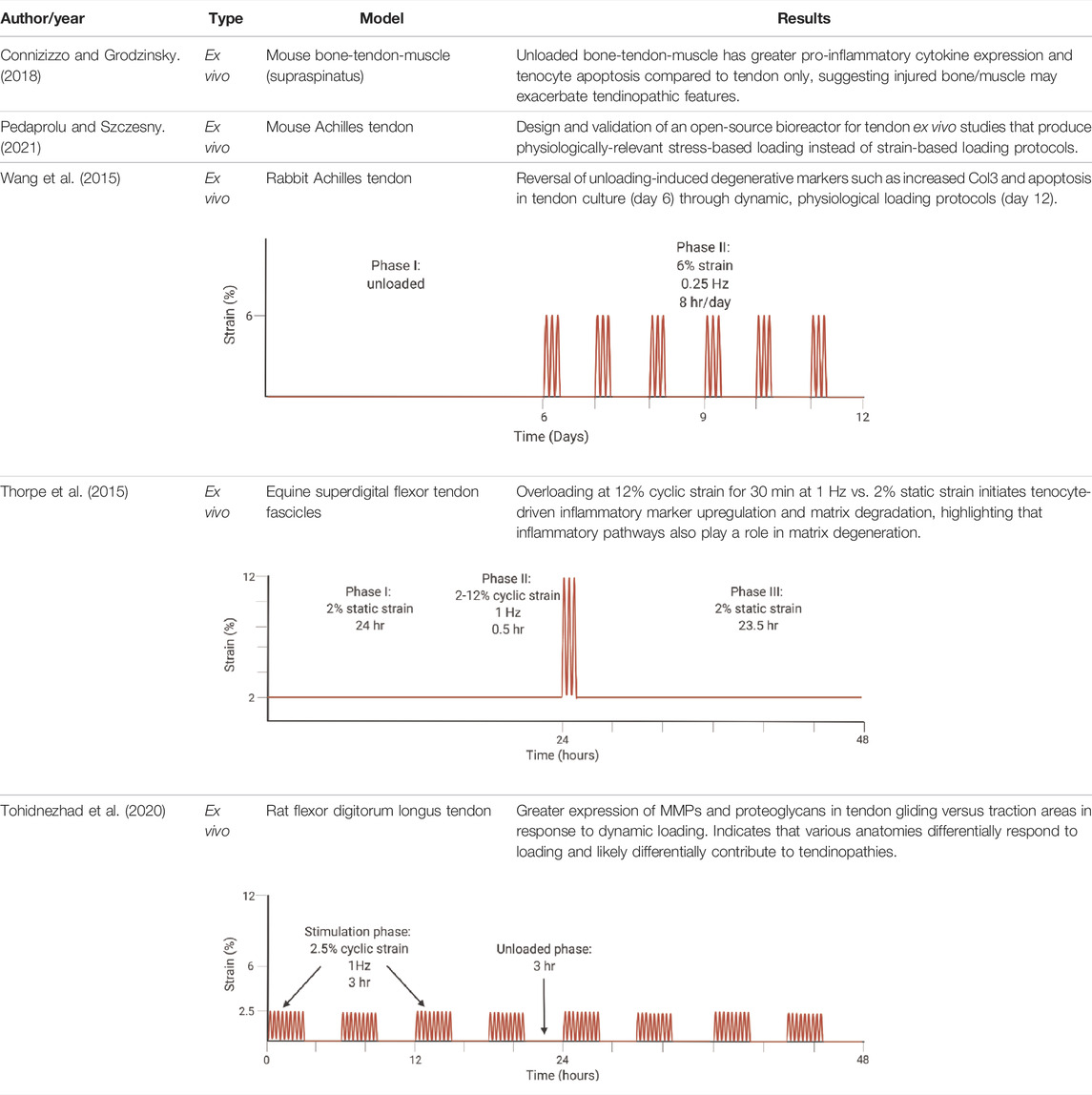
TABLE 1. Highlighted summaries of recent ex vivo studies with associated loading schemes. Images created with Biorender.com.
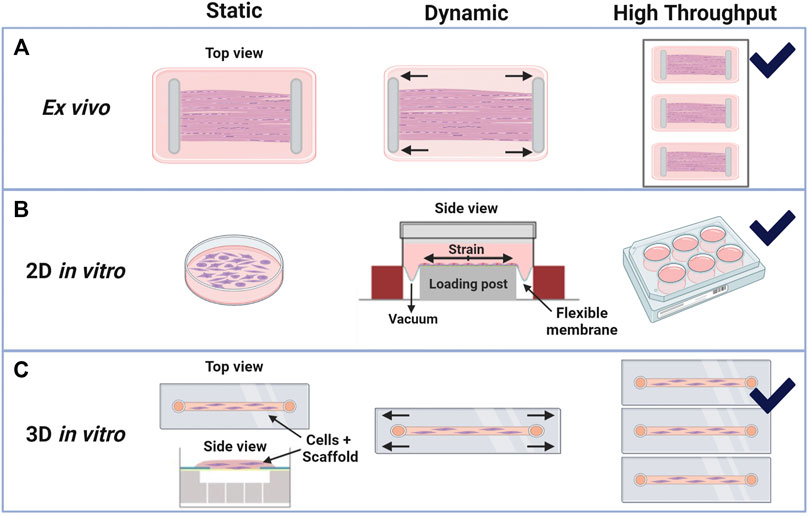
FIGURE 5. Bioreactor schematics for in vitro and ex vivo systems with static or dynamic loading and high-throughput options. Created with Biorender.com. (A) Example ex vivo systems are commonly developed in-house with whole tendon clamping systems that can be uniaxially stretched under set strains or loads (Wang et al., 2015; Tohidnezhad et al., 2020; Pedaprolu and Szczesny, 2021). (B) Tenocyte cell culture monolayers (2D in vitro systems) under static load, or no load, and dynamic loads have the greatest high-throughput capabilities. Dynamic loads on flexible membranes may be produced by mechanical actuators or with vacuum pressure, as illustrated and modified from Flexcell International’s Tension System, Burlington, NC (Arnoczky et al., 2002; Fleischhacker et al., 2020; Gaut et al., 2020; Kubo et al., 2020). (C) Cell-seeded 3D constructs can be gel-like, as shown in the side view (modified from Flexcell’s 3D Tissue Train System), or like whole-tissue if decellularized constructs are used. These 3D systems are generally bound by the dimensions of the material or well in which they are contained, yet there is flexibility in load types (tensile, shear, etc.) that can be analyzed (Zhang et al., 2015; Patel et al., 2017; Wang and Thien, 2018; Sawadkar et al., 2020; Pentzold and Wildemann, 2022).
Many of the previously described ex vivo studies were limited in analyzing longer-term inflammatory effects, especially in overloading of explants. Only Maeda et al., 2011, measured cytokine factors after 7 days of culture with no difference found between unloaded and physiologically loaded groups. Despite this finding, measuring pro-inflammatory factors in hyper-physiologically loaded tissues over at least a 7-day period may help elucidate pathomechanisms associated with tendon matrix modulation by removing other tissue effects. Downstream of TGF-β signaling, hyper-physiological loading is expected to increase IL-1β expression (Figure 2B). It would be helpful to characterize temporal IL-1β expression in hyper-physiological loading scenarios to identify potential feedback loops within the tissue.
Inflammatory cascades within tendon can originate from other sources (Arvind and Huang, 2021). In a rotator cuff muscle, bone, and tendon explant culture model, 7 days in culture results in upregulation of TNF-α and IL-6, atrophy, and tenocyte cell death as compared to the tendon only explant culture (Connizzo and Grodzinsky, 2018). These findings correlate with upregulation of TNF-α, interleukin-6 (IL-6), and IL-1 in bone injuries (Connizzo and Grodzinsky, 2018) and reinforce that unloaded tendon explants alone may produce limited quantities of pro-inflammatory factors (Maeda et al., 2011). This indicates that the muscle and bone likely play a role in the inflammatory signaling and ultimate degeneration of tendon after injury. Culture conditions, specifically serum-supplemented media, likely play a role in directing tenocytes to a pro-inflammatory phenotype as well in both whole tendon explants and 2D culture (van Vijven et al., 2021). Underlying presence of pro-inflammatory factors is generally unknown or varies in serum batches, which may contribute to the pro-inflammatory phenotypes. A primary limitation with these studies is that the tissues were unloaded once explanted; however, these results highlight the need to understand the role of other tissues and culture conditions in inflammatory signaling.
Mechanical loading to a specific strain value is less physiologically relevant as compared to stress-specific loading. This is due to the viscoelastic nature of the tendon, where repeated loading to a certain strain level on the tendon leads to decreasing absolute stress over time. Variations in cross-sectional area lead to differential load distribution across samples when stretched to the same strain as well. An ex vivo bioreactor was designed for this issue specifically, where researchers could characterize the link between tendon explant fatigue loading with tendon degeneration using cyclic loads and stresses instead of strains (Pedaprolu and Szczesny, 2021).
Physiological mechanical loading is necessary for tissue homeostasis though may also have therapeutic effects following injury. In a study by Wang et al., researchers induced early-stage tendinopathy of the rabbit Achilles tendon by unloading the tendon for 6 or 12 days, and then they reversed the effect in both treatment groups via external mechanical stimulation in a previously developed bioreactor (Wang et al., 2015). They stimulated the tendon under 6% strain, 0.25 Hz, and 8 h per day for 6 days based on their previous work estimating loads of 5–6% strain during hopping (schematic illustrated in Table 1). After the 6 days, results indicated reversal of the biochemical, structural, and mechanical changes that were induced by unloading. Significantly, they measured increased Col1 production and decreased cell apoptosis and ECM degradation (Wang et al., 2015). Their work highlights the need for further research on the regenerative capacity of tendon.
Overall, few studies have focused on gliding tendons and the role that compressive and shear forces play in tendon pathologies such as carpal tunnel syndrome (Tse and Keir, 2020). The pathomechanism is widely unknown for such cases. A pilot study by Tohidnezhad et al. measured tenocyte-specific marker expression and ECM remodeling components (MMP-1 and 13 and proteoglycans) in both the traction and gliding, with chondrocyte-like tenocytes, areas of the tendon after 48 hours of uniaxial cyclical loading (refer to Table 1 for the loading schematic). They found that the gliding area of the tendon that is exposed to greater shear and compressive forces had greater upregulation of matrix degradation markers compared to the traction area (Tohidnezhad et al., 2020). This may correlate to clinical observations that tendon ruptures frequently occur in the gliding area of tendons. However, limitations of this study include the short stimulation period (48 h) and limited analysis of the many factors involved in matrix remodeling.
5.2 In Vitro
Previous work indicates that loading type (biaxial versus uniaxial) and structural environment (2D versus 3D) affect cellular responses in vitro. Loading strains of greater than 10–12% induce expected pathways, such as MMP upregulation, inflammatory factors, cell apoptosis, and angiogenic factors in 2D (Arnoczky et al., 2002) and upregulation of inflammatory factors and MMP with decreased tenogenic factors in 3D cultures (Pentzold and Wildemann, 2022). Similarly, underloading led to activation of cellular inflammation and matrix degradation pathways (Arnoczky et al., 2002;; Tsuzaki et al., 2003a; Chen et al., 2009). Physiological loading ranges vary depending if the platform is 2D or 3D as well as 3D construct material (Patel et al., 2017). In general, local strains to the cells in 3D culture are dependent on the scaffold material and crosslinking density of hydrogels (Bryant et al., 2004). Fibrous scaffolds also may lead to higher variability than bulk 3D hydrogels that can more uniformly transfer loads to all cells within the construct (Kloxin et al., 2010). Highlighted tendon mechanobiology in vitro work is summarized in Table 2 for recent findings from years 2015–2020. Bioreactor setups used for in vitro work are outlined in Figures 5B,C.
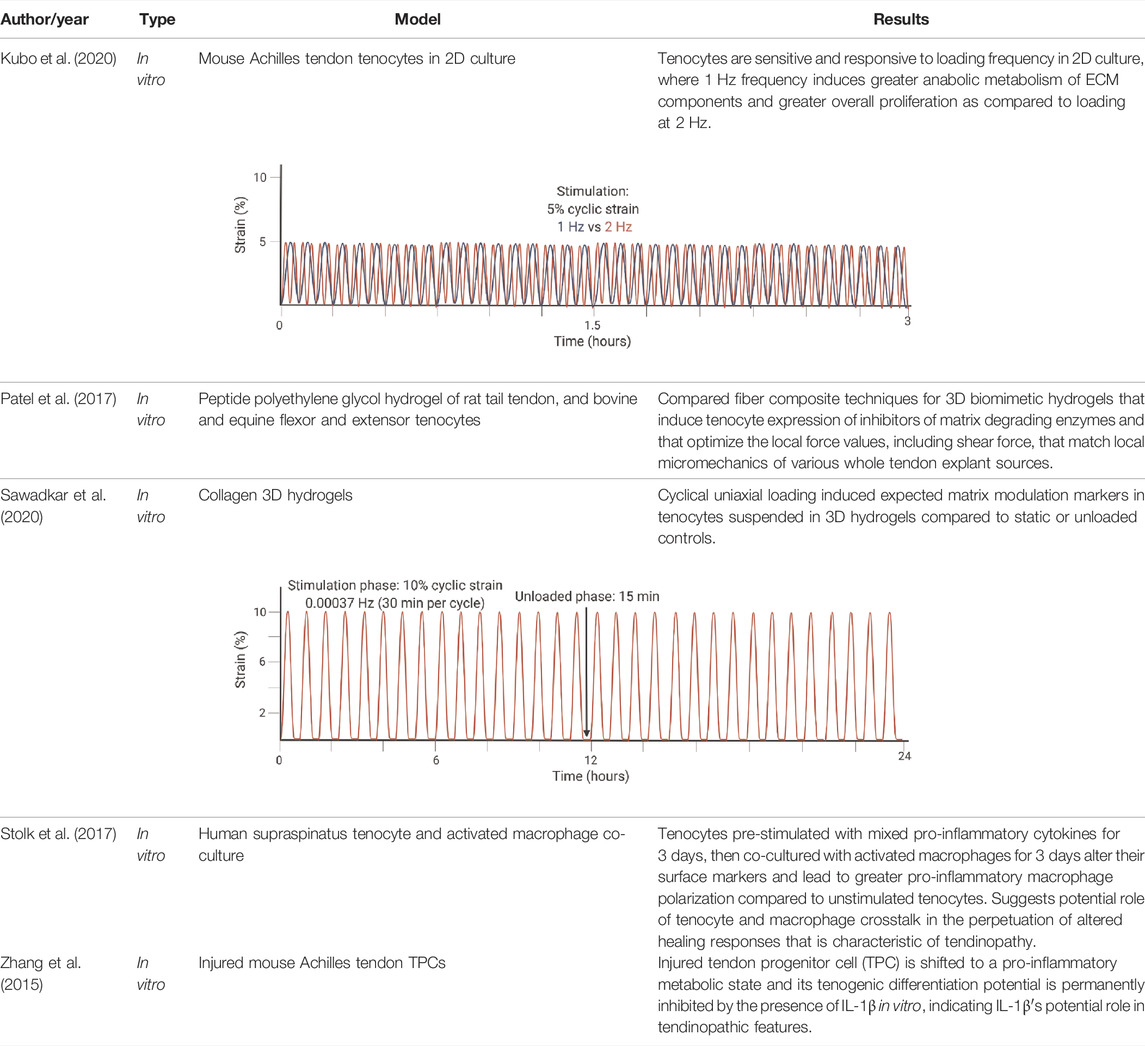
TABLE 2. Highlighted summaries of recent in vitro studies with associated loading schemes. Images created with Biorender.com.
For in vitro studies, loading type and frequency affect catabolic and anabolic pathways. In 2D, a frequency of 1 Hz (similar to walking) compared to 2 Hz (similar to sprinting) lead to greater cell proliferation of 2D cultures, greater cell viability, greater Col 1 expression, reduced expression of MMP-1 and MMP-13, and decreased expression of VEGF (Kubo et al., 2020). The loading schematic is illustrated in Table 2 for Kubo et al., 2020. In 3D cell constructs, dynamic loading type affects TPC differentiation and tendon formation via altering pathway activation. With biaxial loading, tenocytes experience both longitudinal and radial or lateral loading. Uniaxial loading provides a more physiologically relevant stimulation of the 3D cell culture niche that is necessary for tenogenic differentiation (Wang and Thien, 2018). Uniaxial loading in 3D cell culture led to the discovery that the transmembrane integrin protein is activated via the PI3K/AKT pathway (Manning and Cantley, 2007; Paterno et al., 2011). Meanwhile, tissue constructs aim to mimic the collagen fibers loading scheme with the correct ratio of higher magnitude tensile to lower magnitude shear forces (Patel et al., 2017; Sawadkar et al., 2020). In native tendon, whole tissue strain varies from the local fiber strains, with fiber extension reaching only about 40% of the whole tissue (Thorpe et al., 2013; Shepherd et al., 2014). One particular 3D hydrogel was developed to mimic the dynamics of collagen fibers in the native tendon and therefore forces, such that the sliding of fibers produced shear forces and fiber extension as tension (Patel et al., 2017).
As with ex vivo models, there is a need to characterize inflammatory effects in these models of tendon degeneration. Studies found that IL-1β expression is upregulated in tendon cells by exogenous IL-1β (M Tsuzaki et al., 2003b), which triggers the cytokine-induced MMP matrix degradation pathways due to fibroblast activation. In addition, IL-1β alters metabolic pathways and inhibits TPC differentiation potential irreversibly through downregulation of Col1, Col3, Scx, Tnmd, and biglycan (Zhang et al., 2015). Another pilot study by Stolk et al. highlighted that pro-inflammatory pre-stimulated human tenocytes co-cultured with macrophages lead to altered surface markers and increased expression of inflammatory cytokines, such as IL-6 and IL-8, as well as influenced macrophage polarization to a pro-inflammatory state after 3 days (Stolk et al., 2017). Results from this work corroborates cytokine signals originating from other tissues might play a role in tendon degenerative pathways as well as contribute to a positive-feedback loop in tendon cells.
Although 3D cultures facilitate manipulation of the loading protocols, the current limitation of this work is that limited inflammatory analysis is performed on tissue constructs. More specifically, combinatory effects of loading and culture conditions on inflammatory responses needs to be explored. Determining inflammatory effects in vitro is also further complicated by tenocyte response variations associated with the model species used (Oreff et al., 2021), which indicates the importance of selecting a model that most appropriately matches the research question.
5.3 Other Model Systems
Live animal models are commonly used in the study of tendinopathy as they are the most physiologically relevant. Overloading of the Achilles tendons is often performed in rodents as they have homologous anatomy and physiology to humans. They have shown that intensive treadmill running induces degenerative tendon pathologies, including TPC differentiation into chondrocyte-like cells that lead to non-tendinous tissue signaling (Zhang et al., 2020). Meanwhile, more moderate treadmill running promotes TPC differentiation into tenocytes that achieve homeostasis (Zhang et al., 2020). In a recent equine model, researchers found that biomechanical and compositional adaptation that occur from mechanical loading are localized to the non-collagenous extracellular matrix (Zamboulis et al., 2020). However, discrepancies may exist between in vivo and in vitro models. A pilot study illustrated differences between expressions of ECM components for in vitro mouse Achilles tenocytes versus in vivo mouse Achilles tendons. Specifically, in vitro loading led to a higher expression of collagen type III versus in vivo (Fleischhacker et al., 2020). Though successful at modeling tendinopathy, in vivo models are plagued by confounding variables compounded by the difficulty in identifying the underlying mechanisms.
Nevertheless, in vivo models highlight important findings that could shift focus for future investigations, as presented by Zamboulis et al., 2020 with the finding of non-collagenous matrix adaptations. A piezo-bioelectric device used with in vitro and in vivo systems of tendon regeneration suggest the need to further investigate the role of mechanical stimulation in modulating tenogenic-specific phenotypes (Fernandez-Yague et al., 2021). Fernandez-Yague et al. suggests that mechanical stimulation may induce pro-regenerative pathways via modulation of ion channel sensitivity. Furthermore, findings from Passini et al. suggest that shear stress is sensed through the specific PIEZO1 calcium ion channel in rodents and leads to upregulation of collagen cross-linking factors related to tendon stiffness (Passini et al., 2021). The direct effect of various mechanical stimuli in modulating these mechanosensing pathways is important to uncover in future work.
Overall, live models of tendon degeneration and regeneration show age-dependent mechanistic effects (Freedman et al., 2022; Kinitz et al., 2022). Specifically, mouse Achilles tendon following transection recruits Scx-lineage tenocytes to the repair site in neonatal development, but not in adult mice (Howell et al., 2017). The neonatal healing strategy ultimately results in functional restoration, whereas the adult suffers from dysfunction due to fibrotic scarring. Despite this new finding, the underlying mechanism of Scx-lineage tenocyte recruitment is unclear.
Recent advancements in multiscale models have enabled researchers to estimate in vivo stress and strain values (Pizzolato et al., 2020; Devaprakash et al., 2022) as well as investigate the role of mechanical loading (both quasi-static and dynamic) and tendon healing on multiscale mechanical, structural, and compositional properties (Freedman et al., 2018). Patient-specific finite element (FE) models of the human Achilles tendon and an OpenSim neuromusckuloskeletal model may be combined for measuring real-time stress and strain values during various activities (Pizzolato et al., 2020). In another FE analysis, macroscale tendon strain stiffening is reduced with higher magnitude and longer duration loading as well as increased laxity and delayed fiber re-alignment with applied strain (Freedman et al., 2018). Microscale properties during early healing were found to differ greatly from uninjured tendon. Predicted deficits in ECM stress transmission were found following fatigue loading and during healing in FE analysis (Freedman et al., 2018). In general, FE analysis allows for rapid testing that can facilitate experimental designs without the use of live animals, but it also may aid in patient-specific tendon rehabilitation protocols.
5.4 Evaluation of Model Systems
Various model systems are utilized to study tendon mechanobiology. As shown in Table 3, key features of each type of model system (ex vivo, 2D in vitro, 3D in vitro, and in vivo) are semi-quantitatively rated on their overall ability to replicate seven desired features when performing assessments of tendon mechanobiology. These key features include “biological relevance”, “biological controllability”, “biomechanical relevance”, “biomechanical controllability”, “accessibility”, “usability”, and “translatability”. Only mammalians are included in this assessment as they share similar immunological characteristics to humans. Key feature rating values were assigned to the model system types on a relative scale of one to four due to the four types of model systems that are being evaluated. For instance, a value of “one” for “biological relevance” for 2D in vitro systems was assigned as it least mimics the physiological environment as compared to 3D in vitro (second lowest = 2), ex vivo (3), and in vivo (highest = 4).
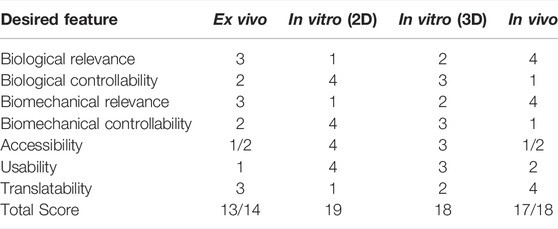
TABLE 3. Summary chart of features desired by various model types for replicating and characterizing tendon mechanoresponses (scale: 1 = lowest to 4 = highest).
Each key feature has particular aspects that were considered in the rating assessment of the model systems though some of their ratings are similar. For “biological relevance”, the presence of other physiological cues was considered, such as immune and cell signaling crosstalk (2D lowest, in vivo highest). Because cells in 2D culture are isolated from other tissues and extended cell culture may alter cell phenotype, 2D in vitro systems rank the lowest for “biological relevance”. For “biological controllability”, the ability to control crosstalk factors was considered and the opposite rating trend was assigned (2D highest, in vivo lowest). Cultures in 2D have the greatest controllability as crosstalk can be isolated to specific cell types or factors using co-culturing techniques. Then cell-matrix interactions can be captured and manipulated with 3D systems, which is why 3D systems rank the second highest for “biological controllability”, followed by ex vivo (2) and in vivo (1) systems. Similarly, “biomechanical relevance” is related to the ability of the investigator to recapitulate the in vivo biomechanical environment (2D lowest, in vivo highest), while “biomechanical controllability” relates to the ability to control the mechanical loading environment (2D highest, in vivo lowest). Explanted tissues, or ex vivo models, maintain the native extracellular matrix, which affects mechanosensing pathways, and this contributes to its higher rating than both 2D and 3D in vitro systems for both biological and biomechanical relevance. In vivo models rank highest in biomechanical relevance because natural variation in loading parameters, gait, and load sharing with other tissues are not recapitulated in ex vivo systems.
Aspects for the other three key features are straightforward. The “accessibility” of a model system relates to the ability to obtain the desired tissue or animal sources for that system. Because primary cell lines are often the most accessible, 2D in vitro systems scored highest (4), followed by 3D in vitro (3), and then ex vivo and in vivo systems share a rating of 1 or 2 as access to whole tissue for ex vivo work or live animals for in vivo work may depend on the institution and regulations. Usability relates to the technical feasibility of developing the model system for dynamic loading experiments (2D highest, followed by 3D, in vivo, and then ex vivo systems). Development of the model system includes aspects such as accruing a bioreactor, troubleshooting, and experimental timelines. Again, these rankings might be institution- or resource-dependent. Last, translatability relates to how well these model systems translate to clinical work, where 2D in vitro has the lowest level of translation while in vivo systems are the most translatable.
In this assessment, each key feature was equally weighted to minimize subjective evaluation; however, this weighting could change based on the research question. For example, “biomechanical controllability” would likely be weighted more heavily than “biomechanical relevance” if an investigator was aiming to determine underlying cell signaling factors for varied loading conditions.
6 Discussion
Mechanical loading of tendon tissue is essential for tendon maturation during development, tendon homeostasis, and degeneration. Many reviews focus in-depth on the history of understanding matrix turnover, tendon biomechanics, and the methods/models used to understand tendon as well as ligament mechanobiology (Lavagnino et al., 2015; Thomopoulos et al., 2015; Wang and Chen, 2018; Dyment et al., 2020; Friese et al., 2020; Gracey et al., 2020; Wang et al., 2020; Bramson et al., 2021). In addition, in vivo models of tendon degeneration are the focus of another review (Theodossiou and Schiele, 2019). This review summarizes the more recent findings relating to tendon mechanobiology using various model systems. In addition, it provides an overall assessment or rating of model systems in their ability to replicate various key features necessary to evaluate tendon mechanobiology. This method of assessing the propriety of a system may facilitate experimental design as it can be tailored for the targeted research question. Based on this assessment, the sum total for 2D in vitro systems was greatest overall (19 out of a max of 28), followed by 3D in vitro (18), in vivo (17/18), and then ex vivo systems (13/14).
Though significant improvements in understanding inflammatory mediators in normal tendon healing have been achieved (Chisari et al., 2019; Arvind and Huang, 2021), inflammation is still not well understood as an effector in tendon pathology, especially its temporal role. For instance, co-culture studies show that tenocyte and macrophage crosstalk likely facilitates macrophage polarization to a pro-inflammatory state after 3 days (Stolk et al., 2017). Yet it is unclear when macrophages polarize to the inflammation-resolving state during healing, if inflammation is fully resolved in pathology, and if tenocytes play a role in this polarization at all. As such, it is necessary to tease apart the inflammatory crosstalk occurring between tissues and the immune system.
Future studies focused on longer-term experiments (Maeda et al., 2011) and decoupling mechanical loading and inflammation crosstalk is recommended to fully characterize model systems of tendon pathology. As it is common for many engineered 3D constructs under dynamic loads to physically weaken after only a few days, the use of explanted tissue or decellularized constructs may help mitigate the limitations associated with experiment length. In addition, decoupling mechanical effects alongside mechanosensing pathways may be accomplished with bioreactor designs that include various mechanical stimulation options, or perhaps through the use of piezo-electric devices in cultivation studies (Fernandez-Yague et al., 2021; Passini et al., 2021). Last, in vivo stress, strain, and frequency values should inform loading paradigms for in vitro and ex vivo bioreactor studies.
The ultimate goal of this work is to improve regenerative therapies and clinical outcomes, or prevent tendon pathologies entirely. Many studies have limited translation due to heterogeneous experimental designs, bioreactor systems, and loading schemes. Addressing these limitations is essential to progressing the field of tendon mechanobiology, though teasing apart the effects of inflammation from the associated mechanical stimulatory effects is not a simple task. Taken together, improvements of in vitro, ex vivo, and in vivo model systems could improve overall translation. Conclusively, considerable attention on performing dynamic loading studies of at least 7 days with inflammatory analysis is paramount due to the scarcity of such explorations currently in the literature.
Author Contributions
LB performed the literature review and wrote the manuscript. JS, MG, and RB provided critical feedback on the manuscript, layout, and figures/tables.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2022.896336/full#supplementary-material
Abbreviations
ECM, extracellular matrix; TPC, tendon progenitor cell; Col 1, collagen type I; Col 3, collagen type III; MMP, matrix metalloproteinases; ADAMT, disintegrin and metalloproteinase with thrombospondin motifs; TGF-β, transforming growth factor β; Mhk, Mohawk; Scx, Scleraxis; Tnmd, Tenomodulin; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor; IL-6, interleukin-6; FEA, finite elemental analysis.
References
Abate, M., Gravare-Silbernagel, K., Siljeholm, C., Di Iorio, A., De Amicis, D., Salini, V., et al. (2009). Pathogenesis of Tendinopathies: Inflammation or Degeneration? Arthritis Res. Ther. 11 (235), 235–315. doi:10.1186/AR2723
Agarwal, S., Loder, S. J., Cholok, D., Peterson, J., Li, J., Breuler, C., et al. (2017). Scleraxis-Lineage Cells Contribute to Ectopic Bone Formation in Muscle and Tendon. NIH Public Access 35 (3), 705–710. doi:10.1002/STEM.2515
Alim, M. A., Peterson, M., and Pejler, G. (2020). Do mast Cells Have a Role in Tendon Healing and Inflammation? Cells 9 (5), 1134–1215. doi:10.3390/cells9051134
Ansorge, H. L., Adams, S., Birk, D. E., and Soslowsky, L. J. (2011). Mechanical, Compositional, and Structural Properties of the Post-natal Mouse Achilles Tendon. Ann. Biomed. Eng. 39 (7), 1904–1913. doi:10.1007/S10439-011-0299-0
Ansorge, H. L., Hsu, J. E., Edelstein, L., Adams, S., Birk, D. E., and Soslowsky, L. J. (2012). Recapitulation of the Achilles Tendon Mechanical Properties during Neonatal Development: a Study of Differential Healing during Two Stages of Development in a Mouse Model. J. Orthop. Res. 30 (3), 448–456. doi:10.1002/JOR.21542
Arnoczky, S. P., Tian, T., Lavagnino, M., Gardner, K., Schuler, P., and Morse, P. (2002). Activation of Stress-Activated Protein Kinases (SAPK) in Tendon Cells Following Cyclic Strain: the Effects of Strain Frequency, Strain Magnitude, and Cytosolic Calcium. J. Orthop. Res. 20 (5), 947–952. doi:10.1016/S0736-0266(02)00038-4
Arvind, V., and Huang, A. H. (2021). Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 9, 1–16. doi:10.3389/FBIOE.2021.719047/BIBTEX
Attia, M., Scott, A., Carpentier, G., Lian, Ø., Van Kuppevelt, T., Gossard, C., et al. (2014). Greater Glycosaminoglycan Content in Human Patellar Tendon Biopsies Is Associated with More Pain and a Lower VISA Score. Br. J. Sports Med. 48 (6), 469–475. doi:10.1136/BJSPORTS-2013-092633
Berthet, E., Chen, C., Butcher, K., Schneider, R. A., Alliston, T., and Amirtharajah, M. (2013). Smad3 Binds Scleraxis and Mohawk and Regulates Tendon Matrix Organization. J. Orthop. Res. 31 (9), 1475–1483. doi:10.1002/JOR.22382
Birk, D. E., and Mayne, R. (1997). Localization of Collagen Types I, III and V during Tendon Development. Changes in Collagen Types I and III Are Correlated with Changes in Fibril Diameter. Eur. J. Cell. Biol. 72, 352–361.
Bramson, M. T. K., Van Houten, S. K., and Corr, D. T. (2021). Mechanobiology in Tendon, Ligament, and Skeletal Muscle Tissue Engineering. J. Biomechanical Eng. 143 (7), 1–15. doi:10.1115/1.4050035/1097189
Bryant, S. J., Chowdhury, T. T., Lee, D. A., Bader, D. L., and Anseth, K. S. (2004)., 32. CA, 407–417. doi:10.1023/B:ABME.0000017535.0060210.1023/b:abme.0000017535.00602.caCrosslinking Density Influences Chondrocyte Metabolism in Dynamically Loaded Photocrosslinked Poly(ethylene Glycol) HydrogelsAnn. Biomed. Eng.
Buono, A. D., Oliva, F., Osti, L., and Maffulli, N. (2013). Metalloproteases and Tendinopathy. Mltj 3 (1), 51–57. doi:10.11138/MLTJ/2013.3.1.051
Burgio, V., Civera, M., Rodriguez Reinoso, M., Pizzolante, E., Prezioso, S., Bertuglia, A., et al. (2022). Mechanical Properties of Animal Tendons: A Review and Comparative Study for the Identification of the Most Suitable Human Tendon Surrogates. Processes 10 (485), 485–520. doi:10.3390/PR10030485
Burk, J. (2019). “Mechanisms of Action of Multipotent Mesenchymal Stromal Cells in Tendon Disease,” in Tendons. Editor H. Sözen (London: IntechOpen), 1–31. doi:10.5772/INTECHOPEN.83745
Chen, Y.-J., Huang, C.-H., Lee, I.-C., Lee, Y.-T., Chen, M.-H., and Young, T.-H. (2009). Effects of Cyclic Mechanical Stretching on the mRNA Expression of Tendon/ligament-Related and Osteoblast-specific Genes in Human Mesenchymal Stem Cells. Connect. Tissue Res. 49 (1), 7–14. doi:10.1080/03008200701818561
Chisari, E., Rehak, L., Khan, W. S., and Maffulli, N. (2019). Tendon Healing in Presence of Chronic Low-Level Inflammation: a Systematic Review. Br. Med. Bull. 132 (1), 97–116. doi:10.1093/BMB/LDZ035
Connizzo, B. K., and Grodzinsky, A. J. (2018). Release of Pro-inflammatory Cytokines from Muscle and Bone Causes Tenocyte Death in a Novel Rotator Cuff In Vitro Explant Culture Model. Connect. Tissue Res. 59 (5), 423–436. doi:10.1080/03008207.2018.1439486
Cosgrove, B. D., Mui, K. L., Driscoll, T. P., Caliari, S. R., Mehta, K. D., Assoian, R. K., et al. (2016). N-Cadherin Adhesive Interactions Modulate Matrix Mechanosensing and Fate Commitment of Mesenchymal Stem Cells. Nat. Mater 15 (12), 1297–1306. doi:10.1038/nmat4725
Dahlgren, L. A., Mohammed, H. O., and Nixon, A. J. (2005). Temporal Expression of Growth Factors and Matrix Molecules in Healing Tendon Lesions. J. Orthop. Res. 23 (1), 84–92. doi:10.1016/j.orthres.2004.05.007
Dakin, S. G., Buckley, C. D., Al-Mossawi, M. H., Hedley, R., Martinez, F. O., Wheway, K., et al. (2017). Persistent Stromal Fibroblast Activation Is Present in Chronic Tendinopathy. Arthritis Res. Ther. 19 (16), 1–11. doi:10.1186/S13075-016-1218-4
Dakin, S. G., Werling, D., Hibbert, A., Abayasekara, D. R. E., Young, N. J., Smith, R. K. W., et al. (2012). Macrophage Sub-populations and the Lipoxin A4 Receptor Implicate Active Inflammation during Equine Tendon Repair. PLoS ONE 7 (2), e32333–12. doi:10.1371/JOURNAL.PONE.0032333
Devaprakash, D., Graham, D. F., Barrett, R. S., Lloyd, D. G., Obst, S. J., Kennedy, B., et al. (2022). Free Achilles Tendon Strain during Selected Rehabilitation, Locomotor, Jumping, and Landing Tasks. J. Appl. Physiology 132 (4), 956–965. doi:10.1152/japplphysiol.00662.2021
Dyment, N. A., Barrett, J. G., Awad, H. A., Bautista, C. A., Banes, A. J., and Butler, D. L. (2020). A Brief History of Tendon and Ligament Bioreactors: Impact and Future Prospects. J. Orthop. Res. 38 (11), 2318–2330. doi:10.1002/JOR.24784
Eliasson, P., Fahlgren, A., Pasternak, B., and Aspenberg, P. (2007). Unloaded Rat Achilles Tendons Continue to Grow, but Lose Viscoelasticity. J. Appl. Physiology 103 (2), 459–463. doi:10.1152/japplphysiol.01333.2006
Eyre, D. R., Paz, M. A., and Gallop, P. M. (1984). Cross-linking in Collagen and Elastin. Annu. Rev. Biochem. 53 (1), 717–748. doi:10.1146/annurev.bi.53.070184.003441
Fernandez‐Yague, M. A., Trotier, A., Demir, S., Abbah, S. A., Larrañaga, A., Thirumaran, A., et al. (2021). A Self‐Powered Piezo‐Bioelectric Device Regulates Tendon Repair‐Associated Signaling Pathways through Modulation of Mechanosensitive Ion Channels. Adv. Mater. 33 (40), 2008788–2008818. doi:10.1002/ADMA.202008788
Fessel, G., and Snedeker, J. G. (2009). Evidence against Proteoglycan Mediated Collagen Fibril Load Transmission and Dynamic Viscoelasticity in Tendon. Matrix Biol. 28 (8), 503–510. doi:10.1016/j.matbio.2009.08.002
Fleischhacker, V., Klatte-Schulz, F., Minkwitz, S., Schmock, A., Rummler, M., Seliger, A., et al. (2020). In Vivo and In Vitro Mechanical Loading of Mouse Achilles Tendons and Tenocytes-A Pilot Study. Ijms 21 (1313), 1313–1315. doi:10.3390/ijms21041313
Fleming, D. M., Cross, K. W., and Barley, M. A. (2005). Recent Changes in the Prevalence of Diseases Presenting for Health Care. Br. J. Gen. Pract. 55 (517), 589–595.
Freedman, B. R., Knecht, R. S., Tinguely, Y., Eskibozkurt, G. E., Wang, C. S., and Mooney, D. J. (2022). Aging and Matrix Viscoelasticity Affect Multiscale Tendon Properties and Tendon Derived Cell Behavior. Acta Biomater. 143 (4), 63–71. doi:10.1016/J.ACTBIO.2022.03.006
Freedman, B. R., Rodriguez, A. B., Leiphart, R. J., Newton, J. B., Ban, E., Sarver, J. J., et al. (2018). Dynamic Loading and Tendon Healing Affect Multiscale Tendon Properties and ECM Stress Transmission. Sci. Rep. 8, 1–13. doi:10.1038/s41598-018-29060-y
Friese, N., Gierschner, M. B., Schadzek, P., Roger, Y., and Hoffmann, A. (2020). Regeneration of Damaged Tendon-Bone Junctions (Entheses)-TAK1 as a Potential Node Factor. Ijms 21 (15), 5177–5221. doi:10.3390/ijms21155177
Fu, S. C., Rolf, C., Cheuk, Y. C., Lui, P. P., and Chan, K. M. (2010). Deciphering the Pathogenesis of Tendinopathy: a Three-Stages Process. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2 (30), 30–12. doi:10.1186/1758-2555-2-30/TABLES/1
Fu, S.-C., Chan, K.-M., and Rolf, C. G. (2007). Increased Deposition of Sulfated Glycosaminoglycans in Human Patellar Tendinopathy. Clin. J. Sport Med. 17 (2), 129–134. doi:10.1097/JSM.0b013e318037998f
Gaut, L., Bonnin, M.-A., Blavet, C., Cacciapuoti, I., Orpel, M., Mericskay, M., et al. (2020). Mechanical and Molecular Parameters that Influence the Tendon Differentiation Potential of C3H10T1/2 Cells in 2D- and 3D-Culture Systems. Biol. Open 9 (2), 1–13. doi:10.1242/bio.047928
Goodship, A. E., Birch, H. L., and Wilson, A. M. (1994). The Pathobiology and Repair of Tendon and Ligament Injury. Veterinary Clin. N. Am. Equine Pract. 10 (2), 323–349. doi:10.1016/S0749-0739(17)30359-0
Gracey, E., Burssens, A., Cambré, I., Schett, G., Lories, R., McInnes, I. B., et al. (2020). Tendon and Ligament Mechanical Loading in the Pathogenesis of Inflammatory Arthritis. Nat. Rev. Rheumatol. 16 (4), 193–207. doi:10.1038/s41584-019-0364-x
Howell, K., Chien, C., Bell, R., Laudier, D., Tufa, S. F., Keene, D. R., et al. (2017). Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci. Rep. 7 (45238), 1–14. doi:10.1038/SREP45238
Itoi, E., Berglund, L. J., Grabowski, J. J., Schultz, F. M., Growney, E. S., Morrey, B. F., et al. (1995). Tensile Properties of the Supraspinatus Tendon. J. Orthop. Res. 13 (4), 578–584. doi:10.1002/jor.1100130413
Järvinen, T. A. H., Järvinen, T. L. N., Kannus, P., ozsa, L., and Järvinen, M. (2004) ‘Collagen Fibres of the Spontaneously Ruptured Human Tendons Display Decreased Thickness and Crimp Angle’, J. Orthop. Res., 22(6), pp. 1303–1309. doi:10.1016/j.orthres.2004.04.003
John, T., Lodka, D., Kohl, B., Ertel, W., Jammrath, J., Conrad, C., et al. (2010). Effect of Pro-inflammatory and Immunoregulatory Cytokines on Human Tenocytes. J. Orthop. Res. 28 (8), a–n. doi:10.1002/JOR.21079
Johnson, G. A., Tramaglini, D. M., Levine, R. E., Ohno, K., Choi, N.-Y., and L-Y. Woo, S. (1994). Tensile and Viscoelastic Properties of Human Patellar Tendon. J. Orthop. Res. 12 (6), 796–803. doi:10.1002/jor.1100120607
Józsa, L., and Kannus, P. (1997). Histopathological Findings in Spontaneous Tendon Ruptures. Scand. J. Med. Sci. Sports 7 (2), 113–118. doi:10.1111/j.1600-0838.1997.tb00127.x
Kannus, P., and Józsa, L. (1991). Histopathological Changes Preceding Spontaneous Rupture of a Tendon. A Controlled Study of 891 Patients. J. Bone & Jt. Surg. 73 (10), 1507–1525. doi:10.2106/00004623-199173100-00009
Kannus, P. (2000). Structure of the Tendon Connective Tissue. Blackwell Munksgaard 10 (6), 312–320. doi:10.1034/J.1600-0838.2000.010006312.X
Khan, K. M., and Scott, A. (2009). Mechanotherapy: How Physical Therapists' Prescription of Exercise Promotes Tissue Repair. Br. J. Sports Med. Assoc. Sport Excercise Med. 43 (4), 247–252. doi:10.1136/BJSM.2008.054239
Kinitz, R., Heyne, E., Koch, L. G., Britton, S. L., Thierbach, M., and Wildemann, B. (2022). The Effect of Age and Intrinsic Aerobic Exercise Capacity on the Expression of Inflammation and Remodeling Markers in Rat Achilles Tendons. Ijms 23 (79), 79–20. doi:10.3390/IJMS23010079
Kjær, M. (2004). Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 84 (2), 649–698. doi:10.1152/PHYSREV.00031.2003
Kloxin, A. M., Tibbitt, M. W., and Anseth, K. S. (2010). Synthesis of Photodegradable Hydrogels as Dynamically Tunable Cell Culture Platforms. Nat. Protoc. 5 (12), 1867–1887. doi:10.1038/nprot.2010.139.Synthesis
Kubo, Y., Hoffmann, B., Goltz, K., Schnakenberg, U., Jahr, H., Merkel, R., et al. (2020). Different Frequency of Cyclic Tensile Strain Relates to Anabolic/catabolic Conditions Consistent with Immunohistochemical Staining Intensity in Tenocytes. Ijms 21 (3), 1082–1114. doi:10.3390/IJMS21031082
Kubow, K. E., Vukmirovic, R., Zhe, L., Klotzsch, E., Smith, M. L., Gourdon, D., et al. (2015). Mechanical Forces Regulate the Interactions of Fibronectin and Collagen I in Extracellular Matrix. Nat. Commun. 6 (1), 1–11. doi:10.1038/ncomms9026
Legerlotz, K., Schjerling, P., Langberg, H., Brüggemann, G.-P., and Niehoff, A. (2007). The Effect of Running, Strength, and Vibration Strength Training on the Mechanical, Morphological, and Biochemical Properties of the Achilles Tendon in Rats. J. Appl. Physiology 102 (2), 564–572. doi:10.1152/japplphysiol.00767.2006
Lavagnino, M., Arnoczky, S. P., Tian, T., and Vaupel, Z. (2003). Effect of Amplitude and Frequency of Cyclic Tensile Strain on the Inhibition of MMP-1 mRNA Expression in Tendon Cells: an In Vitro Study. Connect. Tissue Res. 44 (3–4), 181–187. doi:10.1080/03008200390215881
Lavagnino, M., Wall, M. E., Little, D., Banes, A. J., Guilak, F., and Arnoczky, S. P. (2015). Tendon mechanobiology:Current Knowledge and Future Research Opportunities. J. Orthop. Res. 33 (6), 813–822. doi:10.1002/JOR.22871
Leéjard, V., Brideau, G., Blais, F., Salingcarnboriboon, R., Wagner, G., Roehrl, M. H. A., et al. (2007). Scleraxis and NFATc Regulate the Expression of the Pro-α1(I) Collagen Gene in Tendon Fibroblasts. J. Biol. Chem. 282 (24), 17665–17675. doi:10.1074/JBC.M610113200
Legerlotz, K., Jones, G. C., Screen, H. R. C., and Riley, G. P. (2013). Cyclic Loading of Tendon Fascicles Using a Novel Fatigue Loading System Increases Interleukin‐6 Expression by Tenocytes. Scand. J. Med. Sci. Sports 23 (1), 31–37. doi:10.1111/j.1600-0838.2011.01410.x
Lipman, K., Wang, C., Ting, K., Soo, C., and Zheng, Z. (2018). Tendinopathy: Injury, Repair, and Current Exploration. Dddt Vol. 12, 591–603. doi:10.2147/DDDT.S154660
Liu, S. H., Yang, R. S., al-Shaikh, R., and Lane, J. M. (1995). Collagen in Tendon, Ligament, and Bone Healing. A Current Review. Clin. Orthop. Relat. Res. 1 (318), 265–278.
Lorimer, A. V., and Hume, P. A. (2014). Achilles Tendon Injury Risk Factors Associated with Running. Sports Med. 44 (10), 1459–1472. doi:10.1007/s40279-014-0209-3
Lorimer, A. V., and Hume, P. A. (2016). Stiffness as a Risk Factor for Achilles Tendon Injury in Running Athletes. Sports Med. 46 (12), 1921–1938. doi:10.1007/s40279-016-0526-9
Louis-Ugbo, J., Leeson, B., and Hutton, W. C. (2004). Tensile Properties of Fresh Human Calcaneal (Achilles) Tendons. Clin. Anat. 17 (1), 30–35. doi:10.1002/ca.10126
Maeda, E., Fleischmann, C., Mein, C. A., Shelton, J. C., Bader, D. L., and Lee, D. A. (2010). Functional Analysis of Tenocytes Gene Expression in Tendon Fascicles Subjected to Cyclic Tensile Strain. Connect. Tissue Res. 51 (6), 434–444. doi:10.3109/03008201003597056
Maeda, T., Sakabe, T., Sunaga, A., Sakai, K., Rivera, A. L., Keene, D. R., et al. (2011). Conversion of Mechanical Force into TGF-β-Mediated Biochemical Signals. Curr. Biol. 21 (11), 933–941. doi:10.1016/J.CUB.2011.04.007
Maganaris, C. N., and Paul, J. P. (1999). In Vivo human Tendon Mechanical Properties. J. Physiology 521 (1), 307–313. doi:10.1111/j.1469-7793.1999.00307.x
Manning, B. D., and Cantley, L. C. (2007). AKT/PKB Signaling: Navigating Downstream. Cell. 129 (7), 1261–1274. doi:10.1016/J.CELL.2007.06.009
Matson, A., Konow, N., Miller, S., Konow, P. P., and Roberts, T. J. (2012). Tendon Material Properties Vary and Are Interdependent Among turkey Hindlimb Muscles. J. Exp. Biol. 215 (20), 3552–3558. doi:10.1242/jeb.072728
Mendias, C. L., Gumucio, J. P., and Lynch, E. B. (2012). Mechanical Loading and TGF-β Change the Expression of Multiple miRNAs in Tendon Fibroblasts. J. Appl. Physiology 113 (1), 56–62. doi:10.1152/JAPPLPHYSIOL.00301.2012
Midwood, K. S., Chiquet, M., Tucker, R. P., and Orend, G. (2016). Tenascin-C at a Glance. J. Cell. Sci. 129 (23), 4321–4327. doi:10.1242/JCS.190546
Munger, J. S., and Sheppard, D. (2011). Cross Talk Among TGF- Signaling Pathways, Integrins, and the Extracellular Matrix. Cold Spring Harb. Perspect. Biol. 3 (11), a005017. doi:10.1101/CSHPERSPECT.A005017
Nabeshima, Y., Grood, E. S., Sakurai, A., and Herman, J. H. (1996). Uniaxial Tension Inhibits Tendon Collagen Degradation by Collagenasein Vitro. J. Orthop. Res. 14 (1), 123–130. doi:10.1002/JOR.1100140120
Oreff, G. L., Fenu, M., Vogl, C., Ribitsch, I., and Jenner, F. (2021). Species Variations in Tenocytes' Response to Inflammation Require Careful Selection of Animal Models for Tendon Research. Sci. Rep. 11 (1), 1–14. doi:10.1038/S41598-021-91914-9
Passini, F. S., Jaeger, P. K., Saab, A. S., Hanlon, S., Chittim, N. A., Arlt, M. J., et al. (2021). Shear-stress Sensing by PIEZO1 Regulates Tendon Stiffness in Rodents and Influences Jumping Performance in Humans. Nat. Biomed. Eng. 5 (12), 1457–1471. doi:10.1038/s41551-021-00716-x
Patel, D., Sharma, S., Bryant, S. J., and Screen, H. R. C. (2017). Recapitulating the Micromechanical Behavior of Tension and Shear in a Biomimetic Hydrogel for Controlling Tenocyte Response. Adv. Healthc. Mat. 6 (4), 1601095–1601097. doi:10.1002/ADHM.201601095
Paterno, J., Vial, I. N., Wong, V. W., Rustad, K. C., Sorkin, M., Shi, Y., et al. (2011). Akt-mediated Mechanotransduction in Murine Fibroblasts during Hypertrophic Scar Formation. Wound Repair Regen. 19 (1), 49–58. doi:10.1111/J.1524-475X.2010.00643.X
Pedaprolu, K., and Szczesny, S. E. (2021). A Novel, Open Source, Low-Cost Bioreactor for Load-Controlled Cyclic Loading of Tendon Explants. bioRxiv. doi:10.1101/2021.03.16.435688
Pentzold, S., and Wildemann, B. (2022). Mechanical Overload Decreases Tenogenic Differentiation Compared to Physiological Load in Bioartificial Tendons. J. Biol. Eng. 16 (5), 1–10. doi:10.1186/S13036-022-00283-Y
Pizzolato, C., Shim, V. B., Lloyd, D. G., Devaprakash, D., Obst, S. J., Newsham-West, R., et al. (2020). Targeted Achilles Tendon Training and Rehabilitation Using Personalized and Real-Time Multiscale Models of the Neuromusculoskeletal System. Front. Bioeng. Biotechnol. 8, 1–15. doi:10.3389/FBIOE.2020.00878
Riley, G. P. (2005). Gene Expression and Matrix Turnover in Overused and Damaged Tendons. Scand. J. Med. Sci. Sports 15 (4), 241–251. doi:10.1111/j.1600-0838.2005.00456.x
Robinson, W. H., Lepus, C. M., Wang, Q., Raghu, H., Mao, R., Lindstrom, T. M., et al. (2016). Low-grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 12 (10), 580–592. doi:10.1038/NRRHEUM.2016.136
Sawadkar, P., Player, D., Bozec, L., and Mudera, V. (2020). The Mechanobiology of Tendon Fibroblasts under Static and Uniaxial Cyclic Load in a 3D Tissue Engineered Model Mimicking Native Extracellular Matrix. J. Tissue Eng. Regen. Med. 14 (1), 135–146. doi:10.1002/term.2975
Schulze-Tanzil, G., Al-Sadi, O., Wiegand, E., Ertel, W., Busch, C., Kohl, B., et al. (2011). The Role of Pro-inflammatory and Immunoregulatory Cytokines in Tendon Healing and Rupture: New Insights. Scand. J. Med. Sci. Sports 21 (3), 337–351. doi:10.1111/J.1600-0838.2010.01265.X
Screen, H. R. C., Berk, D. E., Kadler, K. E., Ramirez, F., and Young, M. F. (2015). Tendon Functional Extracellular Matrix. J. Orthop. Res. 33 (6), 793–799. doi:10.1002/jor.22818
Screen, H. R. C., Shelton, J. C., Bader, D. L., and Lee, D. A. (2005). Cyclic Tensile Strain Upregulates Collagen Synthesis in Isolated Tendon Fascicles. Biochem. Biophysical Res. Commun. 336 (2), 424–429. doi:10.1016/J.BBRC.2005.08.102
Shepherd, J. H., Riley, G. P., and Screen, H. R. C. (2014). Early Stage Fatigue Damage Occurs in Bovine Tendon Fascicles in the Absence of Changes in Mechanics at Either the Gross or Micro-structural Level. J. Mech. Behav. Biomed. Mater. 38 (100), 163–172. doi:10.1016/J.JMBBM.2014.06.005
Stauber, T., Blache, U., and Snedeker, J. G. (2020). Tendon Tissue Microdamage and the Limits of Intrinsic Repair. Matrix Biol. 85-86, 68–79. doi:10.1016/j.matbio.2019.07.008
Stolk, M., Klatte-Schulz, F., Schmock, A., Minkwitz, S., Wildemann, B., and Seifert, M. (2017). New Insights into Tenocyte-Immune Cell Interplay in an In Vitro Model of Inflammation. Sci. Rep. 7 (1), 1–14. doi:10.1038/s41598-017-09875-x
Subramanian, G., Stasuk, A., Elsaadany, M., and Yildirim-Ayan, E. (2017). Effect of Uniaxial Tensile Cyclic Loading Regimes on Matrix Organization and Tenogenic Differentiation of Adipose-Derived Stem Cells Encapsulated within 3D Collagen Scaffolds. Stem Cells Int. 2017, 1–16. doi:10.1155/2017/6072406
Thampatty, B. P., and Wang, J. H.-C. (2018). Mechanobiology of Young and Aging Tendons: In Vivo Studies with Treadmill Running. J. Orthop. Res. 36 (2), 557–565. doi:10.1002/jor.23761
Theodossiou, S. K., Pancheri, N. M., Martes, A. C., Bozeman, A. L., Brumley, M. R., Raveling, A. R., et al. (2021). Neonatal Spinal Cord Transection Decreases Hindlimb Weight-Bearing and Affects Formation of Achilles and Tail Tendons. J. Biomech. Eng. 143 (6), 1–9. doi:10.1115/1.4050031/1097185
Theodossiou, S. K., and Schiele, N. R. (2019). Models of Tendon Development and Injury. BMC Biomed. Eng. 1 (32), 1–24. doi:10.1186/s42490-019-0029-5
Thomopoulos, S., Parks, W. C., Rifkin, D. B., and Derwin, K. A. (2015). Mechanisms of Tendon Injury and Repair. J. Orthop. Res. 33 (6), 832–839. doi:10.1002/JOR.22806
Thornton, G. M., Shrive, N. G., and Frank, C. B. (2002). Ligament Creep Recruits Fibres at Low Stresses and Can Lead to Modulus-Reducing Fibre Damage at Higher Creep Stresses: A Study in Rabbit Medial Collateral Ligament Model. J. Orthop. Res. 20 (5), 967–974. doi:10.1016/S0736-0266(02)00028-1
Thorpe, C. T., Chaudhry, S., Lei, I. I., Varone, A., Riley, G. P., Birch, H. L., et al. (2015). Tendon Overload Results in Alterations in Cell Shape and Increased Markers of Inflammation and Matrix Degradation. Scand. J. Med. Sci. Sports 25 (4), e381–e391. doi:10.1111/sms.12333
Thorpe, C. T., Klemt, C., Riley, G. P., Birch, H. L., Clegg, P. D., and Screen, H. R. C. (2013). Helical Sub-structures in Energy-Storing Tendons Provide a Possible Mechanism for Efficient Energy Storage and Return. Acta Biomater. 9 (8), 7948–7956. doi:10.1016/J.ACTBIO.2013.05.004
Tohidnezhad, M., Zander, J., Slowik, A., Kubo, Y., Dursun, G., Willenberg, W., et al. (2020). Impact of Uniaxial Stretching on Both Gliding and Traction Areas of Tendon Explants in a Novel Bioreactor. Ijms 21 (8), 2925–3019. doi:10.3390/ijms21082925
Tom, S., Parkinson, J., Ilic, M. Z., Cook, J., Feller, J. A., and Handley, C. J. (2009). Changes in the Composition of the Extracellular Matrix in Patellar Tendinopathy. Matrix Biol. 28 (4), 230–236. doi:10.1016/j.matbio.2009.04.001
Tse, C. T. F., and Keir, P. J. (2020). External Compression and Partial Ischemia Decrease Human Finger Flexor Tendon and Subsynovial Connective Tissue Relative Motion. J. Orthop. Res. 38 (5), 1038–1044. doi:10.1002/JOR.24540
Tsuzaki, M., Bynum, D., Almekinders, L., Yang, X., Faber, J., and Banes, A. J. (2003a). ATP Modulates Load-Inducible IL-1?, COX 2, and MMP-3 Gene Expression in Human Tendon Cells. J. Cell. Biochem. 89 (3), 556–562. doi:10.1002/JCB.10534
Tsuzaki, M., Guyton, G., Garrett, W., Archambault, J. M., Herzog, W., Almekinders, L., et al. (2003b). IL-1β Induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1β and IL-6 in Human Tendon Cells. J. Orthop. Res. 21 (2), 256–264. doi:10.1016/S0736-0266(02)00141-9
Vijven, M., Wunderli, S. L., Ito, K., Snedeker, J. G., and Foolen, J. (2021). Serum Deprivation Limits Loss and Promotes Recovery of Tenogenic Phenotype in Tendon Cell Culture Systems. J. Orthop. Res. 39 (7), 1561–1571. doi:10.1002/JOR.24761
Wang, H.-N., Huang, Y.-C., and Ni, G.-X. (2020). Mechanotransduction of Stem Cells for Tendon Repair. Wjsc 12 (9), 952–965. doi:10.4252/WJSC.V12.I9.952
Wang, J. H.-C. (2006). Mechanobiology of Tendon. J. Biomechanics 39 (9), 1563–1582. doi:10.1016/J.JBIOMECH.2005.05.011
Wang, J. H.-C., Guo, Q., and Li, B. (2012). Tendon Biomechanics and Mechanobiology-A Minireview of Basic Concepts and Recent Advancements. J. Hand Ther. 25 (2), 133–141. doi:10.1016/J.JHT.2011.07.004
Wang, T., Chen, P., Zheng, M., Wang, A., Lloyd, D., Leys, T., et al. (2018). In Vitro loading Models for Tendon Mechanobiology. J. Orthop. Res. 36 (2), 566–575. doi:10.1002/jor.23752
Wang, T., Lin, Z., Day, R. E., Gardiner, B., Landao-Bassonga, E., Rubenson, J., et al. (2013). Programmable Mechanical Stimulation Influences Tendon Homeostasis in a Bioreactor System. Biotechnol. Bioeng. 110 (5), 1495–1507. doi:10.1002/bit.24809
Wang, T., Lin, Z., Ni, M., Thien, C., Day, R. E., Gardiner, B., et al. (2015). Cyclic Mechanical Stimulation Rescues Achilles Tendon from Degeneration in a Bioreactor System. J. Orthop. Res. 33 (12), 1888–1896. doi:10.1002/jor.22960
Wang, T., Thien, C., Wang, C., Ni, M., Gao, J., Wang, A., et al. (2018). 3D Uniaxial Mechanical Stimulation Induces Tenogenic Differentiation of Tendon‐derived Stem Cells through a PI3K/AKT Signaling Pathway. FASEB J. 32 (9), 4804–4814. doi:10.1096/FJ.201701384R
Weinstein, S. I., Yelin, E. H., and Watkins-Castillo, S. I. (2014). Activity Limitation Due to Select Medical Conditions. Available at: https://www.boneandjointburden.org/fourth-edition/ic0/activity-limitation-due-select-medical-conditions (Accessed: June 30, 2021).
Wren, T. A. L., Yerby, S. A., Beaupré, G. S., and Carter, D. R. (2001). Mechanical Properties of the Human Achilles Tendon. Clin. Biomech. 16 (3), 245–251. doi:10.1016/S0268-0033(00)00089-9
Wu, Y., Han, Y., Wong, Y. S., and Fuh, J. Y. H. (2018). Fibre-based Scaffolding Techniques for Tendon Tissue Engineering. J. Tissue Eng. Regen. Med. 12, 1798–1821. doi:10.1002/term.2701
Xu, H., and Liu, F. (2018). Downregulation of FOXP1 Correlates with Tendon Stem/progenitor Cells Aging. Biochem. Biophysical Res. Commun. 504 (1), 96–102. doi:10.1016/J.BBRC.2018.08.136
Zamboulis, D. E., Thorpe, C. T., Ashraf Kharaz, Y., Birch, H. L., Screen, H. R., and Clegg, P. D. (2020). Postnatal Mechanical Loading Drives Adaptation of Tissues Primarily through Modulation of the Non-collagenous Matrix. eLife 9, 1–23. doi:10.7554/eLife.58075
Zhang, J., Nie, D., Williamson, K., McDowell, A., Hogan, M. V., and Wang, J. H.-C. (2020). Moderate and Intensive Mechanical Loading Differentially Modulate the Phenotype of Tendon Stem/progenitor Cells In Vivo. PLoS ONE 15 (12), e0242640–17. doi:10.1371/journal.pone.0242640
Zhang, J., and Wang, J. H.-C. (2009). Production of PGE2increases in Tendons Subjected to Repetitive Mechanical Loading and Induces Differentiation of Tendon Stem Cells into Non-tenocytes. J. Orthop. Res. 28 (2), a–n. doi:10.1002/JOR.20962
Zhang, J., and Wang, J. H.-C. (2014). Prostaglandin E2 (PGE2) Exerts Biphasic Effects on Human Tendon Stem Cells. PLoS ONE 9 (2), e87706–12. doi:10.1371/JOURNAL.PONE.0087706
Zhang, K., Asai, S., Yu, B., and Enomoto-Iwamoto, M. (2015). IL-1β Irreversibly Inhibits Tenogenic Differentiation and Alters Metabolism in Injured Tendon-Derived Progenitor Cells In Vitro. Biochem. Biophysical Res. Commun. 463 (4), 667–672. doi:10.1016/J.BBRC.2015.05.122
Keywords: tendinopathy, tendon, inflammation, mechanotransduction, tendon pathology, extracellular matix, dynamic loading
Citation: Benage LG, Sweeney JD, Giers MB and Balasubramanian R (2022) Dynamic Load Model Systems of Tendon Inflammation and Mechanobiology. Front. Bioeng. Biotechnol. 10:896336. doi: 10.3389/fbioe.2022.896336
Received: 14 March 2022; Accepted: 22 June 2022;
Published: 15 July 2022.
Edited by:
Cristina Bignardi, Polytechnic University of Turin, ItalyReviewed by:
Stefano Gabetti, Politecnico di Torino, ItalyDavid Putzer, Innsbruck Medical University, Austria
Copyright © 2022 Benage, Sweeney, Giers and Balasubramanian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morgan B. Giers, Morgan.Giers@oregonstate.edu
 Lindsay G. Benage
Lindsay G. Benage James D. Sweeney1
James D. Sweeney1  Morgan B. Giers
Morgan B. Giers