1
Department of Neuroscience, Croatian Institute for Brain Research, School of Medicine, University of Zagreb, Zagreb, Croatia
2
Department of Anatomy, School of Medicine, University of Zagreb, Zagreb, Croatia
3
INSERM U751, Faculté de Médecine Timone, Marseille, France
Gamma-aminobutyric-acidergic (GABAergic) cells form a very heterogeneous population of neurons that play a crucial role in the coordination and integration of cortical functions. Their number and diversity increase through mammalian brain evolution. Does evolution use the same or different developmental rules to provide the increased population of cortical GABAergic neurons? In rodents, these neurons are not generated in the pallial proliferative zones as glutamatergic principal neurons. They are produced almost exclusively by the subpallial proliferative zones, the ganglionic eminence (GE) and migrate tangentially to reach their target cortical layers. The GE is organized in molecularly different subdomains that produce different subpopulations of cortical GABAergic neurons. In humans and non-human primates, in addition to the GE, cortical GABAergic neurons are also abundantly generated by the proliferative zones of the dorsal telencephalon. Neurogenesis in ventral and dorsal telencephalon occurs with distinct temporal profiles. These dorsal and ventral lineages give rise to different populations of GABAergic neurons. Early-generated GABAergic neurons originate from the GE and mostly migrate to the marginal zone and the subplate. Later-generated GABAergic neurons, originating from both proliferative sites, populate the cortical plate. Interestingly, the pool of GABAergic progenitors in dorsal telencephalon produces mainly calretinin neurons, a population known to be significantly increased and to display specific features in primates. We conclude that the development of cortical GABAergic neurons have exclusive features in primates that need to be considered in order to understand pathological mechanisms leading to some neurological and psychiatric diseases.
The cerebral cortex, including the hippocampal formation, is composed of two main classes of neurons: principal and non-principal cells. The first and the more numerous class (around 80% of the neurons) corresponds mainly to pyramidal cells, which are excitatory glutamatergic neurons (for reviews see DeFelipe and Jones, 1988
; Spruston, 2008
). The second class is formed for its vast majority by gamma-aminobutyric acid (GABA) neurons (Freund and Buzsaki, 1996
; Huang et al., 2007
; Ascoli et al., 2008
; Burkhalter, 2008
). GABA is the major inhibitory neurotransmitter in adult brain (Krnjevic and Schwartz, 1967
). These GABAergic non-principal neurons have been also referred as interneurons because most of them have an axon that remains restricted to the local structure. However, several types of such so-called interneurons have an axon that project to distant brain regions (Ribak et al., 1986
; Toth and Freund, 1992
; Jinno et al., 2007
; Tomioka and Rockland, 2007
; for review see Jinno, 2009
).
These GABAergic neurons form a very heterogenous population that plays a crucial role in regulating the activity of neuronal networks and complex interactions among principal cells (Somogyi and Klausberger, 2005
; Skaggs et al., 2007
). Therefore, the coordination and integration of cortical functions depends on the number and diversity of GABAergic neurons.
In fact, dysfunction or cell death of several specific types of GABAergic neurons has been described as a hallmark of various psychiatric and neurological disorders, such as schizophrenia (for reviews see Lewis et al., 2005
; Gonzalez-Burgos and Lewis, 2008
; Maldonado-Aviles et al., 2009
) and epilepsy (Houser, 1999
; DeFelipe, 2004
; Andrioli et al., 2007
) in which defects in neurogenesis or migration can be predisposing factors (Powell et al., 2003
; Poluch et al., 2008
; for reviews see Levitt et al., 2004
; Kostovic et al., 2007
; Leviton and Gressens, 2007
; Metin et al., 2008
). Understanding mechanisms that regulate the development of GABAergic neurons is a crucial pre-requisite step to assess pathological processes that can take place during development and lead to such neurological and psychiatric diseases.
Up to date most of our knowledge regarding GABAergic neuron development are based on studies performed in rodents. However, differences in cortical GABAergic circuitry exist between rodents and primates. Although many types of cortical interneurons appear to be common to all species, some types in primates, like the double bouquet cells (DeFelipe and Jones, 1988
; Yanez et al., 2005
; DeFelipe et al., 2006
; Melchitzky and Lewis, 2008
; Povysheva et al., 2008
; Cruz et al., 2009
; Jones, 2009
; Zaitsev et al., 2009
), or interneurons of cortical layer I (Rakic and Zecevic, 2003
; Kostovic et al., 2005
; Meyer, 2007
; Bystron et al., 2008
), display more elaborate features. These types may represent evolutionary specializations. In addition, several studies suggest an increased proportion of cortical GABAergic neurons between rodents and primates (Hendry et al., 1987
; Gabbott and Bacon, 1996
; DeFelipe, 2002
; Zaitsev et al., 2009
). Such increases in the number and diversity of GABAergic neurons have been suggested to be closely related to the tremendous increased brain complexity that occurs during mammalian evolution (Jones, 2009
; Pierani and Wassef, 2009
; Rakic, 2009
; Rakic et al., 2009
).
Whereas there is a consensus for all species examined that principal glutamatergic neurons originate in the local ventricular (VZ) and subventricular zones (SVZ) of the dorsal telencephalon (pallium) and migrate along radial glia to their target cortical layer (Rakic, 1971
; Noctor et al., 2001
; for reviews see Rakic, 2006
; Bystron et al., 2008
; Metin et al., 2008
; Rakic, 2009
; Rakic et al., 2009
), this seems not to be the case for GABAergic neurons (Molnar et al., 2006
; Wonders and Anderson, 2006
; Metin et al., 2008
; Petanjek et al., 2008
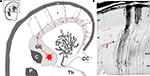
). In rodents, there are compelling evidences that cortical GABAergic neurons (Figure 1
) are not generated in these cortical (pallial) proliferative zones. They are produced in proliferative zones of the ventral (basal) telencephalon (subpallium), the ganglionic eminence (GE). From this region, newly born GABAergic neurons migrate tangentially into the cortex (for review see Wonders and Anderson, 2006
). Surprisingly, data obtained in the human (Letinic et al., 2002
; Rakic and Zecevic, 2003
; Fertuzinhos et al., 2009
) and monkey cortex (Petanjek et al., 2009
) point out significant evolutionary changes with respect to the origin of cortical GABAergic neurons. Therefore, the aim of this paper is to update the literature in this field (see Petanjek et al., 2008
), giving an overview of the main data about the origin and migration of cortical GABAergic neurons in rodents and primates.
Figure 1. Origin and migratory routes differ between the two major classes of cortical neurons. (A) Shematic drawing of migratory routes for cortical neurons in the dorsal telencephalon (pallium). Principal (pyramidal) neurons (excitatory glutamatergic neurons) originate locally in the proliferative ventricular (VZ) and subventricular (SVZ) zones of the dorsal telencephalon. Early-generated neurons display a bipolar morphology and migrate orthogonal to the surface of the brain along radially oriented glial fibres (radial migration). According to data obtained in rodents, hippocampal and cortical GABAergic neurons originate in the ventral proliferative zones, i.e. ganglionic eminence (GE) and migrate tangentially to their target cortical regions (red arrowed lines). The largest stream follows the lower intermediate zone (IZ)/upper subventricular zone (SVZ). In the marginal zone (MZ), the stream of migrating neurons forms a subpial granular layer. Numerous tangentially oriented neurons are present below the cortical plate (CP), in the subplate. Migratory routes are shown on schematized frontal section of human fetus at postconceptual week 15 (Th – thalamus; C – caudate nucleus; P – putamen; CC – corpus callosum). (B) Microphotography of a Golgi impregnated section through the whole thickness of dorsal telencephalon in a human fetus at postconceptual week 10. Bipolar migrating, prospective principal neurons are mostly observed in the VZ and SVZ. Numerous radial glia cells extend their processes from ventricle to pia. Numerous prospective pyramidal neurons at the beginning of differentiation are present in the cortical plate. Red arrows indicate major locations and direction of tangential migration. Modified by permission from Kostovic et al. (2007)
.
In Rodents GABAergic Neurons Originate Exclusively from the Ganglionic Eminence and Migrate Tangentially to the Cortex
Golgi-impregnation studies have first described tangentially migrating cells in the fetal telencephalon (Stensaas, 1968
). Increased attention started when it was established that these cells indeed correspond to GABAergic neurons (Van Eden et al., 1989
; DeDiego et al., 1994
). Although the stream of those cells was observed all along the intermediate zone (IZ) of the dorsal telencephalon, from the GE to the most medial part of dorsal hippocampus, it was difficult at this time to conceive that cortical GABAergic neurons could originate from outside the cortical proliferative zones (DeDiego et al., 1994
). Lineage experiments later established that clones of GABAergic neurons are tangentially dispersed, whereas radially arranged clones are formed primarily of glutamatergic neurons (Tan et al., 1998
; Noctor et al., 2001
; for reviews see Molnar et al., 2006
; Dehay and Kennedy, 2007
; Metin et al., 2008
; Petanjek et al., 2008
; Javaherian and Kriegstein, 2009
). Gorski et al. (2002)
further demonstrated that cortical glutamatergic neurons are produced in proliferative zones of the dorsal telencephalon (pallium), but not GABAergic cells. They suggested that GABAergic neurons originate from progenitors located outside the pallium. In vitro studies directly established that in rodents GABAergic neurons migrate tangentially from the ventral telencephalon (i.e. GE) into the cortex. These migrating neurons follow several routes to reach their target regions (Figure 1
). The major stream of tangentially migrating GABAergic neurons is present at the border of IZ and SVZ (lower part of IZ and upper part of SVZ). In addition, smaller streams of migrating cells are present in the subplate (SP) and the upper part of marginal zone (MZ) (Anderson et al., 1997
; Pleasure et al., 2000
; Heng et al., 2007
; Metin et al., 2007
; for reviews see Molnar et al., 2006
; Wonders and Anderson, 2006
; Metin et al., 2008
). Evidence that GABAergic neurons originate mainly from progenitors in the GE was provided by studies using several lines of knockout mice for genes expressed specifically in the ventral telencephalon. A marked reductions in the number of neocortical GABAergic neurons is observed at birth in all these lines including mutants for the transcription factors Dlx1 and Dlx2 (Anderson et al., 1997
; Pleasure et al., 2000
; Wonders and Anderson, 2005
; Long et al., 2009
), Nkx2.1 (Sussel et al., 1999
; Pleasure et al., 2000
; Butt et al., 2008
; Nobrega-Pereira et al., 2008
) and Mash1 (Casarosa et al., 1999
; Horton et al., 1999
; Guillemot, 2007
; Long et al., 2009
). Together these studies have clearly established that in rodents almost all cortical GABAergic neurons originate from the GE and migrate tangentially to their target cortical layers.
Interestingly, there are compelling evidences that different subdomains within the GE generate different populations of cortical GABAergic neurons. The GE displays two major divisions: the medial and lateral GE (Sidman and Rakic, 1973
; O’Rahilly and Müller, 2006
; Petanjek et al., 2008
). Recent findings demonstrated the existence of two additional components, the caudal and septal divisions (Xu et al., 2004
; Ghanem et al., 2007
; Long et al., 2009
; Petanjek et al., 2009
). In vitro studies using focal electroporation into small, defined regions of the GE, showed that neurons from the medial part migrate laterally and spread widely throughout the cortex, whereas most neurons from the caudal part migrate toward the most caudal end of the telencephalon (Ang et al., 2003
; Liodis et al., 2007
; Kanatani et al., 2008
; Zhao et al., 2008
; for reviews see Wonders and Anderson, 2006
; Metin et al., 2008
; Wonders et al., 2008
). The vast majority of cortical GABAergic neurons are provided by the medial GE and at a lower extent by the caudal GE. However, significant, although smaller numbers of cortical GABAergic neurons originate from the lateral GE, especially at later stages (Xu et al., 2004
; Wonders and Anderson, 2006
; Wonders et al., 2008
). Among the different subpopulations of GABAergic neurons, most of somatostatin-expressing neurons and all parvalbumin-containing cells derive from the medial GE whereas the majority of calretinin-containing cells and half of the NPY-expressing neurons arise from the caudal GE. Even within the medial GE, the different subpopulations of cortical GABAergic neurons have distinct spatial origins. Somatostatin cells are primarily generated within the dorsal part of the medial GE whereas parvalbumin neurons are provided by the ventral part of the medial GE (Wonders et al., 2008
). Therefore, the GE is organized in molecularly different subdomains that produce different subpopulations of cortical GABAergic neurons (Nery et al., 2002
; Bellion and Metin, 2005
; Bielle et al., 2005
; Butt et al., 2005
, 2007
; Cobos et al., 2006
; Wonders and Anderson, 2006
; Flames et al., 2007
; Fogarty et al., 2007
; Ghanem et al., 2007
; Guillemot, 2007
; Kohwi et al., 2007
; Liodis et al., 2007
; Molyneaux et al., 2007
; Batista-Brito et al., 2008
; Bayatti et al., 2008
; Du et al., 2008
; Garcia-Moreno et al., 2008
; Kanatani et al., 2008
; Zhao et al., 2008
; Carney et al., 2009
; Long et al., 2009
). In addition to spatial diversity in the origin of GABAergic neuron subpopulations, the generation of these different subgroups may occur at specific time windows (Butt et al., 2005
, 2008
; Ghanem et al., 2007
; Batista-Brito et al., 2008
; Cai et al., 2009
; Sousa et al., 2009
). However, the precise spatio-temporal pattern of production, for the different GABAergic subtypes, has yet to be established.
Several studies have provided evidence that mammalian evolution uses different developmental rules to afford for cortical GABAergic neurons to an expanding neocortex. In human and non-human primates, cortical GABAergic neurons are produced not only by the GE (as in rodents), but also massively by the proliferative zones of the dorsal telencephalon (Letinic et al., 2002
; Rakic and Zecevic, 2003
; Fertuzinhos et al., 2009
; Petanjek et al., 2009
).
Letinic et al. (2002)
were first to demonstrate the existence of two distinct lineages of cortical GABAergic neurons using retroviral labelling in organotypic slice cultures of the fetal human forebrain. One lineage originates from the GE as in rodents but give rise to only 35% of the cortical GABAergic neurons in human. The second that represents 65% of cortical GABAergic neurons originates from progenitors within the proliferative VZ and SVZ of the dorsal telencephalon. In this study, the authors suggested that the dorsal telencephalic origin of cortical GABAergic neurons might be human-specific (Letinic et al., 2002
), but we demonstrated more recently that this occurs also in the macaque monkey (Macaca rhesus and Macaca fascicularis) (Petanjek et al., 2009
).
Both in human and macaque, neurogenesis of GABAergic neurons within the ventral and dorsal telencephalon occurs with distinct temporal profiles. Whereas at early stages of primate fetal development, GE is an exclusive site of origin for cortical GABAergic neurons, later on the dorsal telencephalic proliferative layers are the major source for these neurons (Letinic et al., 2002
; Petanjek et al., 2009
). In the human fetal brain Mash1, a transcription factor known to be involved in the early specification of GABAergic neurons (Meredith and Johnson, 2000
; Letinic et al., 2002
; Britz et al., 2006
; Guillemot, 2007
; Long et al., 2009
), was expressed only by the progenitors in the GE from postconceptional week (PCW) 10 to PCW13 (Letinic et al., 2002
). In the macaque fetal brain (Figure 2
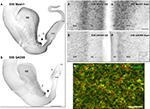
), progenitors labeled for the GABA synthesizing enzyme, glutamic acid decarboxylase 65 (GAD65) and Mash1 were present almost exclusively in the GE and the septal VZ/SVZ (septal eminence) from embryonic day (E) 47 to E55 (Petanjek et al., 2009
).
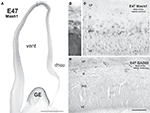
Figure 2. Neurogenesis of GABAergic neurons in the ventral telencephalon of a monkey fetus at embryonic day (E) 55. (A,B) Adjacent sections labeled for Mash1 (A) and GAD65 (B) from a E55 fetal brain at a rostral level in which the lateral ventricle is dilated (paleocortical ventricle indicated by a star). (A) Progenitors labeled for Mash1 were present mainly in the ganglionic eminence (GE) [box magnified in panel (C)] and septal (Sept) proliferative zone [box magnified in panel (D)]. (B) GAD65-containing cells were observed in the same regions of the ventral telencephalon as Mash1, the GE and the Sept [boxes enlarged in panels (E) and (F)]. (C–F) Figures correspond to magnified regions indicated by boxes in (A,B). (C) In the GE many cells were labeled for Mash1 in the ventricular (VZ) and the subventricular zone (SVZ). The intensity of labeling was much higher in the VZ than in the SVZ. (D) In the prospective septal region (Sept), numerous cells highly labeled for Mash1 were observed in the VZ whereas the cells in the SVZ displayed a moderate level of labeling. (E) Many cells labeled for GAD65 were observed in the GE. These cells were mainly located in the SVZ, and only a few cells were present in the VZ. (F) Many cells labeled for GAD65 were present in the SVZ of the Sept. Box: magnified region. (G) Confocal micrographs of an adjacent coronal section double-labeled for Mash1 (red, nuclear) and GAD65 (green, cytoplasmic) showing that in the SVZ of GE numerous Mash1 containing progenitors were labeled for GAD65. Scale bars: 1 mm (A,B), 50 μm (C–G), 10 μm [box in (F)]. Abbreviation: CC – corpus callosum. Modified by permission from Petanjek et al. (2009)
.
At these stages of fetal development, large contingent of GAD65-containing cells with morphological features of tangentially oriented migrating neurons were present in the upper SVZ and lower IZ as well as in the SP and MZ of the dorsal telencephalon (Figure 3
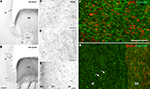
). Such migratory pathways of GABAergic neurons from the GE to the prospective cortical region were observed also in human from PCW10 to PCW13 (Letinic et al., 2002
). So, during the early fetal period both in monkey (E47–E55) and in human (PCW10 – PCW13), migratory cortical GABAergic neurons arise from the GE.
Figure 3. Tangential migration of GABAergic neurons in coronal sections of a monkey fetus at embryonic day (E) 55. (A) Mash1 containing progenitors were present in the ganglionic eminence (GE) but not in the neighboring structures including the caudate nucleus (caud), capsula interna (CI), and adjacent zones of the dorsal telencephalon: ventricular zone (VZ), subventricular zone (SVZ) and intermediate zone (IZ). Arrows indicated the border between GE and adjacent structures. (B) GAD65 containing progenitors were present almost exclusively in the GE. In addition a stream of post-mitotic-like, GAD65-containing neurons extending from the GE to dorsal telencephalon was observed in the lower IZ/upper SVZ. Arrows indicate the deeper border of this stream. Boxes: magnified regions in panels (C) and (D). (C) GAD65-containing cells with a characteristic morphology of post-mitotic migrating neurons, including a leading process (arrows) leaving the GE. (D) In the IZ and upper SVZ of dorsal telencephalon, the majority of GAD65 migrating neurons were tangentially oriented (arrows). (E) Tangentially oriented GAD65 migrating neurons are also observed in the subplate (SP) (arrows), cortical plate (CP) and marginal zone (MZ) of the dorsal telencephalon. (F,G) Confocal micrographs of an adjacent coronal section double-labeled for Ki67 (F) or Mash1 (G) (red, nuclear) and GAD65 (green, cytoplasmic). At E55, in the SVZ of the GE many GAD65-labeled cells were labeled for Ki67 (F) and therefore corresponded to proliferating cells. In contrast none of the migrating GAD65-containing cells in the IZ displayed detectable level of Ki67 or Mash1 (G) and therefore likely corresponded to early post-mitotic neurons. Scale bars: 350 μm (A,B), 50 μm (F,G), 20 μm, (C–E). Modified by permission from Petanjek et al. (2009)
.
Later on during fetal development (E64–E75 in monkey; PCW15–PCW20 in human), cortical GABAergic neurons are, in addition to the GE, massively generated by the proliferative regions of the dorsal telencephalon. In macaque, numerous Mash1-containing progenitors coexpressing GAD65 were present in the SVZ and VZ of the entire dorsal telencephalon (Figure 4
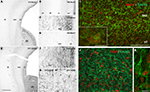
). These double-labeled cells displayed features of proliferative cells. They were clearly morphologically different from adjacent post-mitotic GAD65-containing migrating neurons. Many migrating GAD65-containing neurons displayed nontangential orientation. This is in contrast to earlier stage (E47–E55), when almost all migrating neurons were tangentially oriented (Figure 3
). Similar patterns of GABAergic neuron progenitors and migrating GABAergic neurons were described in human fetuses (Letinic et al., 2002
).
Figure 4. Neurogenesis of GABAergic neurons in the ventral and dorsal telencephalon of monkey fetuses at embryonic day (E) 68, E75 and E88. (A–D) Mash1 expression. (A) At E75, Mash1 containing progenitors were present in the ganglionic eminence (GE) as well as in ventricular (VZ) and subventricular (SVZ) zone of the entire dorsal telencephalon. In both areas, the intensity of labeling for Mash1 decreased from the VZ to the SVZ. (B) At E68, the same pattern of labeling for Mash1 is already present in the VZ and SVZ of the dorsal telencephalon. No labeling is observed in the upper part of intermediate zone (IZ), the subplate (SP) and the cortical plate (CP). (C) Higher magnification of panel (A). The numerous Mash1 highly labeled cells located in the VZ and SVZ of the dorsal telencephalon. (D) At E88, end of neurogenesis, the intensity of labeling for Mash1 was strongly decreased as compared to E68 (B) and E75 (A,C). (E–H) GAD65 expression. (E) At E75, GAD65-containing progenitors were present in the GE, but also in the proliferative VZ and SVZ of the dorsal telencephalon in contrast to earlier stages. The stream of migrating GAD65-containing neurons observed at E55 [compare with panel (B) in Figure 3
in the lower part of IZ and upper part of SVZ was still present (arrows). (F) At E68, similar labeling was already observed. In contrast to previous stages many migrating-like post-mitotic neurons with one leading process were now observed in the VZ and SVZ of the dorsal telencephalon. (G) At E75, in addition to migrating-like post-mitotic neurons, many round-shaped cells without any process were labeled for GAD65 (arrows). These cells displayed a similar morphology to that observed in the GE and septal region. (H) At E75, many migrating-like GAD65-neurons in the SVZ of the dorsal telencephalon displayed multidirectional orientations. (I–J) Confocal micrographs of an adjacent coronal section double-labeled for Mash1 (I) or Ki67 (J,K) (red, nuclear) and GAD65 (green, cytoplasmic). (I) At E75, in the dorsal telencephalon, most Mash1-containing cells of SVZ also contained GAD65, whereas many Mash1-labeled cells in the VZ did not. Insert: aggregates of cells double-labeled for Mash1 and GAD65 were frequently observed in the SVZ of the dorsal telencephalon, suggesting cells undergoing division. (J) At E75 in the SVZ of the dorsal telencephalon [compare with panel (G)] most GAD65-containing cells were labeled for Ki67 and therefore corresponded to progenitors of GABAergic neurons. (K) Migrating-like GAD65-containing cells in the IZ of the dorsal telencephalon were not labeled for Ki67. Scale bars: 550 μm (A,E), 250 μm (B), 60 μm (C,D) and 30 μm (F–H); 50 μm (I,J), 10 μm [insert (I,K)]. Modified by permission from Petanjek et al. (2009)
.
Furthermore, our results strongly support the view that the progenitors of GABAergic neurons in the cortical (dorsal) proliferative zones are generated locally, rather than arriving from the GE. Our main argument is that at E47–E55, no Mash1-containing cells were observed in the dorsal telencephalic wall close to the GE whereas onset of Mash1 expression was already observed in the VZ/SVZ of the most dorsal part of the telencephalon (Figures 5 and 6
B). This pattern of Mash1 expression in dorsal progenitors indicates that these progenitors did not migrate from the GE. However, at earlier stage (E47–E55), the Mash1-containing cells in the dorsal telencephalon did not contain GAD65 and thus may not have a GABAergic phenotype. We cannot exclude the possibility that these Mash1-containing progenitors generate other types of cells such as oligodendrocytes (Jakovcevski and Zecevic, 2005
; Guillemot, 2007
; Parras et al., 2007
) or reflect a regulation of the progenitor division mode not related with specification of GABAergic neurons (Britz et al., 2006
). However, at E64 only few Mash1-containing cells express PDGFR-α, a marker of oligodendrocyte progenitors, and the vast majority of Mash1-containing cells in the VZ/SVZ of the entire dorsal telencephalon expresses GAD65 (Petanjek et al., 2009
). Thus, the expression of Mash1 in the dorsal telencephalon at E47–E55 corresponds likely to symmetrically dividing prospective GABAergic precursors (Rakic, 2009
; Rakic et al., 2009
) and marks the onset of GABAergic neurogenesis in this region.
Figure 5. Mash1 and GAD65 labeling patterns in coronal sections of monkey fetal telencephalon at embryonic day (E) 47 suggest that neurogenesis of GABAergic neurons is induced locally. (A) At E47 Mash1 was highly expressed in the proliferative zone of the ventral telencephalon, the ganglionic eminence (GE). Mash1 immunoreactivity was also observed in the proliferative zones of the dorsal telencephalon. It followed a clear dorso-ventral decreasing gradient in the labeling intensity within the entire cortical wall. Moderate level of labeling was observed in the most dorsal part of the telencephalon [indicated by square and magnified in panel (C)]. The intensity of staining decreased in the lateral and medial wall. No Mash1 immunolabeling was observed close to the GE, nor in the most ventral part of the medial telencephalic wall, that corresponds at this level to dorsal hippocampus (dhipp). (B) Cresyl violet labeled section. Different cellular zones of the most dorsal part of the dorsal telencephalon are delineated. (C) Some cells moderately labeled for Mash1 were observed in the proliferative zones of the most dorsal part of the dorsal telencephalon. Most of these Mash1 labeled cells were present in the upper part of subventricular zone (SVZ), on the border with the intermediate zone (IZ). Some cells were also observed in the deepest part of ventricular zone (VZ). (D) In contrast to Mash1 no GAD65-containing cells were observed in the VZ and SVZ of the most dorsal part of the dorsal telencephalon at E47. However in this region, some GAD65-containing cells with a characteristic morphology of early post-mitotic migrating neurons, including a leading process, and tangentially oriented, were observed in the IZ and the cortical plate (CP) (arrows). Vent – lateral ventricle. Scale bars: 1 mm (A), 50 μm (B–D). Modified by permission from Petanjek et al. (2009)
.
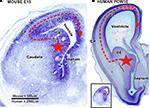
Figure 6. Sources of cortical GABAergic neuron in a mouse fetus and in primate fetuses at early developmental stage (embryonic day (E) 47 to E55 in macaque/postconceptional week (PCW) 10 to PCW13 in human). Nissl staining of frontal brain sections through telencephalic vesicle at the level of septum and paleocortical ventricle (asterix) in a mouse fetus at embryonic day (E) 15 [panel (A) and in a human fetus at postconceptional week 10 panel (B)]. (A) In the rodent, the vast majority of cortical and hippocampal GABAergic neurons are produced by ganglionic eminence (GE) (large star). The neurons migrate tangentially in the cortex (red dashed arrow). A very small percentage of the GABAergic neurons is supposed to be also produced in dorsal proliferative zones (small stars – full red arrows). (B) In the monkey and human brain, the GE is also an important source of cortical GABAergic neurons (large star). Post-mitotic neurons migrate tangentially into the cortex (red dashed arrow). Data from macaque monkey (Petanjek et al., 2009
) suggest that already at this ages (see below) a production of GABAergic neurons started in the proliferative zones of most dorsal part of the pallium (small stars – full red arrows). VZ – ventricular and subventricular zone; CP – cortical plate. Scale bar: 500 μm (A), 2500 μm (B). Small figure [left down in panel (B)] is a mouse section at E15 (A) reduced in size to be comparable with human fetus at PCW10 (B).
Therefore, in human and monkey, cortical GABAergic neurons are generated by two distinct sources: the GE and the proliferative zone of the dorsal telencephalon. In addition, the dorsal proliferative zone is not only an additional but also a major pool of GABAergic progenitors in primates (Figure 7
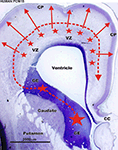
).
Figure 7. Sources of cortical GABAergic neuron in the the primate during middle fetal stage. Nissl staining of frontal brain sections through rostral part of the telencephalic vesicle in a human fetus at postconceptional week (PCW) 15 (at a level of the anterior horn). In human (PCW15 – PCW20) as in macaque monkey [embryonic day (E) 64 to E75] later during gestation, GABAergic neurons are produced massively by the proliferative zones of the dorsal telencephalon (smaller stars – full red arrows). According to Letinic et al. (2002)
these dorsal proliferatives zones generate 65% of the cortical GABAergic neurons. This massive dorsal production of GABAergic neurons is primate-specific. VZ – ventricular and subventricular zone; CP – cortical plate; CC – corpus callosum. Scale bar: 2500 μm.
The Dorsal and Ventral Sites of Neurogenesis Produce Different Populations of Cortical GABAergic Neurons
In the macaque, the dorsal and ventral lineage of GABAergic neurons displayed different cortical distributions. Early-generated GABAergic neurons from the GE populated mainly the MZ and the SP. Indeed, as early as E47, morphologically differentiated GAD-containing neurons were observed first in the MZ and the SP just below the cortical plate (CP). Their number increased in the SP with the development of this layer at a period when progenitors of GABAergic neurons are mainly observed in the ventral telencephalon (Petanjek et al., 2009
). A similar pattern of early-generated GABAergic neurons was described in human fetuses (Zecevic and Milosevic, 1997
; Meyer et al., 2000
; Rakic and Zecevic, 2003
; Meyer, 2007
). It was shown that SP neurons as well as early-generated neurons in the hippocampal MZ are functional and play a crucial role in early-generated network activity (Khazipov et al., 2001
; Hanganu et al., 2002
; Moore et al., 2009
). In human and non-human primates GABAergic neurons in the CP are generated in both GE and dorsal proliferative zones. In macaque, from E64–E75 onwards, GABAergic neurons penetrate into the CP following an inside-out gradient. Their number increases during a period when progenitors of GABAergic neurons are massively present in proliferative regions of the entire dorsal telencephalon. In human, two thirds of CP interneurons are generated in the dorsal telencephalon (Letinic et al., 2002
).
A recent study from Fertuzinhos et al. (2009)
investigates the proportion of different subpopulations of GABAergic neurons in brains of human fetuses or infants affected by holoprosencephaly. This syndrome is characterized by severe striatal hypoplasia and atrophy of the GE (Judas et al., 2003
). Interestingly, in holoprosencephaly, the numbers of cortical GABAergic neurons expressing nitric oxide synthase, neuropeptide Y or somatostatin were significantly reduced in comparison to healthy infants. In contrast, calretinin-containing neurons were present in normal numbers as well as principal neurons. These findings show that, in human, nitric oxide synthase-, neuropeptide Y- and somatostatin-containing neurons originate from the GE whereas calretinin neurons are generated by the dorsal telencephalon.
It is interesting to note that GABAergic neurons identified as missing or significantly reduced (Fertuzinhos et al., 2009
) are those normally found in greatest numbers in derivatives of the MZ and SP (Kostovic and Rakic, 1990
; Delalle et al., 1997
; Rakic and Zecevic, 2003
; Meyer, 2007
; Kostovic et al., 2008
; Petanjek et al., 2009
). In addition, the population of calretinin neurons, generated mainly in the dorsal telencephalon in human, corresponds to the population of GABAergic neurons which proportion increases dramatically in primates (Hendry et al., 1987
; Gabbott and Bacon, 1996
; Zaitsev et al., 2009
) and displays primate-specific subtypes such as double bouquet cells (DeFelipe and Jones, 1988
; DeFelipe, 2002
; Yanez et al., 2005
; DeFelipe et al., 2006
; Jones, 2009
).
Altogether, these studies favor the hypothesis that dorsal production occurs principally as an answer to an increased evolutionary need for specific classes of cortical GABAergic neurons.
Whereas in primates a large majority of cortical GABAergic neurons are produced in the proliferative regions of the dorsal telencephalon (Figure 7
), in rodents cortical GABAergic neurons are generated almost exclusively in the GE (Figure 6
A). A puzzling question is whether this dorsal telencephalic origin of cortical GABAergic neurons is primate-specific or reflects an enhancement of preexisting developmental mechanisms.
We have to stress that mammalian species examined until now included only mouse, rat, ferret, monkey and human. However, in favor of the later hypothesis, a production of GABAergic neurons in dorsal proliferative zones has been reported in mouse (Figure 6
A), but it accounts for a very small fraction of neurons present at maturity (Xu et al., 2004
; Molnar et al., 2006
; Wonders and Anderson, 2006
). In this species, the progenitor cells of the dorsal telencephalon are able to generate GABA-expressing neurons in vitro (He et al., 2001
). However, in the ferret, that has a convoluted neocortex and displays a 2–3 times longer neurogenesis compared to mouse and rat, very few (less than 5%) cortical GABAergic neurons originated from proliferative zones of the dorsal telencephalon (Anderson et al., 2002
). This is about the proportion obtained in rodents (Xu et al., 2004
; Molnar et al., 2006
; Wonders and Anderson, 2006
). However, the massive neurogenesis of cortical GABAergic neurons from dorsal telencephalon is most likely primate-specific.
During tangential migration, prospective GABAergic neurons move parallel to the surface of the brain. They pass by a complex route between numerous growing axon bundles and cross regional boundaries. The length of their trajectory is greater than that of radially migrating neurons (Figure 1
). Therefore, the mechanisms of cellular interactions enabling proper positioning and specification of GABAergic neurons are more complex than for radially migrating neurons (Metin et al., 2006
, 2008
). One can suggests that dorsal production of cortical GABAergic neurons in primates might occur in order to facilitate migration routes through an expanding neocortex (Figures 6 and 7
). An exclusive ventral telencephalic origin of cortical GABAergic neurons in primates would imply extremely long and complex migratory routes for such neurons (Figure 6
B; compare the real size between embryonic mouse and human fetus). In keeping with this hypothesis, it might be expected that the percentage of GABAergic neurons produced dorsally will increase in larger brains in order to keep migratory routes shorter and simpler. However, the data obtained in ferret (Anderson et al., 2002
), which displays a convoluted neocortex significantly larger compared to rodents but shows limited cortical GABAergic neurons generated by the dorsal telencephalon such as described in mouse, do not support this hypothesis. Extensive dorsal production of GABAergic neurons in primates can be related to an increased need in number and/or specific types for GABAergic neurons in brains with more complex cortical circuitries (Fertuzinhos et al., 2009
; Jones, 2009
).
The massive neurogenesis of cortical GABAergic neurons from dorsal telencephalon suggests distinct properties of dorsal telencephalic progenitors in primates compared to rodents. Interestingly, it was demonstrated that the proliferative behavior of cortical neuronal precursors during neurogenesis differs between rodents and primates. This leads to significant differences in morphology and function of their dorsal proliferative zones (Smart et al., 2002
; Kriegstein et al., 2006
; Dehay and Kennedy, 2007
; Noctor et al., 2007
, 2008
; Bystron et al., 2008
; Fish et al., 2008
; Javaherian and Kriegstein, 2009
; Tabata et al., 2009
). In the human embryo, in comparison to the rodent embryo, there is a dramatic increase in surface area and thickness of the VZ at the earliest stage of proliferation (Carney et al., 2007
). In comparison to other species, the primate SVZ is larger and more complex. It displays a different type of cellular organization (Smart et al., 2002
; Zecevic et al., 2005
; Kriegstein et al., 2006
). There is a new, outer compartment within the SVZ, which displays a number of unique features, and exists much longer during development (Kostovic and Vasung 2009
; Molnar et al., 2006
; Dehay and Kennedy, 2007
; Bystron et al., 2008
; Jovanov-Milosevic et al., 2009
). So, it is not unreasonable to suggest that the increase in complexity, or even the specific cellular and laminar organization of dorsal proliferative zones in primates are significantly connected to the massive production of GABAergic neurons. However, no data to prove this hypothesis are currently available.
There has been an impressive amount of research over past years in the field of GABAergic neuron development. The vast majority of this work has been done in rodents, birds and reptiles. These studies give us insight into molecular and cellular mechanisms of GABAergic neuron specification, proper positioning and differentiation. In higher mammals, especially primates, the amount of research in this field is in general disproportionally low. Primate data have pointed out significant changes in the origin (Figures 6
B and 7
), specification and migration of cortical GABAergic neurons compared to lower mammals (Figure 6
A). They strongly support the view that our understanding of GABAergic neuron development in the human brain can not be assessed only on studies performed in rodents.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors wish to thank Prof. Miloš Judaš, Prof. Nataša Jovanov Milošević and Dr. Pascale Quilichini for their comments and technical assistance in preparing the manuscript. This work is supported by MSES 108-1081870-1932 (Zdravko Petanjek), UKF1B 06/07 (Ivica Kostović) and INSERM (Monique Esclapez) grants.
Ascoli, G. A., Alonso-Nanclares, L., Anderson, S. A., Barrionuevo, G., Benavides-Piccione, R., Burkhalter, A., Buzsaki, G., Cauli, B., Defelipe, J., Fairen, A., Feldmeyer, D., Fishell, G., Fregnac, Y., Freund, T. F., Gardner, D., Gardner, E. P., Goldberg, J. H., Helmstaedter, M., Hestrin, S., Karube,F., Kisvarday, Z. F., Lambolez, B., Lewis, D. A., Marin, O., Markram, H., Munoz, A., Packer, A., Petersen, C. C., Rockland, K. S., Rossier, J., Rudy, B., Somogyi, P., Staiger, J. F., Tamas, G., Thomson, A. M., Toledo-Rodriguez, M., Wang, Y., West, D. C., and Yuste, R. (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568.
Bayatti, N., Moss, J. A., Sun, L., Ambrose, P., Ward, J. F., Lindsay, S., and Clowry, G. J. (2008). A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb. Cortex 18, 1536–1548.
Cai, Y., Xiong, K., Chu, Y., Luo, D. W., Luo, X. G., Yuan, X. Y., Struble, R. G., Clough, R. W., Spencer, D. D., Williamson, A., Kordower, J. H., Patrylo, P. R., and Yan, X. X. (2009). Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp. Neurol. 216, 342–356.