The Effector Function of Allergens
- 1Université Paris Saclay, CEA, INRAE, Département Médicaments et Technologies pour la Santé (DMTS), SPI, Gif-sur-Yvette, France
- 2Division of Allergy and Clinical Immunology, Department of Medicine, University of Colorado Denver, Aurora, CO, United States
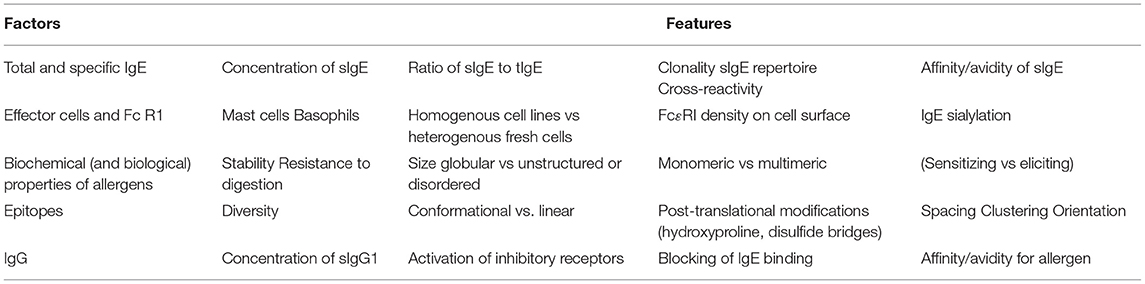
Allergens are antigens that generate an IgE response (sensitization) in susceptible individuals. The allergenicity of an allergen can be thought of in terms of its ability to sensitize as well as its ability to cross-link IgE/IgE receptor complexes on mast cells and basophils leading to release of preformed and newly formed mediators (effector activity). The identity of the allergens responsible for sensitization may be different from those that elicit an allergic response. Effector activity is determined by (1) the amount of specific IgE (sIgE) and in some circumstances the ratio of sIgE to total IgE, (2) the number of high affinity receptors for IgE (FcεR1) on the cell surface, (3) the affinity of binding of sIgE for its epitope and, in a polyclonal response, the collective avidity, (4) the number and spatial relationships of IgE binding epitopes on the allergen and (5) the presence of IgG that can bind to allergen and either block binding of sIgE and/or activate low affinity IgG receptors that activate intracellular inhibitory pathways. This review will discuss these important immunologic and physical properties that contribute to the effector activity of allergens.
Introduction
The word allergen refers to a specific molecule, usually a protein that generates an IgE response as opposed to a more general term, antigen that is a molecule that induces any immune response (1). Allergenicity can refer to the ability of an antigen to elicit the production of specific IgE (sIgE), bind to sIgE antibodies, induce cross-linking of sIgE bound to the high affinity receptor, FcεR1, for IgE (IgE/FcεR1complexes) and trigger cell degranulation that may ultimately lead to an allergic reaction in a sensitized subject (2, 3). Arguably, all of these aspects must be present for an allergen to be clinically relevant although there are exceptions due to cross-reactivity when IgE sensitization is primarily induced by a different allergen (2, 3). The concept of “allergenicity” in general or in a specific context has been reviewed previously, most often focusing on the ability of allergens to elicit an IgE response but also acknowledging the importance of clinical reactions (4–16). This review is focused solely on the effector function of allergens and the ability of an allergen to elicit an allergic reaction by cross-linking of IgE/FcεR1complexes to activate mast cells and basophils. The examples given are frequently regarding peanut allergens as these have been extensively studied. However, these concepts likely apply to most, if not all, allergens.
Before discussing the effector function of allergens, it is important to note that the allergens within an allergenic source (e.g., a food) that are the most potent for sensitization may be different from the allergens that are responsible for elicitation of the allergic response. As an example, sensitization to peanuts may be mediated primarily by Ara h 1, which is a vicilin, also known as a 7S globulin (17, 18). In another mouse model, IgE sensitization to Ara h 1 was induced after oral sensitization to raw or roasted peanut (19). In contrast, production of IgE to Ara h 6 was not induced in mice sensitized to raw peanut and purified native Ara h 6 also displayed limited intrinsic sensitization capacity (19). The formation of large complexes during heat-treatment, notably between Ara h 1 and Ara h 6, were probably required for Ara h 1 to act as carrier for Ara h 6 uptake, activation of dendritic cells, and induction of specific Th2 immune response to Ara h 6. On the other hand, the 2S albumins of peanuts, Ara h 2 and Ara h 6, have been shown to be much more potent than Ara h 1 or Ara h 3, an 11S-globulin, for eliciting IgE-mediated mast cell activation in peanut allergy (16, 17, 20–22). The mechanism whereby one allergenic protein may sensitize and thus generate an IgE response that allows a different but homologous allergen to cause an effector response, likely involves cross-reactive linear and/or conformational epitopes (23, 24). However, for proteins displaying low overall identity such as between Ara h 1 and Ara h 2 or between peanut and tree-nut proteins, the mechanism is less well-understood and may involve discrete sequences similar in physicochemical properties (16, 25–27).
Allergenic proteins are limited to a small number of protein families (28). In some cases, the effector function is predominantly mediated by even a smaller number of allergens that can be specifically depleted by specific high affinity antibodies. This has been tested for peanut allergens, where, following immunodepletion, Ara h 2 and Ara h 6 were found to account for most of the allergenic activity of a peanut whole extract (22, 29). Similarly, immunodepletion of Fel d 1 from an extract of cat allergens demonstrated that Fel d 1 is the most potent cat allergen (30). On the other hand, other allergenic extracts appear to have multiple allergens that contribute to effector function with combinations that vary in clinical relevancy for different individuals (31).
Thus, the ability of an allergen to cross-link IgE/FcεR1complexes on mast cells and basophils is determined by (1) the amount of specific IgE (sIgE) and the ratio of sIgE to total IgE (tIgE), (2) the number of FcεR1 molecules on the cell surface, (3) the affinity of binding of sIgE for its epitope and, in a polyclonal response, the collective avidity, (4) the number and spatial relationships of IgE binding epitopes on the allergen and (5) the presence of IgG that can bind to allergen and either block binding of sIgE and/or activate low affinity IgG receptors (FcγR2 in humans and FcγR2b in mice) that activate intracellular inhibitory pathways (32–36).
Total and Specific IgE
The term specific IgE has evolved over the years. This was initially referring to IgE detected when a saline extract of an allergenic source such as individual animal danders, specific pollens, mold spores or food was used as the capture in an absorbent assay. More recently, specific IgE refers to IgE that binds an individual allergenic protein such as Fel d 1 from cat or Ara h 2 from peanut as discussed in this review. sIgE can be measured in research labs using ELISA assays and commercially in the ImmunoCap® (ThermoFisher) or other similar format. Microarray technology (e.g. ISAC® by ThermoFisher) has allowed simultaneous measurement of IgE binding to a variety of purified or recombinant allergens (37).
As the Cε3 region of the Fc portion of IgE binds to the alpha chain of FcεR1, there is equal binding of all IgE molecules to FcεR1, independent of specificity which resides in the Fab region. However, the density of any given specific IgE on the cell surface is inversely proportional to the amount of IgE of other specificities. Therefore, the effector activity of an allergen is influenced not only by the number of IgE/FcεR1 complexes but also by the ratio of sIgE to tIgE. This becomes important when considering sIgE in a sample with a substantial portion of the IgE directed at other allergens or without known specificity. Consequently, if the ratio of sIgE to tIgE is high, the probability of the same allergen to cross-link with sIgE and meet the threshold of complexes required to generate a measurable effector response is easier to reach than if the ratio of sIgE to tIgE is low (see below). Blanc et al. showed that sera exhibiting a lower ratio sIgE to tIgE required higher concentrations of allergens to trigger RBL SX-38 degranulation, which was not efficiently induced when the ratio was <2% (21). However, using human cultured mast cells, the lowest fraction of sIgE able to activate cells was around 0.3% (38). Hemmings et al. reported that, for peanut allergens, the ratio of sIgE to tIgE, along with the diversity of the sIgE repertoire to peanut allergens, were the major determinants of basophil and mast cell activation (39). In that study, compared to the level of sIgE alone, the ratio sIgE to Ara h 2 and Ara h 6 to tIgE improved the discrimination between patients who were clinically allergic peanut as opposed to those who were only sensitized (39). In addition to the amount of sIgE, the affinity of that IgE for binding to allergens is important (34, 38). This is further discussed below.
Effector Cells and FcεR1
The effector function of an allergen requires that sIgE be bound to FcεR1 on mast cells and basophils. While the half-life of circulating IgE is ~1 day (40), IgE bound to FcεR1, due to the slow off rate of this interaction (KD of 10−9 M), remains on the cell surface for several weeks (41). The function of FcεR1 has been extensively studied on rat basophilic leukemia (RBL) cells using the 2H3 clone (34) and on human basophils (42, 43). Using a monoclonal IgE, crosslinking as few as 100 FcεR1-IgE complexes leads to measurable cell activation and 50% cell activation was seen with crosslinking of 300-2000 FcεR1-IgE complexes (42, 44). Whereas, the number of FcεR1 on the RBL-2H3 cells is stable, the expression of FcεR1 on human basophils is upregulated by the concentration of circulating IgE so that naturally occurring basophils can have from 5 X 104 to 5 X 106 receptors per cell (42). Of note, in addition to their capacity to up-regulate FcεR1 levels on the cell surface, IgE antibodies also enhance mast cell survival and expansion (45). The density of FcεR1 on the cell surface is thus important in assessing the allergen concentration capable to trigger mast cell degranulation.
An interesting caveat that has been recognized for some time is that some patients may produce IgE that has an increased propensity to be cross-linked but the molecular basis of this finding was not understood (46). Recently, characterization by mass spectrometry of serum IgE glycosylation revealed that tIgE from peanut-allergic patients have increased terminal sialylation compared to tIgE from non-atopic controls. Of note, IgE sialylation did not impact the binding of IgE to FcϵRI but was found to modulate the signaling downstream of the receptor (47). The general impact of this observation has not been established.
Not All Allergens That Bind IgE Have Similar Effector Activity
Levels of sIgE can be evaluated through different approaches and assays such as immunoblots, ELISA and competitive inhibition of IgE-binding. However, IgE-binding does not necessarily correlate with relevant clinical activity. To date, 17 peanut proteins have been reported as allergenic by the WHO/IUIS Allergen Nomenclature Sub-Committee. Among them, four peanut allergens, Ara h 1, Ara h 2, Ara h 3 and Ara h 6 bind large amounts of IgE on immunoblots which is is true for the majority of patients (48, 49). However, when studied in functional assays, it was found that Ara h 2 and Ara h 6 account for 70–90% of the allergic effector activity of peanuts (50). In addition, desensitization with only a combination of Ara h 2 and Ara h 6 protected peanut allergic mice from anaphylaxis elicited by whole peanut extract (29). Moreover, although co-sensitization to both Ara h 2 and Ara h 6 generally occurs in patients, IgE cross-reactivity can lead to a high level of IgE-sensitization to both allergens, as determined by ELISA, whereas for some patients, only one of the two 2S-albumins can effectively trigger mast cell degranulation in vitro (51).
The Contribution of Physical Stability and Structure to Effector Function
Inhalant allergens have immediate interactions with the respiratory mucosa and do not have to be particularly stable (12). For example, the PR-10 allergens are generally sensitive to heat and to digestion. Consequently, they often can elicit allergic reactions at mucosal surfaces of the respiratory tract but not of the digestive tract (52). On the other hand, food allergens must be stable enough to withstand the environment of the digestive tract. Moreover, to enter the systemic circulation, soluble allergens are more efficiently transferred across the intestinal epithelial barrier, through transcytosis, than particulate allergens, which are processed through Peyer's patches (53). Therefore, soluble allergens are more potent in triggering anaphylactic reaction while particulate allergens, like aggregates resulting from heat-treatment, food processing or gastric acidity, could be more potent in inducing IgE sensitization (53). For these reasons, physical stability and structural elements such as size, oligomerization, aggregation or formation of micelles are important attributes of food allergens that, when studied, can greatly affect their effector function (6).
For example, Ara h 2 and Ara h 6 are much more resistant to gastric acid and digestive enzymes than are Ara h 1 and Ara h 3. This resistance likely enhances their effector activity (54–60). Furthermore, digestion products of peanut 2S albumins yield a large and a small subunit (5 and 9 kDa) that remain associated by disulfide bridges so that the resulting heterodimer is as immunoreactive as the intact protein. Hence, immunoreactive Ara h 2 and Ara h 6 can be still detected and quantified in the bloodstream and in breast milk of non-peanut allergic human volunteers after peanut consumption or in the blood of peanut allergic patients after oral food challenge (61). These proteins have been shown to bind IgE and to be active in functional assays with RBL SX-38 cells (60, 62–66). On the other hand, the primary allergen in wheat induced anaphylaxis, ω-gliadin, although stable to digestion, is a larger 30–50 kDa protein (67, 68) that requires cofactors that either augment gut permeability or cell responsiveness to cause anaphylaxis (69–71).
IgE-Epitope Interactions
In addition to the number of allergen-specific IgE molecules, the affinity of binding is important (34). The complex relationship between the IgE repertoire and IgE affinity has been recently explored (38, 72, 73) underscoring the importance of tIgE, sIgE, IgE binding affinity and the diversity of the epitopes that bind IgE (72–74). The extent of effector cell activation is also linked to an allergen's oligomeric state, and the valency, spacing/proximity and flexibility of the IgE-binding epitopes (75, 76).
Identification of the IgE-binding epitopes has typically focused on linear sequences as they can be exhaustively and easily characterized by using overlapping peptide libraries and high-throughput technologies (77, 78). Recent diagnostic developments have successfully used peptide microarrays and bead-based epitope assays for profiling epitope-specific IgE repertoire in the context of peanut allergy (79–82). However, conformational epitopes (formed by 3D-folding of the primary amino acid sequences of a protein) are now thought to be critical for high affinity IgE binding to a number of important allergens, including Ara h 1 (83, 84), Ara h 2 (85–87), Ara h 6 (85–87) and others (88–92). The importance of conformational epitopes has been clearly shown by disrupting the disulfide bonds that play an important role in maintaining the structure of the 2S albumins. Chemical reduction and alkylation of these disulfide bonds lead to a loss of secondary, tertiary and quaternary structures with concomitant changes in biochemical as well as immunological characteristics (77, 93, 94).
The importance of conformational structure for Ara h 2 and Ara h 6 was best demonstrated with several reports showing the loss of IgE binding and mast cell activation following reduction and alkylation (77, 94–97). For Ara h 6, IgE-binding was shown to be critically dependent upon conformational epitopes (87, 98). For Ara h 2, around three quarters of 48 peanut allergic patients recognized conformational epitopes to a similar or greater extent than linear ones (99). That said, the contribution of linear vs. conformational epitopes in the overall IgE-binding capacity of Ara h 2 is highly variable among patients (98).
Indeed, the IgE-binding capacity of the entire allergen could be recapitulated by combining a synthetic peptide containing the immunodominant linear epitope plus a mutant of Ara h 2 in which this immunodominant linear epitope had been deleted. That peptide, which is found within an unstructured surface loop of Ara h 2, contains the repeated linear motifs DPYSPOHS, for which IgE-binding is highly dependent upon the presence of hydroxyproline, a post translational modification that occurs commonly in nature (98). On average, IgE-binding to this linear motif account for half of the total IgE-binding capacity of Ara h 2. The remaining IgE binding activity is to conformational epitopes (51, 98).
High-affinity IgE antibodies may appear as a prerequisite because sIgE is 105 times less prevalent than IgG. These IgE antibodies must compete with lower affinity sIgG to bind allergen and initiate the allergic response. Thus, it has been shown that basophil degranulation is enhanced when the allergen is first anchored at the surface of the effector cell by high-affinity IgE bound to the FcεR1. Low-affinity IgE may then contribute almost as efficiently as high-affinity IgE to cell activation when cross-linking occurs with the second IgE (100). Another example is found with the IgE binding to the unstructured region of Ara h 2 that is discussed above. Here, IgE-binding to free peptides found in this region occurs without the large loss of affinity usually observed with free peptide compared to the full allergen (100). This is probably due to an increased avidity caused by the repetition of the motif DPYSPOHS two- and three-times in the isoforms Ara h 2.01 and Ara h 2.02, respectively. Accordingly, the free peptide containing three DPYSPOHS motifs displayed a higher capacity to inhibit IgE-binding to nAra h 2 and a higher potency in mast cell degranulation than the one with a lower valency (98). Of note, the two motifs in the shorter peptide are separated by only one residue. In this regard, the influence of spacing between IgE epitopes was also investigated by using rigidly spaced bi- and trivalent haptens or artificial multivalent allergens (33, 36). In the latter case, a non-allergenic myoglobulin, in which 4 repetitions of the same IgE-reactive peptide were grafted and separated by a linker of 6 Gly residues, triggered RBL degranulation more efficiently than derivatives carrying only 2 repetitions or more distant IgE epitopes (76). In these settings, multivalency of allergens, spatial clustering of IgE epitopes on a particular segment of the allergen and high-avidity interactions can overcome the need for high-affinity interactions to trigger cell degranulation, thus offering an explanation for clinical reactivity induced by unexpected low-affinity IgE cross-reactivity among different allergens (75, 101, 102).
IgG Regulation of Effector Function
IgG to allergens occur naturally and can be further induced by specific immunotherapy. This has been best studied in the field of food allergy. IgG induced by oral immunotherapy for food allergy can bind to allergen and either block binding of IgE and/or activate low affinity IgG receptors (FcγR2 in humans and FcγR2b in mice) that activate intracellular inhibitory pathways (79, 103, 104). So, the effector function of an allergen is affected not only by the amount and affinity of sIgE, but also by the presence of specific IgG that may bind to the same epitope as the IgE (blocking IgG) and IgG that may bind to different parts of the allergen and activate inhibitory pathways (104).
Conclusion
The determinants of the effector function of an allergen are complex and are affected by multiple components (Table 1). These include binding characteristics such as the amount and affinity of the IgE that binds to the allergen, and the presence of either non-specific IgE or IgE of other specificities that may effectively dilute out the allergen-specific IgE. The intrinsic properties of the allergen such as its stability and surface structure (number, spacing and spatial relationships of IgE binding epitopes) also play a vital role. Other contributing factors include the presence of IgG that may either compete with the IgE for specific epitopes or may bind to other epitopes and activate inhibitory receptors. Cellular features such as the numbers of high affinity receptors for IgE may render the same IgE-allergen interaction more clinically severe in one patient compared to another.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported AlimH department of INRAE (SH) and by RO1AI165866 (SCD) and R21AI135397 (SCD).
Author Disclaimer
Contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Conflict of Interest
SCD has received grant support from the National Institutes of Health.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pomes A, Davies JM, Gadermaier G, Hilger C, Holzhauser T, Lidholm J, et al. WHO/IUIS allergen nomenclature: providing a common language. Mol Immunol. (2018) 100:3–13. doi: 10.1016/j.molimm.2018.03.003
2. Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. (2000) 106:228–38. doi: 10.1067/mai.2000.108434
3. Aalberse RC. Structural features of allergenic molecules. Chem Immunol Allergy. (2006) 91:134–46. doi: 10.1159/000090277
4. Traidl-Hoffmann C, Jakob T, Behrendt H. Determinants of allergenicity. J Allergy Clin Immunol. (2009) 123:558–66. doi: 10.1016/j.jaci.2008.12.003
5. Deifl S, Bohle B. Factors influencing the allergenicity and adjuvanticity of allergens. Immunotherapy. (2011) 3:881–93. doi: 10.2217/imt.11.69
6. Masilamani M, Commins S, Shreffler W. Determinants of food allergy. Immunol Allergy Clin North Am. (2012) 32:11–33. doi: 10.1016/j.iac.2011.12.003
7. Masthoff LJ, Hoff R, Verhoeckx KC, van Os-Medendorp H, Michelsen-Huisman A, Baumert JL, et al. A systematic review of the effect of thermal processing on the allergenicity of tree nuts. Allergy. (2013) 68:983–93. doi: 10.1111/all.12185
8. Zhou Y, Wang JS, Yang XJ, Lin DH, Gao YF, Su YJ, et al. Peanut allergy, allergen composition, and methods of reducing allergenicity: a review. Int J Food Sci. (2013) 2013:909140. doi: 10.1155/2013/909140
9. Costa J, Carrapatoso I, Oliveira MB, Mafra I. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. (2014) 44:319–41. doi: 10.1111/cea.12267
10. Dunn SE, Vicini JL, Glenn KC, Fleischer DM, Greenhawt MJ. The allergenicity of genetically modified foods from genetically engineered crops: a narrative and systematic review. Ann Allergy Asthma Immunol. (2017) 119:214–22 e3. doi: 10.1016/j.anai.2017.07.010
11. Stephen JN, Sharp MF, Ruethers T, Taki A, Campbell DE, Lopata AL. Allergenicity of bony and cartilaginous fish - molecular and immunological properties. Clin Exp Allergy. (2017) 47:300–12. doi: 10.1111/cea.12892
12. Pekar J, Ret D, Untersmayr E. Stability of allergens. Mol Immunol. (2018) 100:14–20. doi: 10.1016/j.molimm.2018.03.017
13. Jin Y, Acharya HG, Acharya D, Jorgensen R, Gao H, Secord J, et al. Advances in molecular mechanisms of wheat allergenicity in animal models: a comprehensive review. Molecules. (2019) 24:1142. doi: 10.3390/molecules24061142
14. Caraballo L, Valenta R, Puerta L, Pomes A, Zakzuk J, Fernandez-Caldas E, et al. The allergenic activity and clinical impact of individual IgE-antibody binding molecules from indoor allergen sources. World Allergy Organ J. (2020) 13:100118. doi: 10.1016/j.waojou.2020.100118
15. Costa J, Bavaro SL, Benede S, Diaz-Perales A, Bueno-Diaz C, Gelencser E, et al. Are physicochemical properties shaping the allergenic potency of plant allergens? Clin Rev Allergy Immunol. (2020). doi: 10.1007/s12016-020-08810-9
16. Costa J, Villa C, Verhoeckx K, Cirkovic-Velickovic T, Schrama D, Roncada P, et al. Are physicochemical properties shaping the allergenic potency of animal allergens? Clin Rev Allergy Immunol. (2021). doi: 10.1007/s12016-020-08826-1
17. Smit JJ, Pennings MT, Willemsen K, van Roest M, van Hoffen E, Pieters RH. Heterogeneous responses and cross reactivity between the major peanut allergens Ara h 1, 2,3 and 6 in a mouse model for peanut allergy. Clin Transl Allergy. (2015) 5:13. doi: 10.1186/s13601-015-0056-9
18. Chruszcz M, Maleki SJ, Majorek KA, Demas M, Bublin M, Solberg R, et al. Structural and immunologic characterization of Ara h 1, a major peanut allergen. J Biol Chem. (2011) 286:39318–27. doi: 10.1074/jbc.M111.270132
19. Guillon B, Bernard H, Drumare MF, Hazebrouck S, Adel-Patient K. Heat processing of peanut seed enhances the sensitization potential of the major peanut allergen Ara h 6. Mol Nutr Food Res. (2016) 60:2722–35. doi: 10.1002/mnfr.201500923
20. Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy. (2004) 34:583–90. doi: 10.1111/j.1365-2222.2004.1923.x
21. Blanc F, Adel-Patient K, Drumare MF, Paty E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and Ara h 6 are the most efficient elicitors. Clin Exp Allergy. (2009) 39:1277–85. doi: 10.1111/j.1365-2222.2009.03294.x
22. Porterfield HS, Murray KS, Schlichting DG, Chen X, Hansen KC, Duncan MW, et al. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. (2009) 39:1099–108. doi: 10.1111/j.1365-2222.2009.03273.x
23. Chen X, Negi SS, Liao S, Gao V, Braun W, Dreskin SC. Conformational IgE epitopes of peanut allergens Ara h 2 and Ara h 6. Clin Exp Allergy. (2016) 46:1120–8. doi: 10.1111/cea.12764
24. Chen X, Wang Q, El-Mezayen R, Zhuang Y, Dreskin SC. Ara h 2 and Ara h 6 have similar allergenic activity and are substantially redundant. Int Arch Allergy Immunol. (2013) 160:251–8. doi: 10.1159/000341642
25. Bublin M, Kostadinova M, Radauer C, Hafner C, Szepfalusi Z, Varga EM, et al. IgE cross-reactivity between the major peanut allergen Ara h 2 and the nonhomologous allergens Ara h 1 and Ara h 3. J Allergy Clin Immunol. (2013) 132:118–24. doi: 10.1016/j.jaci.2013.01.022
26. Bublin M, Breiteneder H. Cross-reactivities of non-homologous allergens. Allergy. (2020) 75:1019–22. doi: 10.1111/all.14120
27. Nesbit JB, Schein CH, Braun BA, Gipson SAY, Cheng H, Hurlburt BK, et al. Epitopes with similar physicochemical properties contribute to cross reactivity between peanut and tree nuts. Mol Immunol. (2020) 122:223–31. doi: 10.1016/j.molimm.2020.03.017
28. Scheurer S, Toda M, Vieths S. What makes an allergen? Clin Exp Allergy. (2015) 45:1150–61. doi: 10.1111/cea.12571
29. Kulis M, Chen X, Lew J, Wang Q, Patel O, Murray KS, et al. The 2S albumin allergens of Arachis hypogaea, Ara h 2 and Ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice. Clin Exp Allergy. (2012) 42:326–36. doi: 10.1111/j.1365-2222.2011.03934.x
30. de Groot H, van Swieten P, van Leeuwen J, Lind P, Aalberse RC. Monoclonal antibodies to the major feline allergen Fel d I. I. Serologic and biologic activity of affinity-purified Fel d I and of Fel d I-depleted extract. J Allergy Clin Immunol. (1988) 82:778–86. doi: 10.1016/0091-6749(88)90079-6
31. Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI molecular allergology user's guide. Pediatr Allergy Immunol. (2016) 27(Suppl. 23):1–250. doi: 10.1111/pai.12563
32. Torigoe C, Inman JK, Metzger H. An unusual mechanism for ligand antagonism. Science. (1998) 281:568–72. doi: 10.1126/science.281.5376.568
33. Kane P, Erickson J, Fewtrell C, Baird B, Holowka D. Cross-linking of IgE-receptor complexes at the cell surface: synthesis and characterization of a long bivalent hapten that is capable of triggering mast cells and rat basophilic leukemia cells. Mol Immunol. (1986) 23:783–90. doi: 10.1016/0161-5890(86)90090-8
34. Metzger H, Eglite S, Haleem-Smith H, Reischl I, Torigoe C. Quantitative aspects of signal transduction by the receptor with high affinity for IgE. Mol Immunol. (2002) 38:1207–11. doi: 10.1016/S0161-5890(02)00065-2
35. Ortega E, Schweitzer-Stenner R, Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. EMBO J. (1988) 7:4101–9. doi: 10.1002/j.1460-2075.1988.tb03304.x
36. Sil D, Lee JB, Luo D, Holowka D, Baird B. Trivalent ligands with rigid DNA spacers reveal structural requirements for IgE receptor signaling in RBL mast cells. ACS Chem Biol. (2007) 2:674–84. doi: 10.1021/cb7001472
37. Hamilton RG, Hemmer W, Nopp A, Kleine-Tebbe J. Advances in IgE testing for diagnosis of allergic disease. J Allergy Clin Immunol Pract. (2020) 8:2495–504. doi: 10.1016/j.jaip.2020.07.021
38. Hjort C, Schiotz PO, Ohlin M, Wurtzen PA, Christensen LH, Hoffmann HJ. The number and affinity of productive IgE pairs determine allergen activation of mast cells. J Allergy Clin Immunol. (2017) 140:1167–70 e2. doi: 10.1016/j.jaci.2017.04.014
39. Hemmings O, Niazi U, Kwok M, James LK, Lack G, Santos AF. Peanut diversity and specific activity are the dominant IgE characteristics for effector cell activation in children. J Allergy Clin Immunol. (2021) 148:495–505 e14. doi: 10.1016/j.jaci.2021.02.029
40. Dreskin SC, Goldsmith PK, Strober W, Zech LA, Gallin JI. Metabolism of immunoglobulin E in patients with markedly elevated serum immunoglobulin E levels. J Clin Invest. (1987) 79:1764–72. doi: 10.1172/JCI113017
41. Oettgen HC. Fifty years later: emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. J Allergy Clin Immunol. (2016) 137:1631–45. doi: 10.1016/j.jaci.2016.04.009
42. MacGlashan D Jr. IgE and FcepsilonRI regulation. Clin Rev Allergy Immunol. (2005) 29:49–60. doi: 10.1385/CRIAI:29:1:049
43. MacGlashan D Jr, Lichtenstein LM. Studies of antigen binding on human basophils. I. Antigen binding and functional consequences. J Immunol. (1983) 130:2330–6.
44. Metzger H, Goldstein B, Kent U, Mao SY, Pribluda C, Pribluda V, et al. Quantitative aspects of receptor aggregation. Adv Exp Med Biol. (1994) 365:175–83. doi: 10.1007/978-1-4899-0987-9_18
45. Kanagaratham C, El Ansari YS, Lewis OL, Oettgen HC. IgE and IgG Antibodies as regulators of mast cell and basophil functions in food allergy. Front Immunol. (2020) 11:603050. doi: 10.3389/fimmu.2020.603050
46. Xie L, Schroeder JT, Langdon JM, Sora-Scott RS, Kawakami T, MacDonald SM. Human IgE+ and IgE- are not equivalent to mouse highly cytokinergic IgE. J Allergy Clin Immunol. (2008) 121:1027–33. doi: 10.1016/j.jaci.2007.12.1157
47. Shade KC, Conroy ME, Washburn N, Kitaoka M, Huynh DJ, Laprise E, et al. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature. (2020) 582:265–70. doi: 10.1038/s41586-020-2311-z
48. Koppelman SJ, de Jong GA, Laaper-Ertmann M, Peeters KA, Knulst AC, Hefle SL, et al. Purification and immunoglobulin E-binding properties of peanut allergen Ara h 6: evidence for cross-reactivity with Ara h 2. Clin Exp Allergy. (2005) 35:490–7. doi: 10.1111/j.1365-2222.2005.02204.x
49. Flinterman AE, van Hoffen E, den Hartog Jager CF, Koppelman S, Pasmans SG, Hoekstra MO, et al. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin Exp Allergy. (2007) 37:1221–8. doi: 10.1111/j.1365-2222.2007.02764.x
50. Chen X, Zhuang Y, Wang Q, Moutsoglou D, Ruiz G, Yen SE, et al. Analysis of the effector activity of Ara h 2 and Ara h 6 by selective depletion from a crude peanut extract. J Immunol Methods. (2011) 372:65–70. doi: 10.1016/j.jim.2011.06.031
51. Hazebrouck S, Guillon B, Paty E, Dreskin SC, Adel-Patient K, Bernard H. Variable IgE cross-reactivity between peanut 2S-albumins: the case for measuring IgE to both Ara h 2 and Ara h 6. Clin Exp Allergy. (2019) 49:1107–15. doi: 10.1111/cea.13432
52. Bohle B, Zwolfer B, Heratizadeh A, Jahn-Schmid B, Antonia YD, Alter M, et al. Cooking birch pollen-related food: divergent consequences for IgE- and T cell-mediated reactivity in vitro and in vivo. J Allergy Clin Immunol. (2006) 118:242–9. doi: 10.1016/j.jaci.2006.03.011
53. Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. (2008) 63:882–90. doi: 10.1111/j.1398-9995.2008.01673.x
54. Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. (1996) 14:1269–73. doi: 10.1038/nbt1096-1269
55. Koppelman SJ, Hefle SL, Taylor SL, de Jong GA. Digestion of peanut allergens Ara h 1, Ara h 2, Ara h 3, and Ara h 6: a comparative in vitro study and partial characterization of digestion-resistant peptides. Mol Nutr Food Res. (2010) 54:1711–21. doi: 10.1002/mnfr.201000011
56. Murtagh GJ, Dumoulin M, Archer DB, Alcocer MJ. Stability of recombinant 2 S albumin allergens in vitro. Biochem Soc Trans. (2002) 30:913–5. doi: 10.1042/bst0300913
57. Moreno FJ, Clemente A. 2S albumin storage proteins: what makes them food allergens? Open Biochem J. (2008) 2:16–28. doi: 10.2174/1874091X00802010016
58. Prodic I, Stanic-Vucinic D, Apostolovic D, Mihailovic J, Radibratovic M, Radosavljevic J, et al. Influence of peanut matrix on stability of allergens in gastric-simulated digesta: 2S albumins are main contributors to the IgE reactivity of short digestion-resistant peptides. Clin Exp Allergy. (2018) 48:731–40. doi: 10.1111/cea.13113
59. Koppelman SJ, Smits M, Tomassen M, de Jong GAH, Baumert J, Taylor SL, et al. Release of major peanut allergens from their matrix under various pH and simulated saliva conditions-Ara h2 and Ara h6 are readily bio-accessible. Nutrients. (2018) 10:1281. doi: 10.3390/nu10091281
60. Dreskin SC, Koppelman SJ, Andorf S, Nadeau KC, Kalra A, Braun W, et al. The importance of the 2S albumins for allergenicity and cross-reactivity of peanuts, tree nuts, and sesame seeds. J Allergy Clin Immunol. (2020) 147:1154–63. doi: 10.1016/j.jaci.2020.11.004
61. Bernard H, Turner PJ, Ah-Leung S, Ruiz-Garcia M, Clare Mills EN, Adel-Patient K. Circulating Ara h 6 as a marker of peanut protein absorption in tolerant and allergic humans following ingestion of peanut-containing foods. Clin Exp Allergy. (2020) 50:1093–102. doi: 10.1111/cea.13706
62. Bernard H, Ah-Leung S, Drumare MF, Feraudet-Tarisse C, Verhasselt V, Wal JM, et al. Peanut allergens are rapidly transferred in human breast milk and can prevent sensitization in mice. Allergy. (2014) 69:888–97. doi: 10.1111/all.12411
63. JanssenDuijghuijsen LM, Wichers HJ, van Norren K, Keijer J, Baumert JL, de Jong GA, et al. Detection of peanut allergen in human blood after consumption of peanuts is skewed by endogenous immunoglobulins. J Immunol Methods. (2017) 440:52–7. doi: 10.1016/j.jim.2016.11.002
64. Schocker F, Scharf A, Kull S, Jappe U. Detection of the peanut allergens Ara h 2 and Ara h 6 in human breast milk: development of 2 sensitive and specific sandwich ELISA assays. Int Arch Allergy Immunol. (2017) 174:17–25. doi: 10.1159/000479388
65. Apostolovic D, Stanic-Vucinic D, de Jongh HH, de Jong GA, Mihailovic J, Radosavljevic J, et al. Conformational stability of digestion-resistant peptides of peanut conglutins reveals the molecular basis of their allergenicity. Sci Rep. (2016) 6:29249. doi: 10.1038/srep29249
66. Baumert JL, Taylor SL, Koppelman SJ. Quantitative assessment of the safety benefits associated with increasing clinical peanut thresholds through immunotherapy. J Allergy Clin Immunol Pract. (2018) 6:457–65 e4. doi: 10.1016/j.jaip.2017.05.006
67. Palosuo K, Varjonen E, Nurkkala J, Kalkkinen N, Harvima R, Reunala T, et al. Transglutaminase-mediated cross-linking of a peptic fraction of omega-5 gliadin enhances IgE reactivity in wheat-dependent, exercise-induced anaphylaxis. J Allergy Clin Immunol. (2003) 111:1386–92. doi: 10.1067/mai.2003.1498
68. Scherf KA, Brockow K, Biedermann T, Koehler P, Wieser H. Wheat-dependent exercise-induced anaphylaxis. Clin Exp Allergy. (2016) 46:10–20. doi: 10.1111/cea.12640
69. Ansley L, Bonini M, Delgado L, Del Giacco S, Du Toit G, Khaitov M, et al. Pathophysiological mechanisms of exercise-induced anaphylaxis: an EAACI position statement. Allergy. (2015) 70:1212–21. doi: 10.1111/all.12677
70. Foong RX, Giovannini M, du Toit G. Food-dependent exercise-induced anaphylaxis. Curr Opin Allergy Clin Immunol. (2019) 19:224–8. doi: 10.1097/ACI.0000000000000531
71. Munoz-Cano R, San Bartolome C, Casas-Saucedo R, Araujo G, Gelis S, Ruano-Zaragoza M, et al. Immune-mediated mechanisms in cofactor-dependent food allergy and anaphylaxis: effect of cofactors in basophils and mast cells. Front Immunol. (2020) 11:623071. doi: 10.3389/fimmu.2020.623071
72. Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. (2008) 122:298–304. doi: 10.1016/j.jaci.2008.05.026
73. Christensen LH, Riise E, Bang L, Zhang C, Lund K. Isoallergen variations contribute to the overall complexity of effector cell degranulation: effect mediated through differentiated IgE affinity. J Immunol. (2010) 184:4966–72. doi: 10.4049/jimmunol.0904038
74. Lund G, Willumsen N, Holm J, Christensen LH, Wurtzen PA, Lund K. Antibody repertoire complexity and effector cell biology determined by assays for IgE-mediated basophil and T-cell activation. J Immunol Methods. (2012) 383:4–20. doi: 10.1016/j.jim.2012.05.021
75. Bucaite G, Kang-Pettinger T, Moreira J, Gould HJ, James LK, Sutton BJ, et al. Interplay between affinity and valency in effector cell degranulation: a model system with polcalcin allergens and human patient-derived IgE antibodies. J Immunol. (2019) 203:1693–700. doi: 10.4049/jimmunol.1900509
76. Gieras A, Linhart B, Roux KH, Dutta M, Khodoun M, Zafred D, et al. IgE epitope proximity determines immune complex shape and effector cell activation capacity. J Allergy Clin Immunol. (2016) 137:1557–65. doi: 10.1016/j.jaci.2015.08.055
77. Albrecht M, Kuhne Y, Ballmer-Weber BK, Becker WM, Holzhauser T, Lauer I, et al. Relevance of IgE binding to short peptides for the allergenic activity of food allergens. J Allergy Clin Immunol. (2009) 124:328–36:36 e1–6. doi: 10.1016/j.jaci.2009.05.031
78. Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, et al. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. (1997) 342:244–53. doi: 10.1006/abbi.1997.9998
79. Santos AF, Barbosa-Morais NL, Hurlburt BK, Ramaswamy S, Hemmings O, Kwok M, et al. IgE to epitopes of Ara h 2 enhance the diagnostic accuracy of Ara h 2-specific IgE. Allergy. (2020) 75:2309–18. doi: 10.1111/all.14301
80. Suarez-Farinas M, Suprun M, Bahnson HT, Raghunathan R, Getts R, duToit G, et al. Evolution of epitope-specific IgE and IgG4 antibodies in children enrolled in the LEAP trial. J Allergy Clin Immunol. (2021) 148:835–42. doi: 10.1016/j.jaci.2021.01.030
81. Suarez-Farinas M, Suprun M, Kearney P, Getts R, Grishina G, Hayward C, et al. Accurate and reproducible diagnosis of peanut allergy using epitope mapping. Allergy. (2021) 76:3789–97. doi: 10.1111/all.14905
82. Suprun M, Sicherer SH, Wood RA, Jones SM, Leung DYM, Henning AK, et al. Early epitope-specific IgE antibodies are predictive of childhood peanut allergy. J Allergy Clin Immunol. (2020) 146:1080–8. doi: 10.1016/j.jaci.2020.08.005
83. Bogh KL, Nielsen H, Eiwegger T, Madsen CB, Mills EN, Rigby NM, et al. IgE versus IgG4 epitopes of the peanut allergen Ara h 1 in patients with severe allergy. Mol Immunol. (2014) 58:169–76. doi: 10.1016/j.molimm.2013.11.014
84. Nesbit JB, Hurlburt BK, Schein CH, Cheng H, Maleki SJ. Ara h 1 structure is retained after roasting and is important for enhanced binding to IgE. Mol Nutr Food Res. (2012) 56:1739–47. doi: 10.1002/mnfr.201100815
85. Barre A, Borges JP, Culerrier R, Rouge P. Homology modelling of the major peanut allergen Ara h 2 and surface mapping of IgE-binding epitopes. Immunol Lett. (2005) 100:153–8. doi: 10.1016/j.imlet.2005.03.014
86. Barre A, Sordet C, Culerrier R, Rance F, Didier A, Rouge P. Vicilin allergens of peanut and tree nuts (walnut, hazelnut and cashew nut) share structurally related IgE-binding epitopes. Mol Immunol. (2008) 45:1231–40. doi: 10.1016/j.molimm.2007.09.014
87. Otsu K, Guo R, Dreskin SC. Epitope analysis of Ara h 2 and Ara h 6: characteristic patterns of IgE-binding fingerprints among individuals with similar clinical histories. Clin Exp Allergy. (2015) 45:471–84. doi: 10.1111/cea.12407
88. Gieras A, Cejka P, Blatt K, Focke-Tejkl M, Linhart B, Flicker S, et al. Mapping of conformational IgE epitopes with peptide-specific monoclonal antibodies reveals simultaneous binding of different IgE antibodies to a surface patch on the major birch pollen allergen, Bet v 1. J Immunol. (2011) 186:5333–44. doi: 10.4049/jimmunol.1000804
89. Devanaboyina SC, Cornelius C, Lupinek C, Fauland K, Dall'Antonia F, Nandy A, et al. High-resolution crystal structure and IgE recognition of the major grass pollen allergen Phl p 3. Allergy. (2014) 69:1617–28. doi: 10.1111/all.12511
90. Longo V, Costa MA, Cibella F, Cuttitta G, La Grutta S, Colombo P. Multiple IgE recognition on the major allergen of the Parietaria pollen Par j 2. Mol Immunol. (2015) 63:412–9. doi: 10.1016/j.molimm.2014.09.012
91. Miyaji K, Yurimoto T, Saito A, Yasueda H, Takase Y, Shimakura H, et al. Analysis of conformational and sequential IgE epitopes on the major allergen Cry j 2 of Japanese cedar (Cryptomeria japonica) pollen in humans by using monoclonal antibodies for Cry j 2. J Clin Immunol. (2013) 33:977–83. doi: 10.1007/s10875-013-9880-7
92. Linhart B, Valenta R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine. (2012) 30:4328–35. doi: 10.1016/j.vaccine.2011.11.011
93. Apostolovic D, Luykx D, Warmenhoven H, Verbart D, Stanic-Vucinic D, de Jong GA, et al. Reduction and alkylation of peanut allergen isoforms Ara h 2 and Ara h 6; characterization of intermediate- and end products. Biochim Biophys Acta. (2013) 1834:2832–42. doi: 10.1016/j.bbapap.2013.10.004
94. Bencharitiwong R, van der Kleij HP, Koppelman SJ, Nowak-Wegrzyn A. Effect of chemical modifications on allergenic potency of peanut proteins. Allergy Asthma Proc. (2015) 36:185–91. doi: 10.2500/aap.2015.36.3840
95. Hazebrouck S, Guillon B, Drumare MF, Paty E, Wal JM, Bernard H. Trypsin resistance of the major peanut allergen Ara h 6 and allergenicity of the digestion products are abolished after selective disruption of disulfide bonds. Mol Nutr Food Res. (2012) 56:548–57. doi: 10.1002/mnfr.201100614
96. van Hoffen E, van der Kleij H, Jager SDH, Knol EF, Opstelten DJ. Effect of modificaion of peanut conglutin on IgE and T cell reactivity in adults with peanut allergy. Clin Transl Allergy. (2011) 1(Suppl 1):016. doi: 10.1186/2045-7022-1-S1-O16
97. Starkl P, Felix F, Krishnamurthy D, Stremnitzer C, Roth-Walter F, Prickett SR, et al. An unfolded variant of the major peanut allergen Ara h 2 with decreased anaphylactic potential. Clin Exp Allergy. (2012) 42:1801–12. doi: 10.1111/cea.12031
98. Bernard H, Guillon B, Drumare MF, Paty E, Dreskin SC, Wal JM, et al. Allergenicity of peanut component Ara h 2: Contribution of conformational versus linear hydroxyproline-containing epitopes. J Allergy Clin Immunol. (2015) 135:1267–74 e1–8. doi: 10.1016/j.jaci.2014.10.025
99. Tscheppe A, Palmberger D, van Rijt L, Kalic T, Mayr V, Palladino C, et al. Development of a novel Ara h 2 hypoallergen with no IgE binding or anaphylactogenic activity. J Allergy Clin Immunol. (2020) 145:229–38. doi: 10.1016/j.jaci.2019.08.036
100. Aalberse RC, Crameri R. IgE-binding epitopes: a reappraisal. Allergy. (2011) 66:1261–74. doi: 10.1111/j.1398-9995.2011.02656.x
101. Chang X, Zha L, Wallimann A, Mohsen MO, Krenger P, Liu X, et al. Low-affinity but high-avidity interactions may offer an explanation for IgE-mediated allergen cross-reactivity. Allergy. (2021) 76:2565–74. doi: 10.1111/all.14864
102. Flicker S, Steinberger P, Ball T, Krauth MT, Verdino P, Valent P, et al. Spatial clustering of the IgE epitopes on the major timothy grass pollen allergen Phl p 1: importance for allergenic activity. J Allergy Clin Immunol. (2006) 117:1336–43. doi: 10.1016/j.jaci.2006.02.012
103. Burton OT, Tamayo JM, Stranks AJ, Koleoglou KJ, Oettgen HC. Allergen-specific IgG antibody signaling through FcgammaRIIb promotes food tolerance. J Allergy Clin Immunol. (2018) 141:189–201 e3. doi: 10.1016/j.jaci.2017.03.045
Keywords: allergens, effector function, fcepsilonR1, IgE, mast cells, cross-linking, epitope, degranulation
Citation: Hazebrouck S, Canon N and Dreskin SC (2022) The Effector Function of Allergens. Front. Allergy 3:818732. doi: 10.3389/falgy.2022.818732
Received: 19 November 2021; Accepted: 14 January 2022;
Published: 07 February 2022.
Edited by:
Soheila June Maleki, United States Department of Agriculture, United StatesReviewed by:
Mayte Villalba, Complutense University of Madrid, SpainCopyright © 2022 Hazebrouck, Canon and Dreskin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen C. Dreskin, stephen.dreskin@cuanschutz.edu
 Stéphane Hazebrouck
Stéphane Hazebrouck Nicole Canon2
Nicole Canon2  Stephen C. Dreskin
Stephen C. Dreskin