Analysis of Nitrogenase Fe Protein Activity in Transplastomic Tobacco
- 1Centro de Biotecnología y Genómica de Plantas, Universidad Politécnica de Madrid (UPM), Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), Madrid, Spain
- 2Departamento de Biotecnología-Biología Vegetal, Escuela Técnica Superior de Ingeniería Agronómica, Alimentaría y de Biosistemas, UPM, Madrid, Spain
Integration of prokaryotic nitrogen fixation (nif) genes into the plastid genome for expression of functional nitrogenase components could render plants capable of assimilating atmospheric N2 making their crops less dependent of nitrogen fertilizers. The nitrogenase Fe protein component (NifH) has been used as proxy for expression and targeting of Nif proteins within plant and yeast cells. Here we use tobacco plants with the Azotobacter vinelandii nifH and nifM genes integrated into the plastid genome. NifH and its maturase NifM were constitutively produced in leaves, but not roots, during light and dark periods. Nif protein expression in transplastomic plants was stable throughout development. Chloroplast NifH was soluble, but it only showed in vitro activity when isolated from leaves collected at the end of the dark period. Exposing the plant extracts to elevated temperatures precipitated NifM and apo-NifH protein devoid of [Fe4S4] clusters, dramatically increasing the specific activity of remaining NifH protein. Our data indicate that the chloroplast endogenous [Fe-S] cluster biosynthesis was insufficient for complete NifH maturation, albeit a negative effect on NifH maturation due to excess NifM in the chloroplast cannot be excluded. NifH and NifM constitutive expression in transplastomic plants did not affect any of the following traits: seed size, germination time, germination ratio, seedling growth, emergence of the cotyledon and first leaves, chlorophyll content and plant height throughout development.
Introduction
Under optimal climate and water availability, crop yields correlate directly with chemical fertilizer inputs. Among these, nitrogen (N) fertilizers are critical to maximize commercial crop yields (Mueller et al., 2012). However, production and application of N fertilizers are major sources of pollution (Erisman et al., 2015). First, conversion of dinitrogen gas (N2) into ammonia (NH3) by the Haber-Bosh process is energetically costly and uses fossil fuels. Then, once applied in the field, NH3 is converted to nitrate () by nitrification in the soil. This causes N runoff into ground waters and aquatic systems and is often followed by toxic algal and cyanobacterial blooms. Finally, some of the is denitrified into the potent greenhouse gas nitrous oxide (N2O). As the world's population is expected to reach 9.7 billion by 2050 and as the agricultural yield overall should increase with 60% from 2007 levels to meet coming food demand (Alexandratos and Bruinsma, 2012), new technologies could be important tools to reduce future agricultural impact on the environment.
Many of the effects from intense N fertilization could be avoided if crops could directly utilize atmospheric N2 as N source by using biological N2 fixation (BNF) (Curatti and Rubio, 2014; Oldroyd and Dixon, 2014), a trait only performed by a small (albeit phylogenetically diverse) group of prokaryotes collectively called diazotrophs. These select bacteria and archaea reduce N2 to NH3 using nitrogenase, a metalloenzyme that in its most prevalent form is composed of the Fe protein (encoded by nifH) and the MoFe protein (encoded by nifD and nifK) [for recent reviews see (Burén et al., 2020; Seefeldt et al., 2020)]. Progress has already been achieved in the path to engineer N2 fixation by transfer of nif genes to unicellular eukaryotes (Cheng et al., 2005; López-Torrejón et al., 2016; Burén et al., 2017, 2019; Burén and Rubio, 2018) and to higher plants (Ivleva et al., 2016; Allen et al., 2020; Eseverri et al., 2020; Jiang et al., 2021).
Genetically modified plants are most often created by gene insertion into the nuclear genome (nuclear transformation) or the plastid genome (transplastomic, plastid transformation) (Bock, 2013). Currently, there is no standard methodology to insert exogenous DNA into the genome of plant mitochondria, although recent work in this direction is encouraging (Kazama et al., 2019). While nuclear transformation is relatively straightforward and can be achieved either by A. tumefaciens-mediated transformation or by particle bombardment, manipulation of chloroplast genome is only possible through bombardment (Ruf et al., 2019). Plastid genome transformation offers some important advantages over nuclear transformation, e.g. “in house” expression of proteins destined for the plastid, the possibility to organize genes into operons and to obtain high transgene expression, and it is devoid of gene silencing (Adem et al., 2017).
Importantly, many Nif proteins, including the nitrogenase structural components, carry metal clusters that are extremely sensitive to O2. The NifH protein in particular is rapidly and irreversibly inactivated by O2 (Shah and Brill, 1973). Due to this extreme sensitivity, and because NifH function involves fewer ancillary proteins than NifDK, the former has been used as proxy for nitrogenase engineering in eukaryotic cells. Proof of concept was obtained when NifH and NifM (a putative peptidyl-prolyl cis-trans isomerase involved in NifH maturation) were targeted to the mitochondria of aerobically cultured yeast cells (López-Torrejón et al., 2016). Active NifH has also been isolated from Agrobacterium tumefaciens-infiltrated tobacco leaves expressing chloroplast or mitochondria targeted NifH (Eseverri et al., 2020; Jiang et al., 2021). In these studies, NifU and NifS where included, in addition to NifM, as they are involved in the assembly of [Fe-S] clusters destined for Nif proteins (Burén et al., 2020).
Previously, Ivleva and colleagues generated transplastomic tobacco plants for the expression of Azotobacter vinelandii NifH and NifM, and demonstrated NifH protein activity in extracts from plants incubated at 10% O2 (Ivleva et al., 2016). Although NifH protein activity was very low, these results were encouraging and deserved further investigation. In the present work we have studied these transplastomic tobacco plants in more detail. We show that NifH and NifM proteins were constitutively expressed in leaves, but not roots, and accumulated in both light and darkness throughout the plant development. Importantly, we show that NifH protein was active when isolated from plants grown under standard air atmosphere if they were collected at the end of the dark period. Exposing the plant extracts to elevated temperatures precipitated NifM and apo-NifH protein devoid of [Fe4S4] clusters, which significantly increased the specific activity of remaining NifH protein. Finally, constitutive expression of NifH and NifM in the chloroplast did not cause pleiotropic effects that could otherwise impact the agronomic relevance of the attempts to engineer nitrogenase in crops.
Materials and Methods
Plant Material and Growth Conditions
Transplastomic tobacco plants (Nicotiana tabacum) with A. vinelandii nifH and nifM genes integrated in the chloroplast genome downstream of rbcL using plasmid pMON261406 (carrying the aadA gene cassette) were kindly provided by Bayer Cropscience. In short, spectinomycin resistant transplastomic lines were generated by biolistic bombardment of tobacco leaves with pMON261406 and recovered in tissue culture after plant regeneration steps. Presence of the desired DNA insert was verified by Southern blot analysis (Ivleva et al., 2016). The plants were designed to constitutively express NifH and NifM proteins from a chimeric two-gene operon controlled by the ribosomal RNA promoter Prrn. Two transplastomic lines were recovered that carried nifH and nifM integrated in the same location of the chloroplast genome and expressed identical amounts of NifM and NifH polypeptides. One line was used for the present study.
Seeds were sown in soil inside a growth chamber set at 25°C (day, 17 h) and 23°C (night, 7 h), and 55% humidity. Four-week-old plants were irrigated with Sequestrene G100 (1 g·L−1) plus FeSO4 (1 g·L−1) starting one week before leaves harvesting. Leaves were harvested either at the end of the dark period, or at least after five hours into the light period.
Preparation of NifH-Enriched Samples From Tobacco Leaves
About 100 g of leaves from four-week-old tobacco plants were used for each preparation. Harvested leaves were immediately snap-frozen and maintained in liquid N2. The material was ground into powder using a mortar and pestle in liquid N2, and then transferred into an anaerobic glovebox to avoid O2 exposure during protein extraction.
The leaf powder was resuspended in anaerobic lysis buffer containing 50 mM Tris-HCl (pH 7.6), 2 mM sodium dithionite (DTH), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg·ml−1 leupeptin, and 5 μg·ml−1 DNase I, at a 1:2 (leaf weight to buffer volume) ratio. For heat treatment, the total extract was incubated at 50°C in a closed bottle submerged in a water bath with stirring for 5 min, filtered through Miracloth (Merck) and centrifuged at 4°C and 58,000 × g for 1 h. The resulting soluble cell free extract (CFE) was passed through a Minisart 0.45 μm CA filter (Sartorius AG) and loaded into a 5 ml Q-Sepharose column (GE healthcare) at 1.5 ml·min−1. The column was washed with five column volumes of buffer A (50 mM Tris-HCl, pH 7.6, 2 mM DTH) at the same flow rate before applying a linear gradient spanning 0–500 mM NaCl (gradient volume of 100 ml mixing buffer A and B (50 mM Tris, pH 7.6, 500 mM NaCl, 2 mM DTH)). The flow rate during the gradient was set at 2 ml·min−1 and fractions were collected with 2.5 ml intervals. Attempts to reduce contamination of Rubisco with NifH included: extended washing with five column volumes of 100 or 150 mM NaCl before starting the linear gradient, NifH elution using stepwise gradients (five column volumes each of 100, 200, 300, 400 and 500 mM NaCl), and changes in the chromatography flow rates. However, we were not able to remove Rubisco from NifH-enriched fractions.
The NifH elution profile was determined by immunoblot analysis. Fractions containing NifH were pooled and concentrated using Amicon Ultra 30K centrifugal filters (Merck), snap-frozen and then kept in liquid N2 until further analysis.
Immunoblots
Samples were mixed with Laemmli buffer, heated for 5 min at 90°C, and centrifuged at 10,000 × g for 30 s to remove cell debris. Protein samples (final volume 20 μl) were resolved by SDS-PAGE at 200 V for 40 min. Gels were either stained directly with Coomassie or transferred to nitrocellulose membranes using a Trans-Blot Semi-Dry system (BioRad) and then stained with Ponceau. Membranes were blocked in phosphate buffer saline (PBS) containing 5% skimmed milk at room temperature for 1 h, washed 3 times for 15 min with PBS, and then incubated overnight at 4°C in an orbital shaker with polyclonal antibodies (1:5,000 dilution) detecting either A. vinelandii NifH (López-Torrejón et al., 2016) or A. vinelandii NifM (Burén and Rubio, 2018). Membranes were washed 3 times for 15 min with PBS before addition of secondary antibody (A0545-1ML, Sigma-Aldrich) at 1:50,000 dilution in PBS and incubated for 1 h at room temperature. Finally, membranes were washed 3 times for 15 min in PBS, and then 1 ml of ECL reagent were added to the membrane. Signals were recorded using an iBright FL1000 Imaging System (Thermo Fisher Scientific).
Quantification of NifH levels in the final samples was performed as previously described (Poza-Carrión et al., 2014). Membranes from gels loaded with increasing amounts of sample were probed with NifH-targeting antibodies, scanned at high resolution, and analyzed using the ImageJ software. NifH band intensities were compared to membranes (processed in parallel) originating from gels loaded with known amounts of NifH purified from A. vinelandii.
Heat Treatment Analysis
Two types of samples were subjected to heat treatment: (i) tobacco NifH-enriched fractions (containing NifH and NifM) and (ii) purified A. vinelandii NifH or apo-NifH. Protein samples were incubated either at 35°C or 55°C for 5 min and then centrifuged at 12,000 × g for 10 min. Soluble (corresponding to the supernatant remaining after centrifugation) and pellet (corresponding to the pellet after resuspension in 1 ml lysis buffer) fractions were analyzed by immunoblotting. For heat treatment experiments using purified A. vinelandii NifH or apo-NifH, 1 ml of tobacco WT total protein extract from the corresponding dark or light periods were added to 12 μg apo-NifH or holo-NifH before the treatment.
Nitrogenase Activity
In vitro NifH protein activity was assayed in 9 ml serum vials sealed with serum stoppers according to Shah and Brill with minor modifications (Shah and Brill, 1973). Nitrogenase activity was measured by the acetylene reduction assay (ARA) using a NifH:NifDK protein molar ratio of 9:1. For this, 8.2 μg of NifH was mixed with 3 μg of NifDK and an ATP-regenerating mixture (1.23 mM ATP, 18 mM phosphocreatine, 2.2 mM MgCl2, 3 mM DTH and 40 μg of creatine phosphokinase). Reactions (in a total volume of 0.8 ml) were incubated at 30°C for 30 min in a rotary shaker, and then stopped by addition of 0.1 ml of 8 M NaOH. The produced ethylene was measured by injecting 500 μl of the head space gas into a gas chromatograph (GC2014, Shimadzu) equipped with a Porapak N 80/100 column. Positive control reactions were performed with NifH and NifDK proteins purified from A. vinelandii (Curatti et al., 2007). Negative control reactions were performed using enriched samples from WT plants, processed and collected identically to those from the transplastomic plants. For each experiment, nitrogenase activities were measured in at least three independent biological replicates.
Apo-NifH Generation and Reconstitution
Apo-NifH was prepared in vitro according to Rangaraj and colleagues (Rangaraj et al., 1997) with slight modifications. To remove the [Fe4S4] cluster from the NifH dimers, 50 μM of NifH purified from A. vinelandii was incubated with 2.5 mM MgATP, 2 mM DTH, and 40 mM 2,2′-bipyridyl in 22 mM Tris-HCl (pH 7.4) for 30 min at 25°C. The protein was desalted twice using Sephadex G-25 PD-10 columns (Amersham Biosciences) and collected in buffer containing 200 mM NaCl, 10% glycerol, 2 mM DTH and 100 mM Tris-HCl (pH 8.0). Finally, the generated apo-NifH was concentrated using Amicon Ultra 10K centrifugal filters (Merck) and quantified using the BCA protein assay (Pierce).
Reconstitution assays using tobacco extracts were carried out with holo-NifH purified from A. vinelandii or with apo-NifH prepared as described above. Total protein extracts from WT tobacco leaves harvested at the end of the dark period were separately mixed with 12.33 μg NifH (holo-NifH or apo-NifH) in a final volume of 200 μl, and then either directly used for ARA or first heat-treated at 55°C for 5 min and then used for ARA. In parallel and following the same procedure, apo-NifH activation was performed using WT tobacco total extracts supplemented with NifU that had been previously loaded with [Fe-S] clusters as described in Dos Santos et al. (2004) and López-Torrejón et al. (2016). Apo-NifH reconstitution was estimated by ARA following the addition of 5 μg of NifDK protein and ATP-regenerating mixture as described above.
NifH Specific Activity Determination After Reconstruction of Co-existence of Apo- and Holo-NifH in Tobacco Extracts
A mixture of NifH polypeptides (12.33 μg total at a 1:6 ratio of holo-NifH to apo-NifH) were mixed with 80 μl of tobacco cell-free extract. Four hundred eighty μl of this mix was prepared and split into two tubes of 240 μl each. Samples were heated either at 35°C or 55°C for 5 min and centrifuged at 12,000 × g for 5 min to separate soluble and precipitated protein. Eighty μl of each supernatant was mixed with ATP-regeneration mixture containing 5 μg of purified NifDK, and ARA was performed as described above. The remaining supernatant solution and the pellets left after centrifugation from each heat treatment condition were used for protein quantification based on immunoblotting assays.
Plant Development and Seed Size Analyses
Three independent experiments were performed to determine emergence of the cotyledon and the first pair of leaves. For each experiment at least 60 WT or transplastomic seeds were sown in soil and daily monitored. Plant height and chlorophyll content (CCM-200plus, OPTI-SCIENCES) were measured from 3 to 6 weeks after sowing seeds.
Seed sizes were analyzed following a previously established protocol (Herridge et al., 2011). Approximately 200 randomly selected seeds from 4 different WT or transplastomic plants were photographed using a Leica M165FC microscope, and then measured using the ImageJ software (version 1.48v).
Results
Accumulation of A. vinelandii NifH and NifM Polypeptides in Transplastomic Tobacco
Accumulation of the nitrogenase NifH protein polypeptide was analyzed in leaf (aerial) and root (lower oxygen concentration below soil) tissues of transplastomic tobacco plants. The protein migrated similarly to native A. vinelandii NifH in SDS-PAGE gels and was almost exclusively detected in the soluble fraction of leaf extracts, with only a faint band being associated to the membrane fraction after centrifugation (Figure 1A). That NifH accumulated as soluble protein was indicative of successful maturation by NifM, as previous reports indicate that absence of NifM renders insoluble NifH polypeptides (Roberts et al., 1978; Howard et al., 1986; Eseverri et al., 2020). No NifH was detected in root tissue of transplastomic plants.
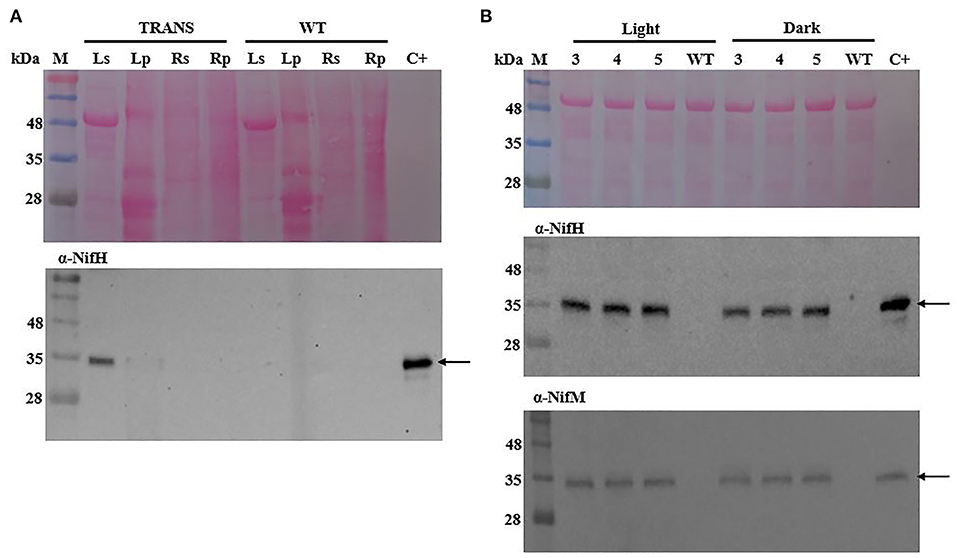
Figure 1. Immunoblot analysis of NifH and NifM polypeptide accumulation in transplastomic tobacco. (A) NifH accumulation in soluble (s) or membrane-associated pellet (p) fractions from leaf (L) and root (R) tissues of transplastomic (TRANS) or wild-type (WT) tobacco. (B) Accumulation of soluble NifH and NifM in leaves of transplastomic tobacco harvested during light or dark periods at three (3), four (4) and five (5) weeks after sowing. Arrows point to polypeptides detected with antibodies against the A. vinelandii NifH and NifM proteins. C+, pure A. vinelandii NifH and NifM proteins; M, molecular mass markers (kDa); WT, total protein extract from 4-week-old WT tobacco leaves. Ponceau staining of membranes are shown as control of protein loading and transfer.
To analyze the accumulation of NifH and NifM proteins during the development of the transplastomic plants, protein extracts were prepared from leaves harvested in both light and darkness at three, four, and five weeks after sowing. The levels of NifH and NifM in soluble extracts were analyzed by immunoblotting and were found to be constant over time which indicated stable and consistent expression (Figure 1B).
Effect of Light and Darkness on Function of Plastid-Expressed NifH Protein
The metabolic status of plants varies during light and darkness (Ort and Oxborough, 1992; Dodd et al., 2015). The circadian cycle affects both physical and biochemical factors that are critical to NifH protein maturation, stability and activity, e.g., O2 levels, availability of ATP and reducing power (Bulen and LeComte, 1966; Shah and Brill, 1973). Ivleva and colleagues previously demonstrated low NifH protein activity in chloroplasts enriched from transplastomic tobacco (Ivleva et al., 2016). However, these plants were incubated under atmosphere with 10% O2. Understanding how plant metabolic and physiological conditions, such as those imposed by light and darkness, affects NifH function is therefore of major importance to engineer nitrogen fixation in planta.
For this analysis, anion exchange chromatography was employed to enrich NifH from tobacco soluble protein extracts prepared from leaves harvested either in the light or at the end of the dark period (Figure 2A). Two main protein peaks were observed in the chromatogram, where the second peak was dominated by the large (55 kDa) and small (13 kDa) subunits of Rubisco (Figure 2B). Immunoblot analysis using antibodies recognizing NifH was required to unambiguously identify which fractions contained the NifH protein. NifH was normally found in fractions 27–40 of the linear NaCl gradient, which corresponded to 325–475 mM NaCl (25–39 mS cm−1) (Figure 2C).
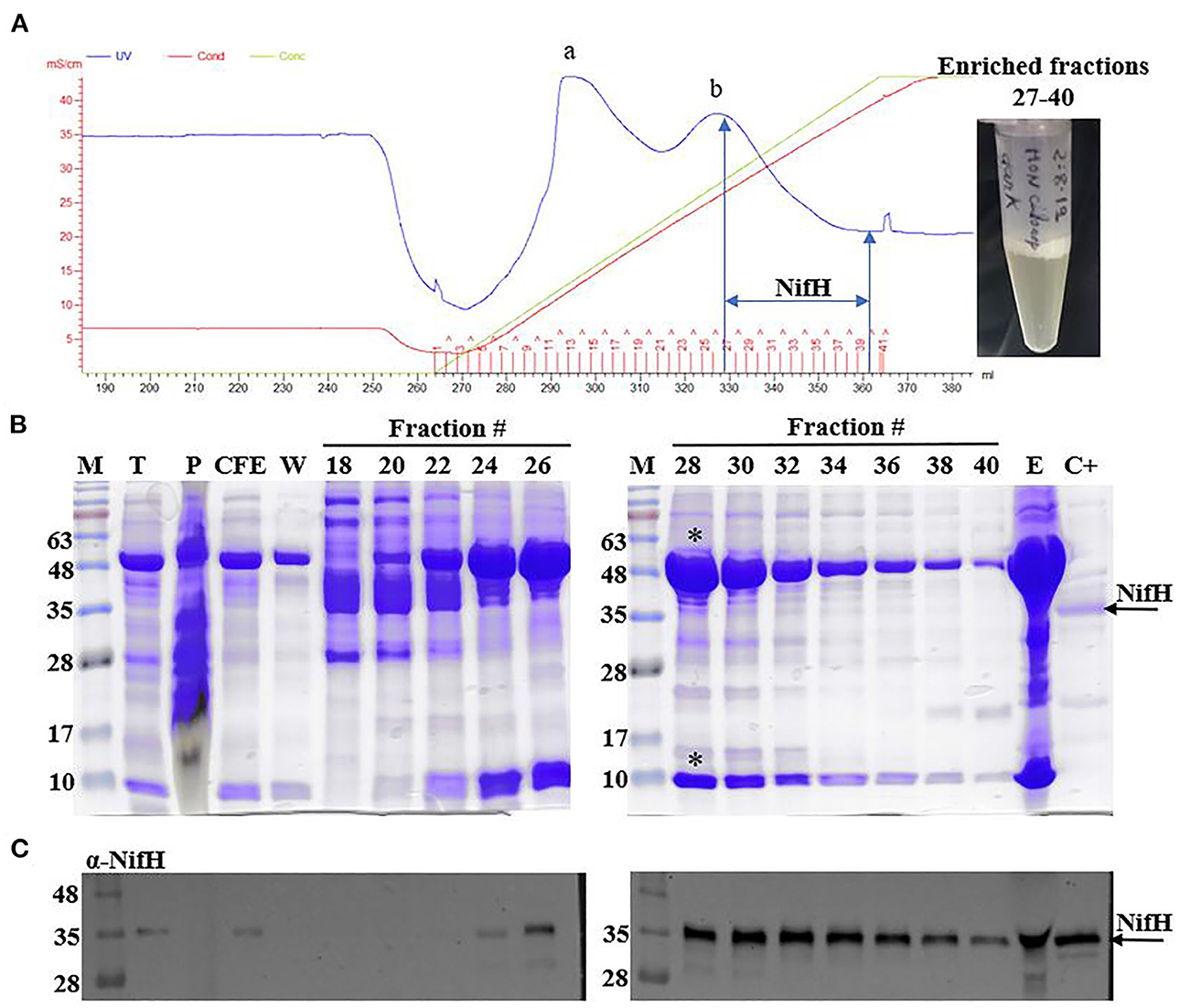
Figure 2. Enrichment of NifH from tobacco leaf extracts. (A) Chromatogram showing a typical procedure for NifH enrichment using Q-Sepharose chromatography. Two main protein elution peaks (a and b) are indicated. Red line, conductivity; blue line, UV-vis absorption; green line, NaCl concentration. Fractions containing NifH (27–40) were pooled and concentrated (final preparation shown to the right). (B, C) Coomassie staining (B) and immunoblots developed with antibodies against NifH (C) of SDS gels loaded with selected elution fractions. The large (55 kDa) and small (13 kDa) subunits of Rubisco eluting in peak b are highlighted (*). CFE, soluble protein fraction; C+, pure A. vinelandii NifH; E, pooled NifH-containing fractions 27–40; M, molecular mass markers (kDa); P, membrane fraction (insoluble protein aggregates and proteins associated with membranes); T, total protein extract; W, wash fraction.
To reduce protein contaminants in fractions containing NifH, transplastomic leaf extracts were heat-treated for 5 mins and centrifuged prior to chromatography. Heat treatment can denature proteins and this method has been described to remove approximately 60% of the soluble proteins in A. vinelandii extracts at the cost of only minor NifH loss (Burgess et al., 1980). We therefore employed this protocol expecting that this procedure would reduce the amounts of Rubisco in leaf extracts. However, Rubisco was still prominent in NifH enriched fractions under all conditions tested (see Material and Methods), which impaired isolating pure NifH leading us to determine NifH concentration in the enriched samples using immunoblotting (Supplementary Figure 1).
The Fe protein activity of NifH enriched preparations was determined by the ARA, in which NifDK reduces acetylene to ethylene using electrons donated by NifH. NifDK for ARA was purified from A. vinelandii, and so was the NifH protein used in positive control reactions (Supplementary Figure 2). Since the amount of NifH protein in the enriched fractions was a limiting factor, activity assays were carried out at 1:9 (NifDK:NifH) ratio. When NifH was isolated from leaves collected during the light period the measured NifH protein activity was low (8.2 nmol C2H4·min−1·mg NifDK−1), which corresponded to about twice the amount of ethylene measured using samples isolated from WT tobacco leaves (Figure 3A, inset). A further two-fold increase in ethylene production was observed when NifH from heat-treated leaves (also harvested in light) was used (Figure 3B). Similarly, NifH samples obtained from leaves collected at the end of the dark period showed low NifH protein activity (9.2 nmol C2H4·min−1·mg NifDK−1) (Figure 3A), but heat-treatment increased NifH protein specific activity 13-fold. Importantly, heat treatment was more effective in increasing NifH activity in samples from dark-collected leaves than those from light-exposed leaves (Figure 3B).
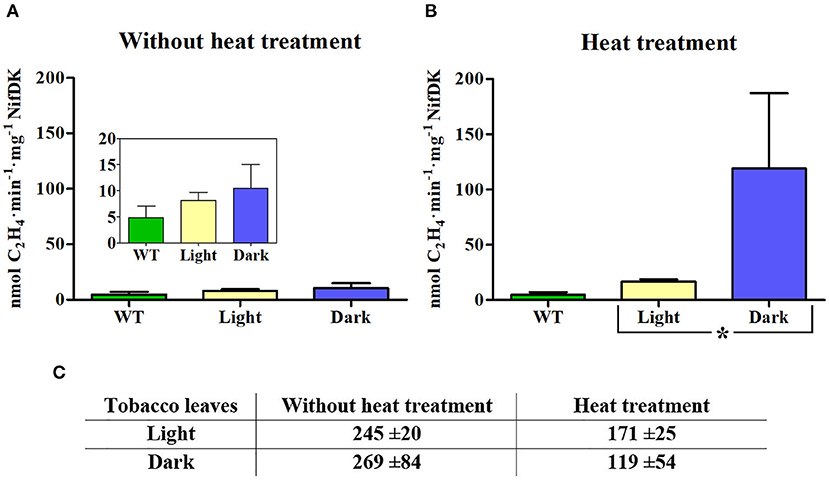
Figure 3. Activity of NifH enriched from light- and dark-incubated leaves. (A, B) ARA at a 9:1 molar ratio of NifH:NifDK using transplastomic NifH isolated from leaves without (A) or with heat treatment (B) of extracts prior chromatography. Protein samples from leaves of wild-type (WT) plants subject to the same enrichment procedure were used as control. Inserts shows activities at zoomed y-axis scale. Average activities ± SD (n = 3) are shown. Statistically significant differences based on Student's t-test is shown; *P < 0.05. (C) Total μg of NifH protein per 100 g of leaves present in samples from enrichment experiments. Values represent average content ± SD (n = 3).
As previously reported for A. vinelandii extracts (Burgess et al., 1980), heat treatment reduced the amount of soluble NifH in transplastomic leaf extracts (Figure 3C; Supplementary Figures 1 and 3). By comparing the specific activity of NifH protein enriched from chloroplasts to that of NifH purified from A. vinelandii (Supplementary Figure 2), it was estimated that about half of the heat resistant NifH isolated from transplastomic leaves at the end of the dark period was active. Although this result does not necessarily encompass all active NifH present in transplastomic leaves (some active holo-NifH protein could have precipitated), it strongly suggests that darkness improved the cellular environment for NifH protein maturation and/or stability.
Heat-Precipitation of NifM Does Not Increase NifH Protein Activity
NifH is highly abundant in N2-fixing A. vinelandii and Klebsiella oxytoca cells, about 50-fold higher than NifM (Poza-Carrión et al., 2014; Yang et al., 2018). One limitation with the transplastomic plants used is the expected equimolar stoichiometry of NifH and NifM, which could impair NifH activity. Incorrect Nif protein stoichiometry has been shown to affect nitrogenase maturation in vitro (Curatti et al., 2007; Hernandez et al., 2011) and in vivo (Temme et al., 2012). Analysis of the NifH-enriched samples showed that NifM was absent when the leaf extracts had been heated prior to chromatography (Figure 4A; Supplementary Figure 3). Data in Figure 4A shows that while some NifH was resistant to the elevated temperature all NifM precipitated to the pellet. We therefore speculated that NifH function was impaired by NifM present in non-heated samples. To test this possibility, ARA were performed in which increasing amounts of NifM were added to the reaction mixtures. No inhibitory effect from NifM but rather a slight increase in nitrogenase activity was observed in the ARA (Figure 4B). This result argued against inhibition of NifH activity by NifM in non-heated samples. Thus, heat mediated NifM precipitation did not explain the increase in NifH activity.
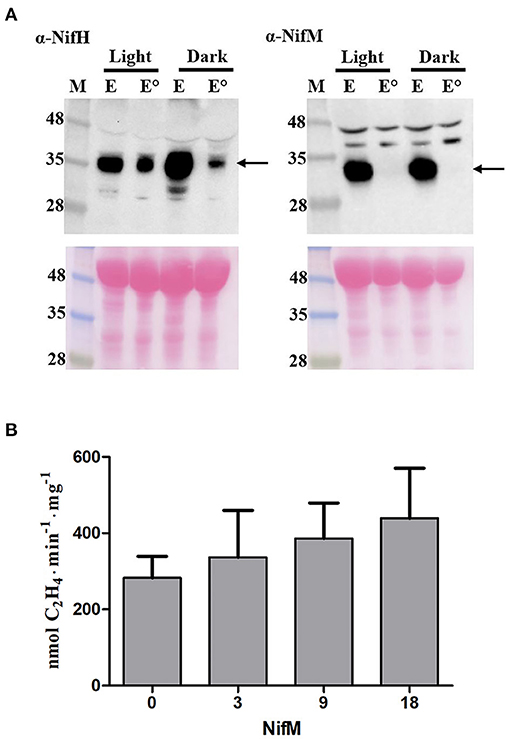
Figure 4. NifM does not inhibit NifH activity. (A) Immunoblot analysis of NifH and NifM levels in NifH-enriched samples (E) prepared from leaves harvested under light (Light) or darkness (Dark) with (°) or without heat treatment. M, molecular mass markers (kDa). Arrows indicate positions of NifH and NifM in the membrane. Ponceau staining of membrane is shown at the bottom as control of protein loading and transfer. (B) ARA using a 9:1 molar ratio of NifH:NifDK in the presence of increasing amounts of NifM (values in the x-axis indicate the ratio of NifM over NifDK). Average activities ± SD (n = 3) are shown.
Apo-NifH Removal by Heat Treatment Correlates With Increased NifH Protein Activity
The NifH activity increase upon heat treatment could be explained if [Fe4S4] clusters were liberated from (other) denatured plant metalloproteins and incorporated into cluster-deficient apo-NifH. To test this hypothesis, purified A. vinelandii apo-NifH was added to WT tobacco leaf extracts and then subject to the same heat treatment. No apo-NifH activation was observed indicating lack of [Fe4S4] cluster transfer (Figure 5A). A similar experiment was performed in the presence of pure NifU, a physiological donor of [Fe-S] clusters to nitrogenase proteins that had been previously purified from E. coli and loaded with [Fe4S4] clusters in vitro. NifU-mediated reconstitution of apo-NifH has been previously demonstrated (Dos Santos et al., 2004; López-Torrejón et al., 2016). Consistently, NifU was able to activate apo-NifH presumably by transferring [Fe4S4] clusters (Figure 5B). In all experiments, the heat treatment decreased the level of apo-NifH activation in the extract, likely due to partial NifH precipitation (Figure 5). Thus, no direct positive effect on NifH activity was obtained by heating the extract. We note that the NifU-reconstituted apo-NifH appeared to be more sensitive to heat than the holo-NifH protein.
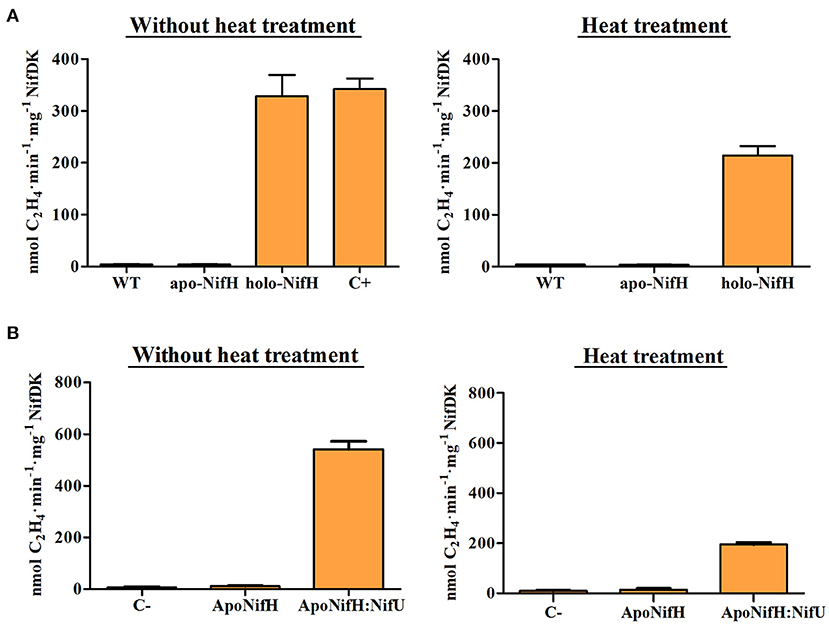
Figure 5. Reconstitution of apo-NifH added to tobacco protein extracts. (A) Pure apo-NifH and NifH (holo-NifH) isolated from A. vinelandii were mixed with tobacco WT tobacco protein extracts without a heat treatment (left) or subjected to heat treatment (right). NifH activity was determined by ARA using 9:1 molar ratio of NifH:NifDK. WT, control reactions containing only WT tobacco leaf extract. C+, control reactions including the same amount of holo-NifH but lacking WT tobacco protein extracts. Average activities ± SD (n = 4) are shown. (B) Reconstitution of apo-NifH by NifU added to WT tobacco total protein extracts without (left) and with (right) heat treatment. Pure A. vinelandii apo-NifH was mixed with WT tobacco leaf extracts and then, [Fe-S] cluster-loaded NifU was added to the mixtures. After 10 min incubation at room temperature, pure NifDK was added and samples were analyzed for ARA. C-, WT tobacco leaf extracts without added NifH. Average activities ± SD (n = 4) are shown.
We also tested whether native tobacco proteins present in the extracts were inhibiting NifH activity, whose disappearance upon heating could explain improved NifH functionality. However, pure holo-NifH mixed with WT tobacco protein extract showed the same activity as control reactions lacking the tobacco extracts (Figure 5A).
The yield of NifH in enriched fractions was reduced when tobacco protein extracts had been previously heated, although the specific activity was higher (Figure 3). This inverse correlation between yield and activity suggested that extract-heating positively selected for functional NifH protein. We therefore tested whether holo-NifH was more heat-resistant than apo-NifH. While about half of the holo-NifH remained in solution upon heating at 55°C for 5 min (Figure 6A) most apo-NifH precipitated. We also tested whether the presence of apo-NifH inhibited holo-NifH activity. When half of holo-NifH was replaced with apo-NifH nitrogenase activity was also reduced by half (Figure 6B), indicating that apo-NifH did not compete with holo-NifH for NifDK (and inhibit NifDK) during ARA under the tested conditions. Finally, a reconstruction experiment was conducted in which known mixtures of apo-NifH and holo-NifH (6:1 ratio) were added to tobacco extracts, incubated at either 35°C or 55°C, and analyzed for NifH precipitation and activity (Supplementary Figure 4). Again, a correlation between NifH precipitation and NifH specific activity was observed with activities of samples incubated at 55°C being significantly higher than those incubated at 35°C. Taken together, these results suggest that the NifH protein enriched from heated tobacco extracts was more active due to precipitation of non-functional and inactive apo-NifH.
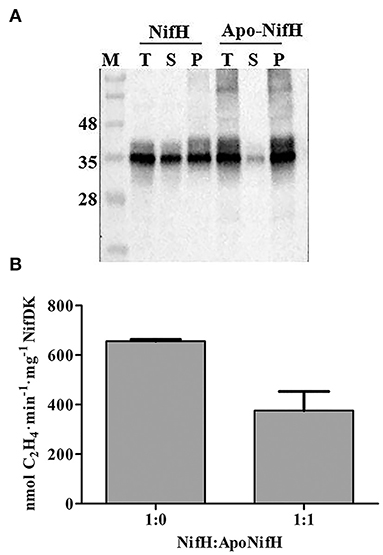
Figure 6. Increased NifH specific activity correlates with apo-NifH precipitation after heat treatment. (A) Pure A. vinelandii apo-NifH or NifH (holo-NifH) were mixed with WT tobacco leaf extracts (T), heated at 55°C for 5 min and then detected in soluble (S) or insoluble membrane-associated pellet (P) fractions by immunoblotting. (B) Pure NifH (8.2 μg), or a 1:1 mixture of NifH (4.1 μg) and apo-NifH (4.1 μg), were mixed with WT tobacco leaf extract and used for ARA determinations. A 9:1 molar ratio of NifH:NifDK or NifH/apo-NifH:NifDK were used. Average activities ± SD (n = 3) are shown.
Agronomic Traits Are Not Affected by Constitutive Expression of NifH and NifM in Transplastomic Plants
Plastids possesses an impressive capacity to synthesize and accumulate proteins of industrial and pharmaceutical importance (Oey et al., 2009; Scotti et al., 2009; Petersen and Bock, 2011). However, transplastomic plants can show pleiotropic effects that reduce crop yield (Scotti and Cardi, 2014). We therefore examined some physiological parameters of transplastomic tobacco plants expressing NifH and NifM. Transplastomic seed germination time and ratio were not affected compared to WT plants. This was expected as NifH and NifM expression is driven by the ribosomal Prrn promoter which is not active in plastids of non-green tissues (Zhang et al., 2012). NifH and NifM expression did neither affect seedling growth nor emergence of the cotyledon and first leaves (Figure 7A), suggesting that plant development was not impaired. Since chlorosis has been described in transplastomic plants (Oey et al., 2009; Scotti et al., 2009; Petersen and Bock, 2011), the chlorophyll content was quantified in leaves from plants three to six weeks after sowing (Table 1). It is well established that photosynthetic efficiency directly correlates with crop yield (Long et al., 2015; Wu et al., 2019). No effect on chlorophyll content was observed suggesting that constitutive expression of NifH and NifM did not affect photosynthesis. Other traits such as plant height and seed size were also determined. Growth of transplastomic plants expressing NifH and NifM was similar to that of WT plants (Table 2) and no morphological differences were observed. Moreover, the seed size was not affected (Figure 7B). Altogether, these results indicate that relevant agronomical traits were not affected by constitutive expression of NifH and NifM in the chloroplast.
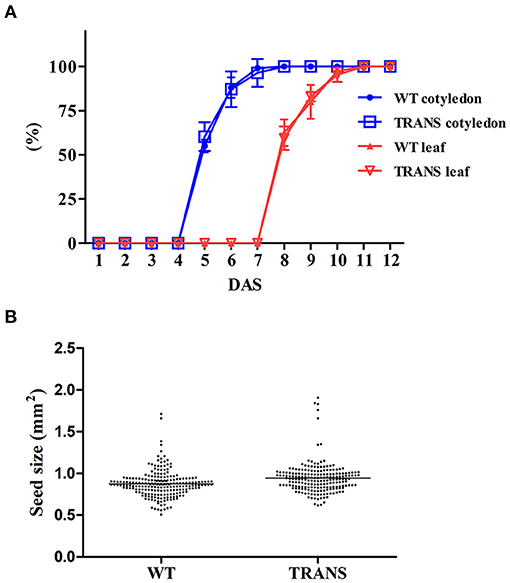
Figure 7. Transplastomic expression of NifH and NifM does not cause pleiotropic effects. (A) Germination of NifH-expressing transplastomic tobacco plants. Seed germination (blue line) and seedling development (red line) of wild type (WT) and transplastomic (TRANS) plants. Germination was determined by cotyledon emergence (cotyledon) and seedling growth (leaf) by the emergence and elongation of the first leaves. Data represent average values ±SD of three independent experiments using seeds harvested from different batches of plants. DAS; days after sowing. (B) Seed size from four different plants were photographed using a Leica M165FC microscope and calculated using ImageJ software (version 1.48v). Tobacco wild type (WT) seeds were used as control. The size (mm2) of seeds harvested from WT (0.89 ±0.17) and transplastomic (0.94 ±0.21) tobacco plants did not display statistically significant differences (Student's t-test).
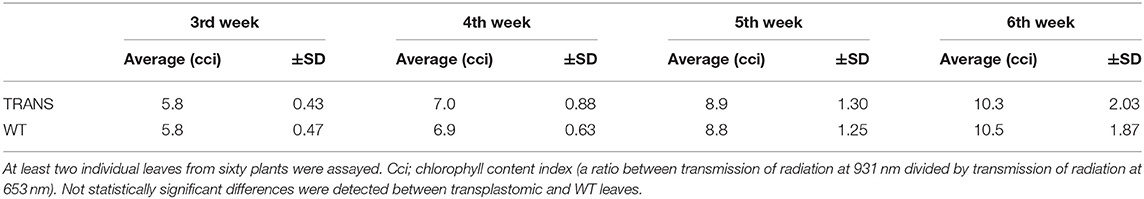
Table 1. Chlorophyll content in transplastomic (TRANS) and wild type (WT) tobacco leaves from 3, 4, 5 and 6 weeks after seed sowing.
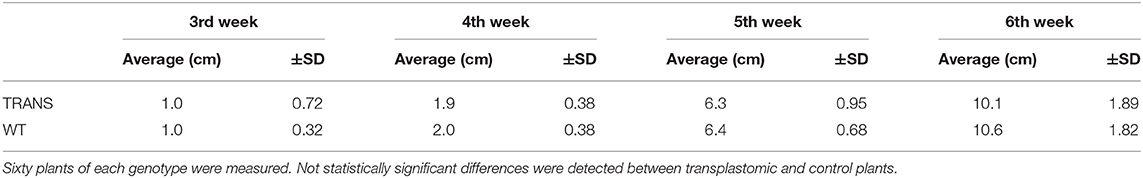
Table 2. Height of transplastomic (TRANS) and wild type (WT) tobacco plants at 3, 4, 5 and 6 weeks after seed sowing.
Discussion
In this work, we have characterized in more detail a previously generated transplastomic tobacco line that expresses the A. vinelandii nifH and nifM genes in the chloroplast. These plants showed very low NifH protein activity in chloroplast-enriched samples when plants were transiently incubated at low O2 concentrations (Ivleva et al., 2016). In contrast, here we analyzed NifH protein activity from these plants grown at standard atmosphere. We have used anion exchange chromatography, which is a well-established method to isolate NifH, that takes advantage of the relative strong interaction between the NifH polypeptide and this type of resins. Unfortunately, the presence of Rubisco -the most abundant protein in the green tissues of plants- in NifH-enriched fractions could not be avoided (Figure 2B). This contaminant precluded direct NifH polypeptide quantitation and other biochemical analyses such as spectroscopy and metal content determination.
The O2 levels at which the plants were grown were not manipulated in this study, as the atmosphere cannot be manipulated in field conditions. Instead, we investigated the effect of oxygenic photosynthesis on NifH accumulation and activity. NifH and NifM accumulation in chloroplasts was similar during light and darkness indicating stability of NifH polypeptides. No Nif protein expression was detected in plastids of non-green root tissue (Figure 1), consistent with other studies using the Prrn promoter (Zhang et al., 2012).
Significant NifH activity was only obtained using extracts from leaves harvested at the end of the dark period and required heating of the leaf protein extract prior to enrichment by chromatography. The data suggest that the heat treatment had two immediate consequences over NifH. First, NifM and non-functional apo-NifH precipitated out of the soluble extract. Second, while apo-NifH removal reduced the total NifH yield it increased the apparent specific activity because NifH preparations were more enriched in holo-NifH after the treatment. Assuming that NifH folding was not affected by light, the most plausible explanation for higher NifH activity observed in leaves harvested in darkness is [Fe4S4] cluster loading or stability. Lower internal O2 levels at night (Hitoshi and Hideo, 1985) is likely to increase NifH [Fe4S4] cluster stability. Moreover, NifH has shown functional during darkness for chlorophyll biosynthesis in the unicellular algae Chlamydomonas reinhardtii (Cheng et al., 2005). Activity of chloroplast targeted NifH has also been observed in leaves harvested at the end of the dark period (Eseverri et al., 2020).
If [Fe4S4] cluster occupancy was the limiting factor for NifH maturation it would be interesting to co-express NifU and NifS together with NifH and NifM in the transplastomic tobacco line. NifU and NifS are proteins involved in assembly of [Fe-S] clusters destined for Nif proteins. Although NifU and NifS were not required for NifH maturation in mitochondria of Saccharomyces cerevisiae (López-Torrejón et al., 2016), they were required to produce active yeast NifB (Burén et al., 2019). In a recent study from our laboratory, we also showed that tobacco plants transiently expressing high levels of chloroplast-targeted NifH and NifM required NifU and NifS (Eseverri et al., 2020). There are some important differences in the experimental approach between the two studies that might explain this contradiction. First, transient expression from the strong 35S promoter results in a sudden burst of protein production. Although the Prrn promoter used in the transplastomic plants also results in high protein expression, endogenous chloroplast iron-sulfur cluster biosynthesis could perhaps deal better with constitutive and stable rather than transient expression. Second, the transplastomic tobacco plants were grown in soil supplemented with FeSO4, which could alleviate the requirement for NifU and NifS. While it is difficult to directly compare NifH specific activities from the two studies, it would be interesting to determine the specific activity of heat-treated NifH when expressed together with NifU and NifS, as there is a good chance that the activities in the previous study (Eseverri et al., 2020) were underestimated because of presence of apo-NifH.
Only one transplastomic line was used in this study. Although results must be interpreted with care, a number of conclusions can be drawn. This study shows that functional nitrogenase NifH protein can be expressed in tobacco chloroplasts during the dark period, even if plants are grown at standard atmospheric O2 levels. In contrast to mitochondria-targeted K. oxytoca NifH, which is insoluble even when expressed together with NifM (Okada et al., 2020), the A. vinelandii NifH expressed with NifM inside chloroplasts was soluble overcoming a major obstacle for nitrogenase engineering in plants. Chloroplast metabolism in darkness appears to permit NifH protein maturation, which opens new avenues for controlling Nif protein expression by the circadian cycle or for using promoters for expression in root plastids. Although no positive effects on yield are expected from NifH expression alone (as nitrogenase is a two-component enzyme), we show that NifH and NifM expression did not induce any apparent pleiotropic effects demonstrating that accumulation of nitrogenase components in the chloroplast does not necessarily cause unwanted secondary effects.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
JAAM, XJ, and LMR designed experiments. XJ purified and reconstituted NifU, performed apo-NifH reconstitutions. JAAM performed all other experiments. JAAM, XJ, SB, and LMR analyzed data and wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
Funding for this research was provided by the Bill & Melinda Gates Foundation (BMGF) Grant OPP1143172 (to LMR). JAAM is supported by Juan de la Cierva fellowship (IJCI-2017-31937) from the Ministerio de Economía, Industria y Competitividad. XJ is supported by a doctoral fellowship from the Universidad Politécnica de Madrid.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Bayer Cropscience for sharing the transplastomic tobacco plant used in this study under the terms of BMGF Global Access policy. We thank Carlos Echavarri-Erasun for assistance in protein purifications and critically reading the article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2021.657227/full#supplementary-material
References
Adem, M., Beyene, D., and Feyissa, T. (2017). Recent achievements obtained by chloroplast transformation. Plant Methods 13, 30–30. doi: 10.1186/s13007-017-0179-1
Alexandratos, N., and Bruinsma, J. (2012). World agriculture towards 2030/2050: the 2012 revision. ESA Working Papers 12–03.
Allen, R. S., Gregg, C. M., Okada, S., Menon, A., Hussain, D., Gillespie, V., et al. (2020). Plant expression of NifD protein variants resistant to mitochondrial degradation. Proc. Natl. Acad. Sci. U.S.A. 117, 23165–23173. doi: 10.1073/pnas.2002365117
Bock, R. (2013). Strategies for metabolic pathway engineering with multiple transgenes. Plant Mol. Biol. 83, 21–31. doi: 10.1007/s11103-013-0045-0
Bulen, W., and LeComte, J. (1966). The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc. Natl. Acad. Sci. U.S.A. 56:979. doi: 10.1073/pnas.56.3.979
Burén, S., Jiménez-Vicente, E., Echavarri-Erasun, C., and Rubio, L. M. (2020). Biosynthesis of nitrogenase cofactors. Chem. Rev. 120, 4921–4968. doi: 10.1021/acs.chemrev.9b00489
Burén, S., Pratt, K., Jiang, X., Guo, Y., Jimenez-Vicente, E., Echavarri-Erasun, C., et al. (2019). Biosynthesis of the nitrogenase active-site cofactor precursor NifB-co in andlt;emandgt;Saccharomyces cerevisiaeandlt;/emandgt. Proc. Nat. Acad. Sci. 116:25078. doi: 10.1073/pnas.1904903116
Burén, S., and Rubio, L. M. (2018). State of the art in eukaryotic nitrogenase engineering. FEMS Microbiol. Lett. 365:fnx274. doi: 10.1093/femsle/fnx274
Burén, S., Young, E. M., Sweeny, E. A., Lopez-Torrejón, G., Veldhuizen, M., Voigt, C. A., et al. (2017). Formation of nitrogenase NifDK tetramers in the mitochondria of Saccharomyces cerevisiae. ACS Synth. Biol. 6, 1043–1055. doi: 10.1021/acssynbio.6b00371
Burgess, B. K., Jacobs, D. B., and Stiefel, E. I. (1980). Large-scale purification of high activity Azotobacter vinelandii nitrogenase. Biochim. Biophys. Acta (BBA) Enzymol 614, 196–209. doi: 10.1016/0005-2744(80)90180-1
Cheng, Q., Day, A., Dowson-Day, M., Shen, G.-F., and Dixon, R. (2005). The Klebsiella pneumoniae nitrogenase Fe protein gene (nifH) functionally substitutes for the chlL gene in Chlamydomonas reinhardtii. Biochem. Biophys. Res. Commun. 329, 966–975. doi: 10.1016/j.bbrc.2005.02.064
Curatti, L., Hernandez, J. A., Igarashi, R. Y., Soboh, B., Zhao, D., and Rubio, L. M. (2007). In vitro synthesis of the iron-molybdenum cofactor of nitrogenase from iron, sulfur, molybdenum, and homocitrate using purified proteins. Proc. Natl. Acad. Sci. U.S.A. 104, 17626–17631. doi: 10.1073/pnas.0703050104
Curatti, L., and Rubio, L. M. (2014). Challenges to develop nitrogen-fixing cereals by direct nif-gene transfer. Plant Sci. 225, 130–137. doi: 10.1016/j.plantsci.2014.06.003
Dodd, A. N., Belbin, F. E., Frank, A., and Webb, A. A. R. (2015). Interactions between circadian clocks and photosynthesis for the temporal and spatial coordination of metabolism. Front. Plant Sci. 6, 245–245. doi: 10.3389/fpls.2015.00245
Dos Santos, P. C., Smith, A. D., Frazzon, J., Cash, V. L., Johnson, M. K., and Dean, D. R. (2004). Iron–sulfur cluster assembly: NifU-directed activation of the nitrogenase Fe protein. J. Biol. Chem. 279, 19705–19711. doi: 10.1074/jbc.M400278200
Erisman, J. W., Galloway, J. N., Dise, N. B., Sutton, M. A., Bleeker, A., Grizzetti, B., et al. (2015). Nitrogen: Too Much of a Vital Resource: Science Brief. Zeist: WWF Netherlands.
Eseverri, Á., López-Torrejón, G., Jiang, X., Burén, S., Rubio, L. M., and Caro, E. (2020). Use of synthetic biology tools to optimize the production of active nitrogenase Fe protein in chloroplasts of tobacco leaf cells. Plant Biotechnol. J. 18, 1882–1896. doi: 10.1111/pbi.13347
Hernandez, J. A., Phillips, A. H., Erbil, W. K., Zhao, D., Demuez, M., Zeymer, C., et al. (2011). A Sterile α-Motif domain in NafY targets apo-NifDK for iron-molybdenum cofactor delivery via a tethered domain. J. Biol. Chem. 286, 6321–6328. doi: 10.1074/jbc.M110.168732
Herridge, R. P., Day, R. C., Baldwin, S., and Macknight, R. C. (2011). Rapid analysis of seed size in Arabidopsis for mutant and QTL discovery. Plant Methods 7:3. doi: 10.1186/1746-4811-7-3
Hitoshi, S., and Hideo, C. (1985). Photosynthesis measurement by oxygen electrode as a simple bioassay method. JARQ 18, 252–259.
Howard, K. S., McLean, P. A., Hansen, F. B., Lemley, P. V., Koblan, K. S., and Orme-Johnson, W. H. (1986). Klebsiella pneumoniae nifM gene product is required for stabilization and activation of nitrogenase iron protein in Escherichia coli. J. Biol. Chem. 261, 772–778. doi: 10.1016/S0021-9258(17)36161-6
Ivleva, N. B., Groat, J., Staub, J. M., and Stephens, M. (2016). Expression of active subunit of nitrogenase via integration into plant organelle genome. PLoS ONE 11:e0160951. doi: 10.1371/journal.pone.0160951
Jiang, X., Payá-Tormo, L., Coroian, D., García-Rubio, I., Castellanos-Rueda, R., Eseverri, Á., et al. (2021). Exploiting genetic diversity and gene synthesis to identify superior nitrogenase NifH protein variants to engineer N2-fixation in plants. Commun. Biol. 4:4. doi: 10.1038/s42003-020-01536-6
Kazama, T., Okuno, M., Watari, Y., Yanase, S., Koizuka, C., Tsuruta, Y., et al. (2019). Curing cytoplasmic male sterility via TALEN-mediated mitochondrial genome editing. Nat. Plants 5, 722–730. doi: 10.1038/s41477-019-0459-z
Long, S. P., Marshall-Colon, A., and Zhu, X. G. (2015). Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66. doi: 10.1016/j.cell.2015.03.019
López-Torrejón, G., Jiménez-Vicente, E., Buesa, J. M., Hernandez, J. A., Verma, H. K., and Rubio, L. M. (2016). Expression of a functional oxygen-labile nitrogenase component in the mitochondrial matrix of aerobically grown yeast. Nat. Commun. 7:11426. doi: 10.1038/ncomms11426
Mueller, N. D., Gerber, J. S., Johnston, M., Ray, D. K., Ramankutty, N., and Foley, J. A. (2012). Closing yield gaps through nutrient and water management. Nature 490, 254–257. doi: 10.1038/nature11420
Oey, M., Lohse, M., Kreikemeyer, B., and Bock, R. (2009). Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 57, 436–445. doi: 10.1111/j.1365-313X.2008.03702.x
Okada, S., Gregg, C. M., Allen, R. S., Menon, A., Hussain, D., Gillespie, V., et al. (2020). A synthetic biology workflow reveals variation in processing and solubility of nitrogenase proteins targeted to plant mitochondria, and differing tolerance of targeting sequences in a bacterial nitrogenase assay. Front. Plant Sci. 11:1388. doi: 10.3389/fpls.2020.552160
Oldroyd, G. E., and Dixon, R. (2014). Biotechnological solutions to the nitrogen problem. Curr. Opin. Biotechnol. 26, 19–24. doi: 10.1016/j.copbio.2013.08.006
Ort, D. R., and Oxborough, K. (1992). In situ regulation of chloroplast coupling factor activity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 269–291. doi: 10.1146/annurev.pp.43.060192.001413
Petersen, K., and Bock, R. (2011). High-level expression of a suite of thermostable cell wall-degrading enzymes from the chloroplast genome. Plant Mol. Biol. 76, 311–321. doi: 10.1007/s11103-011-9742-8
Poza-Carrión, C., Jiménez-Vicente, E., Navarro-Rodríguez, M., Echavarri-Erasun, C., and Rubio, L. M. (2014). Kinetics of andlt;emandgt;nifandlt;/emandgt; gene expression in a nitrogen-fixing bacterium. J. Bacteriol. 196:595. doi: 10.1128/JB.00942-13
Rangaraj, P., Shah, V. K., and Ludden, P. W. (1997). ApoNifH functions in iron-molybdenum cofactor synthesis and apodinitrogenase maturation. Proc. Natl. Acad. Sci. U.S.A. 94, 11250–11255. doi: 10.1073/pnas.94.21.11250
Roberts, G. P., MacNeil, T., MacNeil, D., and Brill, W. J. (1978). Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J. Bacteriol. 136, 267–279. doi: 10.1128/jb.136.1.267-279.1978
Ruf, S., Forner, J., Hasse, C., Kroop, X., Seeger, S., Schollbach, L., et al. (2019). High-efficiency generation of fertile transplastomic Arabidopsis plants. Nat. Plants 5, 282–289. doi: 10.1038/s41477-019-0359-2
Scotti, N., Alagna, F., Ferraiolo, E., Formisano, G., Sannino, L., Buonaguro, L., et al. (2009). High-level expression of the HIV-1 Pr55 gag polyprotein in transgenic tobacco chloroplasts. Planta 229, 1109–1122. doi: 10.1007/s00425-009-0898-2
Scotti, N., and Cardi, T. (2014). Transgene-induced pleiotropic effects in transplastomic plants. Biotechnol. Lett. 36, 229–239. doi: 10.1007/s10529-013-1356-6
Seefeldt, L. C., Yang, Z.-Y., Lukoyanov, D. A., Harris, D. F., Dean, D. R., Raugei, S., et al. (2020). Reduction of substrates by nitrogenases. Chem. Rev. 120, 5082–5106. doi: 10.1021/acs.chemrev.9b00556
Shah, V. K., and Brill, W. J. (1973). Nitrogenase IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim. Biophys. Acta Bioenerg. 305, 445–454. doi: 10.1016/0005-2728(73)90190-4
Temme, K., Zhao, D., and Voigt, C. A. (2012). Refactoring the nitrogen fixation gene cluster from Klebsiella oxytoca. Proc. Natl. Acad. Sci. U.S.A. 109, 7085–7090. doi: 10.1073/pnas.1120788109
Wu, A., Hammer, G. L., Doherty, A., von Caemmerer, S., and Farquhar, G. D. (2019). Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 5, 380–388. doi: 10.1038/s41477-019-0398-8
Yang, J., Xie, X., Xiang, N., Tian, Z.-X., Dixon, R., and Wang, Y.-P. (2018). Polyprotein strategy for stoichiometric assembly of nitrogen fixation components for synthetic biology. Proc. Nat. Acad. Sci. U.S.A. 115:E8509. doi: 10.1073/pnas.1804992115
Keywords: nifH, chloroplast, nitrogen fixation, crop improvement, transplastomic plants, fertilizer, biotechnology
Citation: Aznar-Moreno JA, Jiang X, Burén S and Rubio LM (2021) Analysis of Nitrogenase Fe Protein Activity in Transplastomic Tobacco. Front. Agron. 3:657227. doi: 10.3389/fagro.2021.657227
Received: 22 January 2021; Accepted: 28 June 2021;
Published: 21 July 2021.
Edited by:
Juan Imperial, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Zhenhua ZHANG, Hunan Agricultural University, ChinaRay Dixon, John Innes Centre, United Kingdom
Copyright © 2021 Aznar-Moreno, Jiang, Burén and Rubio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis M. Rubio, lm.rubio@upm.es
†ORCID: Jose A. Aznar-Moreno orcid.org/0000-0002-4474-8710
Xi Jiang orcid.org/0000-0002-1819-9041
Stefan Burén orcid.org/0000-0002-8487-2732
Luis M. Rubio orcid.org/0000-0003-1596-2475
 Jose A. Aznar-Moreno
Jose A. Aznar-Moreno Xi Jiang
Xi Jiang Stefan Burén
Stefan Burén Luis M. Rubio
Luis M. Rubio