- 1Department of Psychology, The University of British Columbia, Vancouver, BC, Canada
- 2Department of Psychology, Ohio State University, Columbus, OH, United States
- 3Department of Psychology, University of California, Berkeley, Berkeley, CA, United States
- 4Department of Psychology, University of Toronto Scarborough, Toronto, ON, Canada
Our study was designed to examine whether the pain reliever acetaminophen impacts the normal ebb-and-flow of off-task attentional states, such as captured by the phenomenon of mind wandering. In a placebo-controlled between-groups design, participants performed a sustained attention to response task while event-related potentials (ERPs) to target events were recorded. Participants were queried at random intervals for their attentional reports – either “on-task” or “off-task.” The frequency of these reports and the ERPs generated by the preceding target events were assessed. Behaviorally, the frequency of off-task attentional reports was comparable between groups. Electrophysiologically, two findings emerged: first, the amplitude of the P300 ERP component elicited by target events was significantly attenuated during off-task vs. on-task attentional states in both the acetaminophen and placebo groups. Second, the amplitude of the LPP ERP component elicited by target events showed a significant decrease during off-task attentional states that was specific to the acetaminophen group. Taken together, our findings support the conclusion that acetaminophen doesn’t impact our relative propensity to drift into off-task attentional states, but it does affect the depth of neurocognitive disengagement during off-task attentional states, and in particular, at the level of post-categorization stimulus evaluations indexed by the LPP.
Introduction
Acetaminophen—also known as paracetamol or Tylenol®—is a common non-prescription, centrally-acting pain reliever that has been recognized in recent years to have a number of substantive cognitive and affective side-effects. In the original study on the topic it was found that social pain was attenuated by chronic doses of acetaminophen taken over a period of 3 weeks (DeWall et al., 2010). Since then, the side effects of acetaminophen have been expanded on in a number of different ways, all demonstrating that it attenuates various aspects of cognitive-affective processes — including reduced emotional reactions to affectively polarized images (Durso et al., 2015); reduced social anxiety (Fung and Alden, 2017); reduced affective reactions to negative events (DeWall et al., 2015); reduced empathy (Mischkowski et al., 2016); reduced distrust of others (Roberts et al., 2018); reduced affective responses to cognitively threatening events (Randles et al., 2013); and reduced implicit analysis of behavioral errors (Randles et al., 2016).
Why might acetaminophen induce such effects? The available evidence from functional neuroimaging has demonstrated that acetaminophen modulates responsivity in the dorsal anterior cingulate cortex (dACC) during tasks invoking both social (DeWall et al., 2010) and physical pain (Pickering et al., 2015). Given that dACC function has been tied to behavioral performance monitoring (Holroyd et al., 2004; Brown and Braver, 2005; Sheth et al., 2012) and the evaluative analysis of behaviorally-relevant events (Bush et al., 2002; Blair et al., 2006; Cai and Padoa-Schioppa, 2012; Kolling et al., 2012), acetaminophen’s attenuating effects on reactivity to behavioral errors, poor decision outcomes, emotionally-charged stimuli, social rejection and the like (Randles et al., 2013, 2016; DeWall et al., 2015, 2010; Mischkowski et al., 2016) all functionally align with this possibility.
At the same time, the dACC has also been implicated in modulating the depth of our attentional engagement with the external task environment (Heilbronner and Hayden, 2016), and this is the point of focus in our study. In particular, the dACC has been identified as one of two cortical hubs in the brain’s “salience network” (Sridharan et al., 2008; Menon and Uddin, 2010), a network which is believed to control the depth of our attention to the external task environment through its modulatory influence on the brain’s default mode network (or DMN). Specifically, when our attention transiently drifts away from the given task at hand such as during periods of mind wandering, it correlates with a systematic up-regulation of activity in the DMN (Mason et al., 2007; Christoff et al., 2009; Andrews-Hanna et al., 2010; Andrews-Hanna, 2012). This has supported the idea that DMN activation is associated with spontaneous, task-independent thought (Christoff et al., 2016; Dixon et al., 2017). Against this background, the salience network has been shown to transiently suppress activity in the DMN, leading to the proposal that this modulatory influence maintains “on-task” attentional states because the neural circuitry associated with “off-task” attentional states — the DMN — is being directly inhibited (Bonnelle et al., 2012; Jilka et al., 2014; Menon, 2015).
Given the collective evidence, our study thus addressed the following question: might acetaminophen impact the normal ebb and flow of on-task vs. off-task attentional states? We suggest that the question warrants consideration for two central reasons.
First, when our thoughts drift or wander away from the on-going task at hand, there is a transient but widespread attenuation in the cortical processing of external events during the off-task attentional state that extends across the sensory-perceptual (Kam et al., 2011; Smallwood et al., 2011; Baird et al., 2014), cognitive (Smallwood et al., 2008; Barron et al., 2011; Kam et al., 2013), affective (Kam et al., 2014), and motor/performance-monitoring (Kam et al., 2012) domains. Such effects are believed to reflect a basic functional tension that persists in the brain between prioritizing external vs. internal inputs for processing by capacity-limited resources over time, as we naturally vacillate between on-task and off-task attentional states (Smallwood et al., 2011; Kam and Handy, 2013; Handy and Kam, 2015). In light of this, if acetaminophen does in fact alter the normal ebb and flow between on-task vs. off-task attentional states — such as by increasing the relative proportion of time spent in off-task attentional states, and/or by increasing the depth of our processing attenuation during off-task attentional states — it raises potential health and safety concerns. For example, falling in older individuals, a leading cause of death and disability in seniors, is associated with higher rates of mind wandering (Nagamatsu et al., 2013).
Second, taken together, the neurocognitive and neuroaffective side effects of acetaminophen as reviewed above have supported the hypothesis that the drug influences the brain’s social-affective evaluative processes (DeWall et al., 2015). While our intention here is not to inform on the validity of this hypothesis, if acetaminophen is found to alter normative patterns of on- vs. off-task attentional states, it would represent an important expansion in our basic understanding of acetaminophen and the breadth of its neurocognitive effects. That is, to our knowledge there has been no evidence reported to date indicating that acetaminophen alters attentional processes such as those associated with visual target identification, for example, processes impacted by off-task attentional states (Smallwood et al., 2008; Barron et al., 2011; Kam et al., 2013) that precede more evaluative stages of analyses in the afferent stream of visual stimulus processing.
To address our primary question, we combined a canonical event-related potential (ERP) paradigm we have used previously for assessing on- vs. off-task attentional states (Smallwood et al., 2008; Kam et al., 2011) with a between-group, double-blind, placebo-controlled procedure for administering acetaminophen that we have also used previously, both with (Randles et al., 2016) and without (Randles et al., 2013) ERPs as a dependent measure. Participants were asked to perform a simple visual target detection task while we recorded their ERP responses to target events. At random intervals they were then stopped and prompted to report on their attentional state just prior to the stoppage—either “on-task” vs. “off-task.” Participants in the “acetaminophen” condition consumed a 1000 mg capsule of acetaminophen approximately 1 h prior to the initiation of data collection, while participants in the “placebo” condition consumed an identical capsule containing granulated sugar. Our primary dependent measures were (1) the relative frequencies of on- vs. off-task attentional reports, to assess acetaminophen’s impacts on the relative proportion of time spent in off-task attentional states, and (2) the ERPs elicited by target events, to assess acetaminophen’s possible impacts on the depth of our attentional and evaluative processing attenuation during off-task attentional states, as indexed by the P300 and LPP ERP components respectively.
Materials and Methods
Participants
Participants were recruited through the community via posting at the Paid Participants Study List hosted by Psychology Graduate Student Council website, and remunerated $20 (CAD) for their participation. A total of 60 participants from the community were recruited, but data from 20 participants was excluded from final analyses due to excessive eye movement and other recording artifacts identified in their data during initial data analysis (see below). Of the remaining forty participants (26 females; M = 23.4 years old, SD = 6.61; 39 were right-handed), all had no history of neurological problems, and had normal or corrected-to-normal vision. In the placebo group, participants had a mean age of 23.9 (SD = 6.77; 15 females), and in the acetaminophen group, the mean age was 22.9 (SD = 6.57; 11 females). Participants were asked to not participate if they had a history of alcoholism or consume on average more than two alcoholic drinks a day, were a smoker, were pregnant or breastfeeding, were asthmatic or diabetic, had been diagnosed with liver, stomach, gastrointestinal, heart, or kidney disease or an allergy or family history of allergy to Tylenol (acetaminophen), had experienced intracranial bleeding or had a tendency to bleed excessively, or were at the time of participation taking any medication other than oral contraceptives. We did not assess the participants’ daily pain medication use. However, when participants’ alcohol, cigarette, and cannabis use over the past 24 h was assessed, we found no significant difference between the two experimental groups (p ≥ 0.15). Participants provided written informed consent to the experimental procedure, and the UBC Behavioral Review Ethics Board approved all procedures and protocols of this experiment.
Stimuli and Task
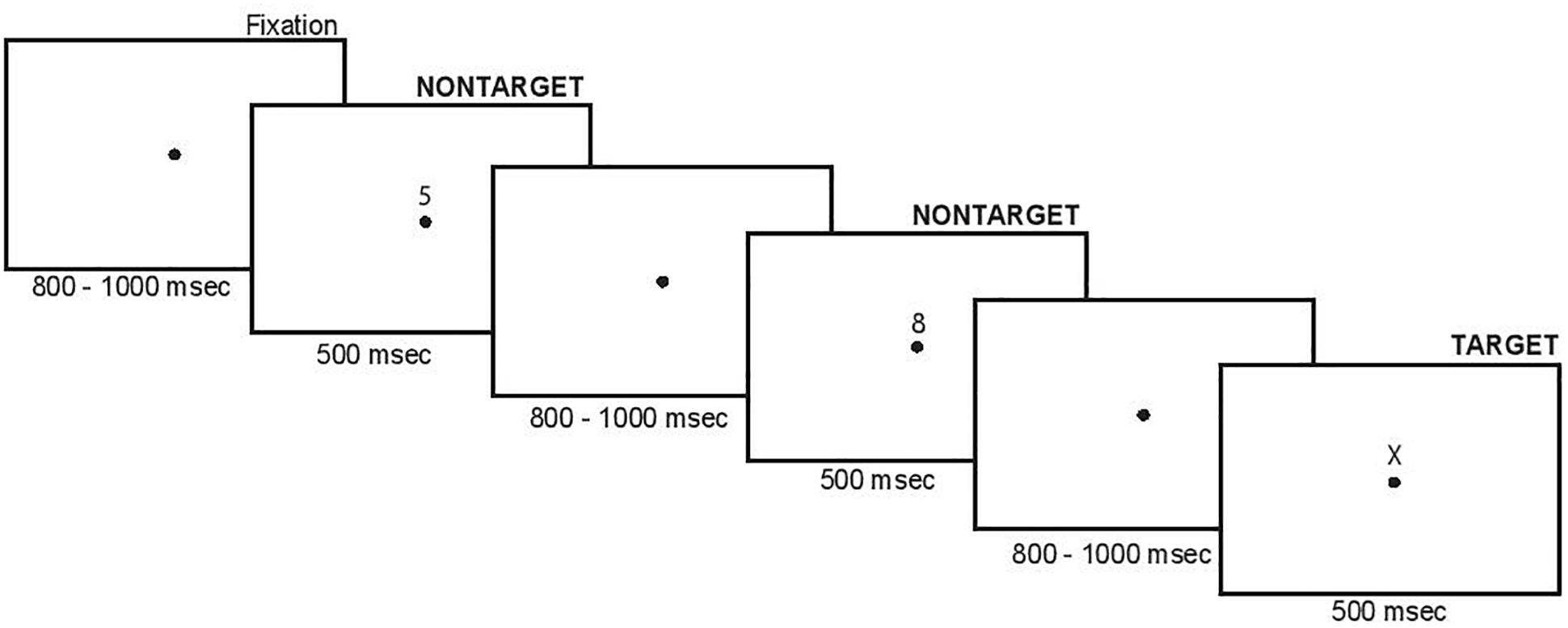
Participants performed a SART adapted from Smallwood et al. (2008) and Kam et al. (2011). The task involved presentation of a serial stream of stimuli at foveal centered fixation dot. The stimuli were black numbers or a letter on a white background. Participants were asked to make a manual button press for “non-targets” (numbers 0–9, which were presented frequently), and were asked to withhold a button press response when presented with a “target” (letter X, which was presented infrequently). The timing and sequence of stimuli are shown in Figure 1. Within each block of stimuli, target probability was quasi-randomized, with the constraints that (1) one to two targets were presented during each block, and (2) for blocks having two targets, the targets would be separated by at least 10 non-target events, and (3) the last six trials of each block included no target events. The block duration was randomly varied between 30 and 90 s, meaning each block had anywhere from 15 to 45 trials, and each participant completed a minimum of 20 blocks in total.
To measure task-related attention, participants were instructed to report their “attentional state” at the end of each trial block. Specifically, they were asked to identify their state immediately prior to the block termination as either being “on-task” (fully attentive to task performance), or “off-task” (inattentive to the task). Importantly, participants were provided with verbal descriptions and examples of these two “attentional states” prior to starting the testing session. On-task states were defined as when one’s attention is firmly directed toward the task, whereas off-task states were described as when one is aware of other things than just the task at hand. Examples of these attentional states were given in the context of reading, during which “one may be fully attentive to the content of the reading material, or thinking about something completely unrelated to the content, reflective of on-task and off-task states, respectively.” Attentional reports were recorded by the investigator at the conclusion of each trial block, and these reports were then used to sort ERP data based on on-task versus off-task states as described below. The block duration itself was randomly varied between 30 and 90 s to (1) minimize predictability of block completion and (2) maximize variability of attentional state at the time of block completion.
Experimental Manipulation
Acetaminophen is a widely used non-opiate, analgesic, and antipyretic drug, that is believed to impact the brain in complex ways. Comparably to non-steroidal anti-inflammatory drugs (NSAID), acetaminophen suppresses prostaglandin synthesis by the inhibitory effect on COX-1 and COX-2 activity (Ouellet and Percival, 2001; Graham and Scott, 2005). However, unlike NSAIDs, acetaminophen produces weak anti-inflammatory effects due to its lack of significant influence on the peripheral COX enzymes (Clissold, 1986). Acetaminophen when studied in combination with 5-HT3 receptors demonstrates stimulation of the descending serotoninergic pathway (Pickering et al., 2008). Moreover, acetaminophen is regarded as a prodrug that due to its active metabolization results in a fatty acid amid (N-arachidonoyl phenol amine; AM404) that indirectly increases activity in the endocannabinoid system (Högestätt et al., 2005). Thus, the pain sensation of the tissue receptors through the spinal cord to the thalamus and the cerebral cortex are impacted by acetaminophen (Jóźwiak-Bebenista and Nowak, 2014). Our procedures for the between-group pharmacological manipulation replicated Randles et al. (2016) such that participants in the experimental condition consumed two capsules each containing 500 mg tablets of Kirkland-brand acetaminophen (1000 mg total), while participants in the placebo condition consumed two identical-looking capsules that were filled with white granulated sugar. Participants were informed that they were being randomly assigned to an experimental condition through double-blind procedure, where each participant’s dose was assigned a unique ID prior to the study that matched it to the correct condition. While the researcher running the study was blind to condition assignment, they could request access to identify the condition if it became medically necessary. Participants were sorted into their assigned condition beginning at the group averaging stage of data analysis. The testing took place between 10 am and 7 pm, and there was no significant difference when comparing the two experimental groups for the time the testing took place [t(38) = 0.82, p = 0.42].
Procedure
Participants provided written consent and then consumed their assigned capsules. The participant was then prepared for recording his or her electroencephalogram (EEG), and instructed in the SART while seated at the task computer running MATLAB R2010a (The MathWorks, Inc.) with Psychtoolbox. This task began approximately 60 min after consuming the pills, ensuring that most participants experienced peak pharmacological activation; typically requiring 45–60 min for adults consuming acetaminophen orally (Bertolini et al., 2006). The half-life of acetaminophen in healthy subjects has been reported to be 1.9 to 2.5 h (Forrest et al., 1982). Once commenced, the EEG testing session itself took approximately 1 h, giving participants a total study time of approximately 2 h.
Electrophysiological Recording and Analysis
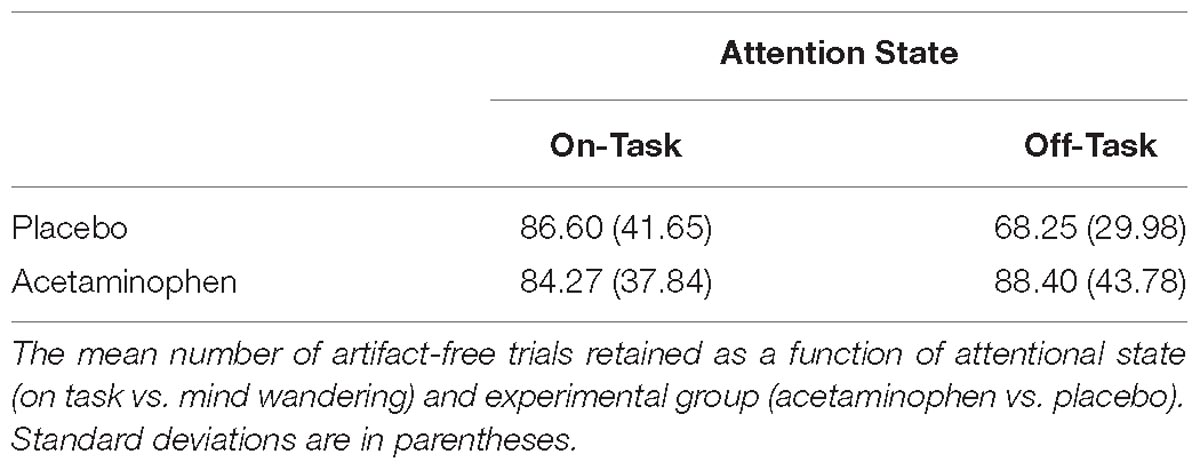
Continuous EEG was recorded during the task via 64 Ag/AgCl active electrodes mounted in an elastic cap (BioSemi Active-Two amplifier system; BioSemi, Amsterdam, Netherlands) in spatial accordance with the international 10–20 system. Two additional electrodes located over the medial-parietal cortex (Common Mode Sense and Driven Right Leg) were used as ground electrodes. Recordings were digitized at 256 Hz, digitally filtered offline between 0.1 and 30 Hz (zero phase-shift Butterworth filter) and then referenced offline to the average of two mastoid electrodes. EEG data processing was performed using ERPLAB, a toolbox within MATLAB 2012a (The MathWorks, Inc.) used in conjunction with EEGLAB. To ensure proper eye fixation and allow for the removal of events associated with eye movement artifacts, vertical and horizontal electrooculograms (EOGs) were also recorded—the vertical EOGs from an electrode inferior to the right eye, and the horizontal EOGs from two electrodes on the right and left outer canthus. Offline, computerized artifact rejection was used to eliminate trials during which detectable eye movements and blinks occurred. These eye artifacts were detected by identifying the minimum and maximum voltage values on all recorded EOG channels from −200 to 800 ms post-stimulus for each event epoch, and then removing the trial from subsequent signal averaging if that value exceeded 200 —V, a value calibrated to capture all blinks and saccades. This was followed by visual inspection of the data. If additional artifacts (e.g., muscle movements and loose connections) were observed, the threshold was reduced by 25 —V until artifacts were not present or a minimum of 100 —V was reached. Participants (N = 20) with more than 50% rejected trials were excluded from analysis; the remainder of participants (N = 40) had an average of 17.84% trials rejected due to these signal artifacts; the percentage of rejected trials did not significantly differ between individuals in the acetaminophen vs. placebo conditions (p = 0.53), and the mean number of artifact-free trials retained in each experimental group and attentional state are reported in Table 1. Of the removed participants, 10 were in the acetaminophen experimental group.
The ERP waveforms for the two attentional conditions of interest were derived by averaging together the EEG epochs for the six non-target events preceding each categorical instance of a subjective attentional report (on-task vs. off-task). Although it is never certain how long participants have actually been in a particular attentional state at the time a subjective report is given, analyses were based on the assumption that the 12 s prior to each report (the time window capturing the six preceding non-targets) would, on average, reliably capture the given attentional state—an assumption consistent with the presumed time course of off-task thinking (e.g., Sonuga-Barke and Castellanos, 2007; Christoff et al., 2009) and the time windows of analyses we have adopted previously (Smallwood et al., 2008; Kam et al., 2011, 2012; Kirschner et al., 2012; Kam and Handy, 2013). Although a shorter pre-report time window for averaging non-target EEG epochs would more accurately capture attentional state, it would also reduce the number of events included in the ERP analysis. The choice of how many pre-report events to include in the averages was therefore an attempt to maximize the number of events in each waveform average while not extending the window back so far in time as to consistently capture the preceding attentional state or transition between states.
Results
As outlined above, the goal of our study was to examine whether acetaminophen disrupts the normal ebb and flow of on-task vs. off-task attentional states. Our primary dependent measures were (1) the relative frequencies of on- vs. off-task attentional reports, to assess acetaminophen’s impacts on the relative proportion of time spent in on- vs. off-task attentional states, and (2) the ERPs elicited by target events, to assess acetaminophen’s impacts on the depth of our attentional and evaluative processing attenuation during off-task attentional states, as indexed by the mean amplitudes of the P3 and LPP ERP components elicited by target events, respectively.
Attentional Reports
Overall, participants completed an average of 36.2 trial blocks during the 1-h testing session, with 49.4% of the attentional reports being given as “off-task.” However, there was no significant between-group difference in the reported frequency of “off-task” attentional states [acetaminophen = 51.20%, placebo = 47.85%; t(38) = 0.59, p = 0.56].
Event-Related Potentials
Analyses of the ERPs elicited by the non-target events in the 12 s immediately preceding each attentional report focused on two components of interest: the P300, which increases in amplitude with the degree of attention allocated to the ERP-eliciting stimulus (Donchin, 1981; Polich, 2007), and the late positive potential (or LPP) that follows the P3 in time, which increases in amplitude with the degree of post-attentional stimulus evaluation (e.g., Cacioppo and Berntson, 1994; Crites et al., 1995; Cacioppo et al., 1996; Cuthbert et al., 2000; Ito and Cacioppo, 2000) even for implicit evaluative analyses (Handy et al., 2010). The P300 was chosen a priori for analysis because previous research has demonstrated that the amplitude of the P300 elicited by task-relevant events systematically decreases during off-task attentional states (Smallwood et al., 2008; Barron et al., 2011); at issue in the current study was whether acetaminophen might increase the depth of attentional disengagement during off-task attentional states, as indexed by the P300. Likewise, the LPP was chosen a priori for analysis because acetaminophen has been associated with reductions in the evaluative analysis of external events (DeWall et al., 2010, 2015; Randles et al., 2013, 2016; Mischkowski et al., 2016); at issue in the current study was whether there might be an increase in the depth of this evaluative disengagement during off-task attentional states. For both ERP components, the analyses reported below were all based on mean amplitude measures centered on the approximate peak of the component of interest as identified in the group-averaged waveforms, measured relative to a −200 to 0 ms pre-stimulus baseline.
Attentional Disengagement: P300
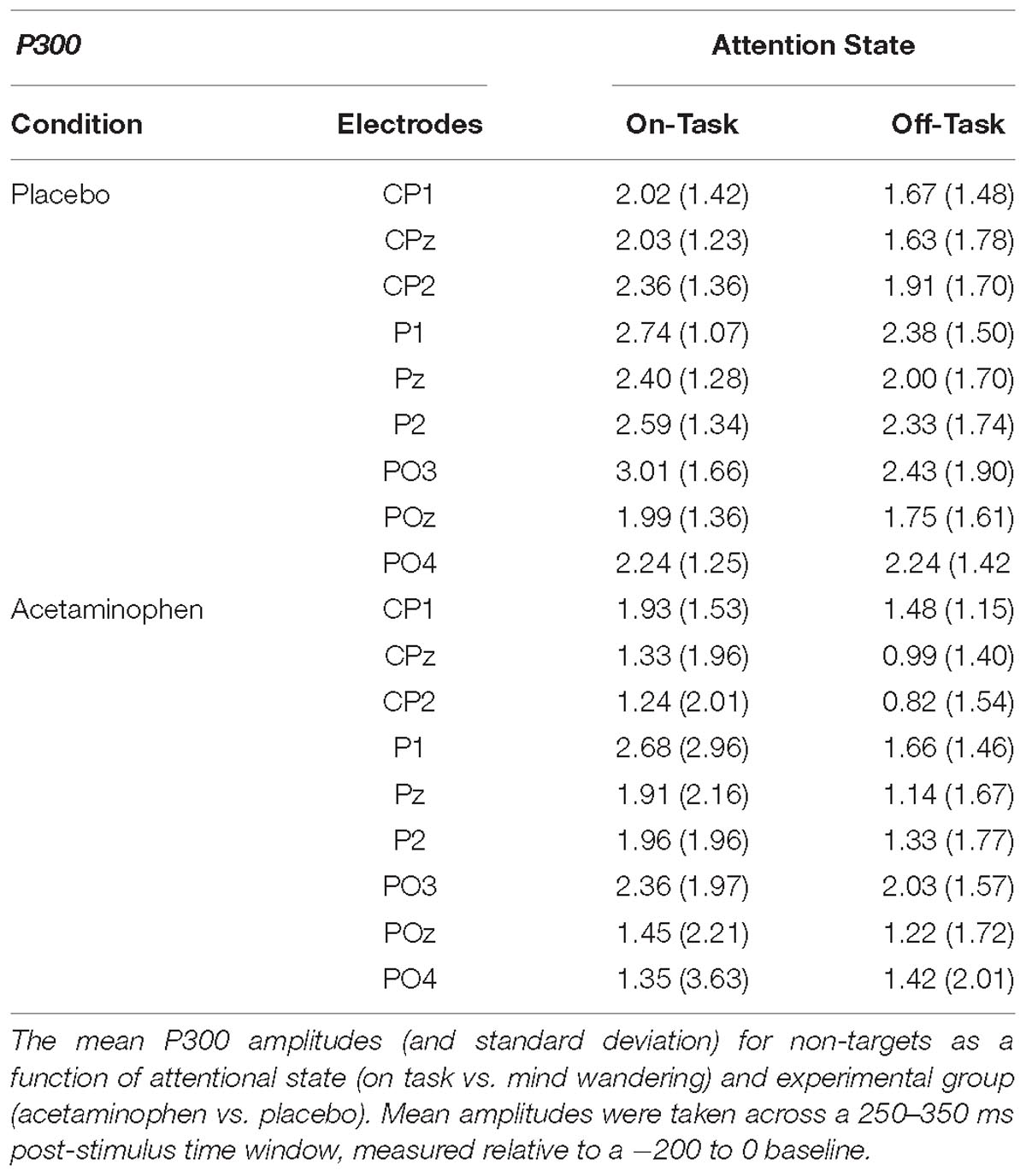
The P300 elicited by non-target events is shown in Figure 2 at the midline-posterior scalp electrode locations CPz, Pz, and POz where it was maximal in amplitude, along with the immediately distal locations to each over the left (CP1, P1, and PO1) and right (CP2, P2, and PO2) cerebral hemispheres. The mean amplitude of the P300 at each of these electrode sites is reported in Table 2 as a function of attentional report (on-task vs. off-task) and experimental condition (acetaminophen vs. placebo), as measured across a 250 to 350 ms time window capturing the approximate P300 peak. It appeared that there was a general reduction in P300 amplitude during off-task relative to on-task attentional states, but no impact of acetaminophen on this effect. This data pattern was confirmed by an omnibus, repeated-measures ANOVA that included experimental group (acetaminophen vs. placebo) as a between-groups factor, and attentional report (on-task vs. off-task) and electrode site included as within-groups factors. There was an overall significant main effect of attentional report [F(1,38) = 4.64, p = 0.038, = 0.11] with an overall greater amplitude during on-task attentional reports, but there was no significant main effect of experimental group [F(1,38) = 2.22, p = 0.14] or a significant interaction between attentional report and experimental group [F(1,38) = 0.11, p = 0.75]. As well, although a main effect of electrode site indicated an overall difference in P300 amplitude across scalp electrode site [F(8,31) = 6.38, p < 0.001], there was no significant interaction between electrode site and experimental group [F(8,31) = 1.28, p = 0.29], or interaction of electrode site with attentional state [F(8,31) = 1.15, p = 0.36].
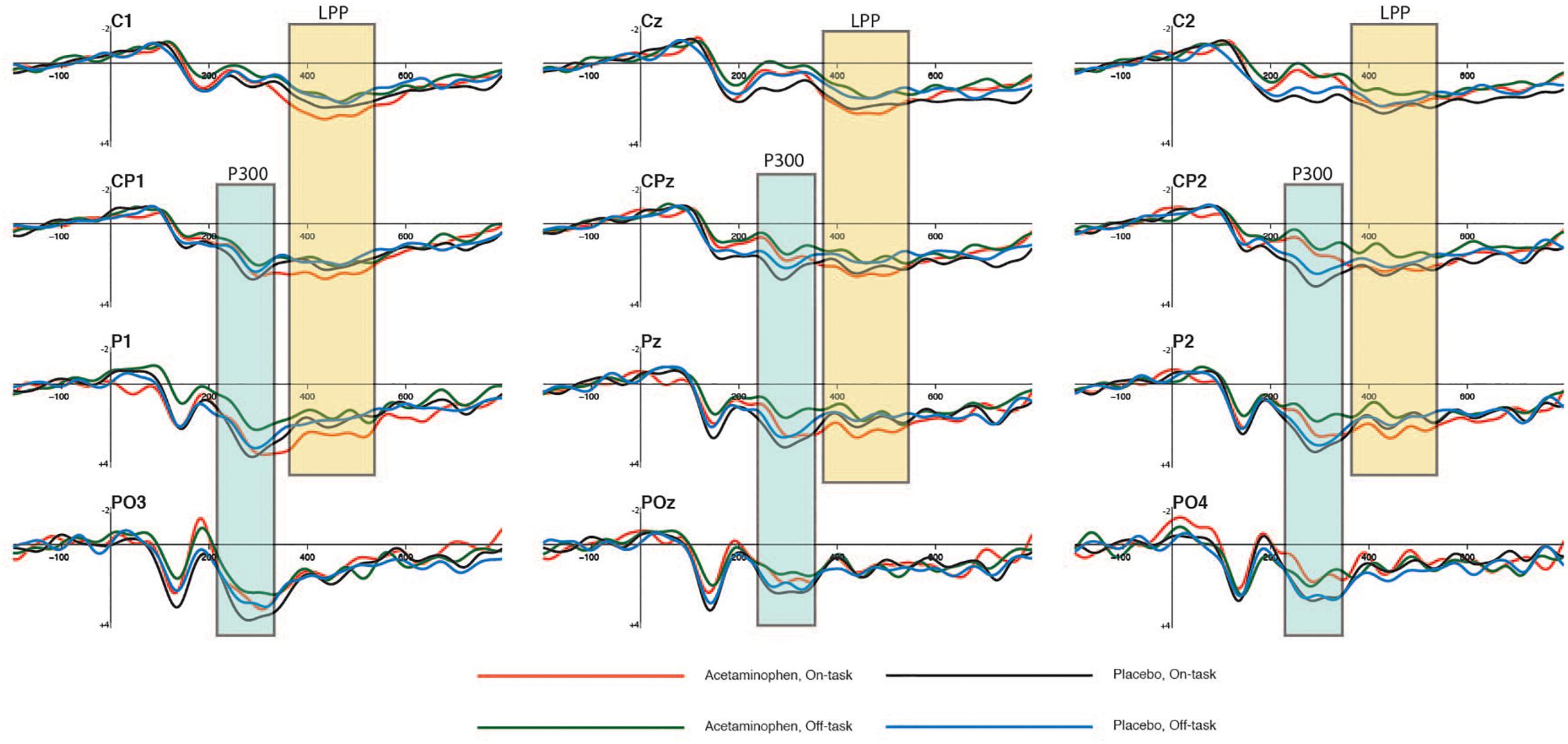
Figure 2. Averaged waveforms showing cognitive effects of P300 and LPP ERP components elicited by non-targets at 12 electrode sites as a function of attentional state and experimental condition. The time-windows used in the P300 amplitude analyses are highlighted in blue (250–350 ms), and the ones used in the LPP amplitude analyses are highlighted in yellow (375–525 ms). These ERP waveforms are time-locked to visual non-target events in the 12 s preceding an attentional report.
Evaluative Disengagement: LPP
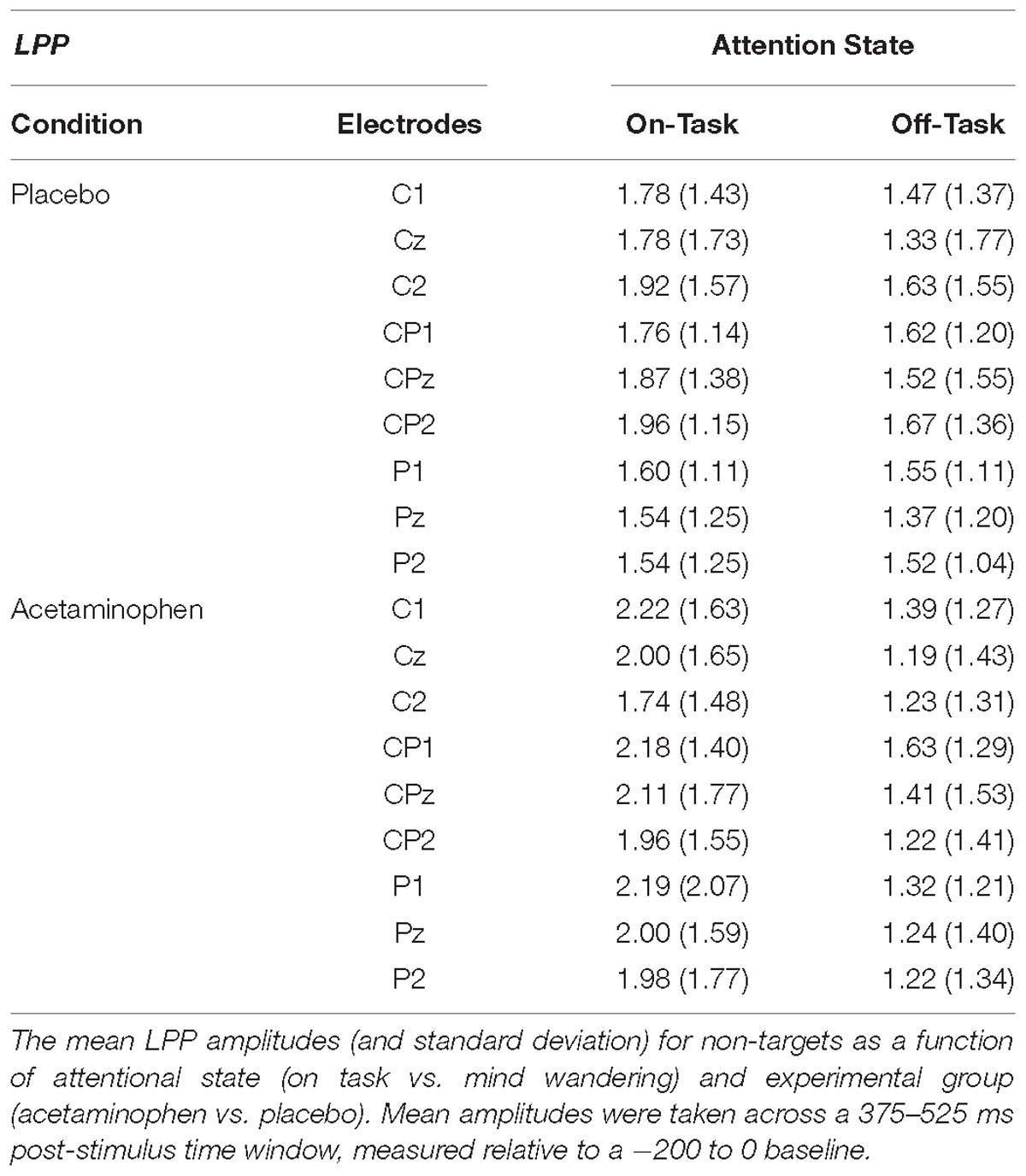
The LPP elicited by non-target events is shown in Figure 2 over the midline-posterior scalp electrode locations where it was maximal (Cz, CPz, and Pz) along with the immediately distal locations to each over the left (C1, CP1, and P1) and right (C2, CP2, and P2) cerebral hemispheres. The mean amplitude of the LPP at each of these electrode sites is reported in Table 3 as a function of attentional report (on-task vs. off-task) and experimental condition (acetaminophen vs. placebo), as measured across a 375 to 525 ms time window capturing the approximate LPP maximum. Like the P300, there appeared to be a general reduction in LPP amplitude during off-task relative to on-task attentional states, but unlike the P300, this effect also appeared to be greater in the acetaminophen relative to placebo group. This data pattern was confirmed via an omnibus, repeated-measures ANOVA that included experimental group (acetaminophen vs. placebo) as a between-groups factor, and attentional report (on-task vs. off-task) and electrode site as within-groups factors. Specifically, there was an overall significant main effect of attentional report [F(1,38) = 21.66, p < 0.001, = 0.36] and a significant group × attentional report interaction [F(1,38) = 5.84, p = 0.021, = 0.13], but there was no significant main effect of group [F(1,38) = 0.13, p = 0.91]. Separate ANOVAs within each group confirmed a main effect of attentional report for the acetaminophen group [F(1,38) = 25.00, p < 0.001, = 0.40], but not for the placebo group [F(1,38) = 2.50, p = 0.12]. However, separate between-groups comparisons within each attentional condition indicated that there was no significant between-group effect in either the on-task [F(1,38) = 0.53, p = 0.47] or off-task attentional condition [F(1,38) = 0.27, p = 0.61]. As well, there was also no significant main effect of electrode site [F(8,31) = 1.44, p = 0.22], or interaction of electrode site with experimental group [F(8,31) = 1.32, p = 0.27], or interaction of electrode site with attentional state [F(8,31) = 0.97, p = 0.48].
Additional Analyses
In addition to the above planned analyses, we conducted an additional set of analyses aimed at following up on two specific issues arising from our findings. First, given the significant interaction found between group and attentional state in the LPP data, we wanted to determine whether the impacts of acetaminophen might have also extended to behavioral performance. Second, given that one out of three participants had sufficient residual noise in their EEG data to preclude them from data analysis, we wanted to confirm that there were no systematic behavioral or demographic differences between the included vs. excluded participants that may have been biasing our data outcomes.
Behavioral Performance
For assessing the hit rate for the non-targets button-presses, an omnibus, repeated-measures ANOVA, with experimental group (acetaminophen vs. placebo) as a between-groups factor, and attentional report (on-task vs. off-task) as within-groups factors was conducted. There was no significant main effect of experimental group [F(1,38) = 0.79, p = 0.38], or a significant main effect of attentional report [F(1,38) = 0.09, p = 0.77], or a significant interaction between attentional report and experimental group [F(1,38) = 0.006, p = 0.94]. We were not able to perform a false alarm analyses for incorrect button-presses for target events (“X”) in the 12 s preceding an attentional report, as these preceding trials were always non-targets (numbers).
For evaluating the reaction time for the button presses to non-targets, we conducted another omnibus, repeated-measures ANOVA, with experimental group as a between-groups factor, and attentional report as within-groups factors, and revealed no significant main effect of experimental group [F(1,38) = 0.10, p = 0.76], or a significant main effect of attentional report [F(1,38) = 1.92, p = 0.17], or a significant interaction between attentional report and experimental group [F(1,38) = 0.81, p = 0.37]. Participants were given a time of 1 s to respond following the non-target appearance to make a button-press response. Any button-presses that were made past this allotted time were thereby considered as a “miss” and recorded as a “0” in the accuracy analyses, and were omitted from the reaction time analyses.
For evaluating the reaction time variability for the button presses to non-targets, we conducted another omnibus, repeated-measures ANOVA, with experimental group as a between-groups factor, and attentional report as within-groups factors, and revealed a significant main effect of attentional report [F(1,34) = 4.68, p = 0.04, = 0.12]. A post hoc Tukey test revealed that participants varied significantly more when they reported being in off-task vs. on-task attentional state [p < 0.05]. However, there was no significant main effect of experimental group [F(1,34) = 1.14, p = 0.29], or a significant interaction between attentional report and experimental group [F(1,34) = 0.01, p = 0.92]. Note: there was omission of participants data that had only one or less reaction time during the experimental blocks as we were not able to assess their variability.
Removed Participants
We also examined the 30% subject attrition from our analyses due to excessive ERP artifacts, to assess whether there was any meaningful difference between the subjects retained (n = 40) and the subjects removed (n = 19) on both demographic and behavioral information. We are unable to assess one participants’ data in our analyses, as they did not provide the researchers with consent for their data. For the remaining subjects, difference in age between the retained group of participants (M = 23.40, SD = 6.61), and the removed group of participants (M = 22.42, SD = 9.65) was non-significant [t(57) = 0.46, p = 0.65]. The retained group had a total of 26 females, whereas the removed group consisted of 16 females, however this group difference was not statistically significant [t(57) = −1.53, p = 0.13]. When comparing the retained and the removed groups on various behavioral analyses, there was no significant difference for the hit rate for button-presses to non-targets [t(57) = 0.43, p = 0.67], reaction time for button-presses to non-targets [t(57) = 0.69, p = 0.50], or false alarm rate for button-presses to target events [t(57) = 0.21, p = 0.83].
Discussion
Our study examined the potential impacts of acetaminophen on off-task attentional states. Participants were randomly assigned to either an acetaminophen or placebo condition, and then performed a SART as they were queried on their attentional states and the ERPs elicited by non-target events were recorded. In terms of behavior, we found no significant differences in the frequency of off-task attentional reports for acetaminophen vs. placebo experimental groups. This suggests that acetaminophen did not influence the relative proportion of time spent in off-task attentional states. In terms of ERPs, there were two main findings of interest. First, there was an overall main effect of attentional state on the mean amplitude of the P300 elicited by non-target events, but no interaction in the P300 between attentional state and group. Given prior evidence showing that the P300 attenuates during off-task relative on on-task attentional states (Smallwood et al., 2008; Barron et al., 2011; Kam et al., 2011), this basic replication provided a critical measure of normative validity to our data.
More importantly however, there was a significant interaction between group and attentional state in the mean amplitude of the LPP elicited by non-target events, such that the LPP was significantly reduced in amplitude during off-task relative to on-task attentional states in the acetaminophen group, but no comparable attention effect was observed in the placebo group. This suggests that our null between-group effects in the frequency of attentional reports and the P300 amplitude cannot simply be ascribed to an ineffective dose of acetaminophen. Rather, our findings support the conclusion that acetaminophen can in fact impact the depth of neurocognitive disengagement during off-task attentional states, but that this effect appears to be restricted to the level of stimulus processing indexed by the LPP ERP component. Given the conclusions and findings, several key questions follow.
First, how should the differential effect of acetaminophen on the P300 vs. LPP be construed at a functional level? At issue here is understanding how these components differ in terms of what they are believed to capture in terms of neurocognitive processing. The P300 is taken to index the degree to which a stimulus is initially discriminated or categorized at a basic cognitive level (Donchin, 1981; Polich, 2007), while the LPP is believed to index deeper or more contemplative aspects of evaluative analysis (e.g., Cacioppo and Berntson, 1994; Crites et al., 1995; Cacioppo et al., 1996; Cuthbert et al., 2000; Ito and Cacioppo, 2000). For example, while the P300 modulates in amplitude with stimulus frequency (van Dinteren et al., 2014), the LPP modulates with emotional intensity (Brown et al., 2012) and implicit esthetic preference (Handy et al., 2010; Miller et al., 2011). Given this distinction, our findings here suggest that acetaminophen impacts neurocognitive disengagement during off-task attentional states not at the level of more basic stimulus categorization processes as indexed by the P300, but rather, at the level of post-categorization stimulus evaluation as indexed by the LPP. To be clear, however, whether this effect is restricted to implicit evaluative analysis as captured in the current paradigm, or whether it extends to situations where stimuli are being explicitly evaluated on emotional and/or esthetic dimensions remains an open question.
Second, how do our current findings extend our understanding of acetaminophen and its psychopharmacological side effects? As reviewed above, previous studies on the topic have converged on the conclusion that acetaminophen attenuates the evaluative analysis of affectively salient events. For example, individuals on acetaminophen present as less empathetic toward the physical plight of others (Mischkowski et al., 2016), less sensitive to the pain of social rejection (DeWall et al., 2010), less prone to cognitive dissonance with respect to behavioral outcomes (DeWall et al., 2015), less affected by existential threats (Randles et al., 2013), less reactive to emotionally valanced imagery (Durso et al., 2015), and less perturbed by performance errors (Randles et al., 2016). All of these situations can be described as having some degree of affective salience of one form or another. By way of contrast, the stimuli used in the current study were affectively neutral visual events presented in the context of a simple target detection task. Nevertheless, acetaminophen was found to induce a significant decrease in the implicit evaluative analysis of these stimuli, as captured in the LPP ERP component. This thus suggests that the psychological impacts of acetaminophen can extend to affectively benign stimuli. That is, the effects of acetaminophen on evaluative processing may not be specific to affectively-valenced stimuli and events.
Finally, to what extent if at all might our findings inform on the functional interactions between the putative site of acetaminophen’s effect in cortex — the dACC (DeWall et al., 2010; Pickering et al., 2015) — and the DMN, which upregulates in activity during off-task attentional states (Mason et al., 2007; Christoff et al., 2009; Andrews-Hanna et al., 2010; Andrews-Hanna, 2012)? To be certain, the ability to make neuroanatomically definitive statements in this regard go beyond the scope of what our study and methods allow. But given that qualifier, our findings do support the possibility that whatever the dACC’s role in the analysis of on-going external events (Heilbronner and Hayden, 2016), the evaluative functions impacted by acetaminophen (DeWall et al., 2015) may be functionally dissociable from those dACC processes involved in modulating DMN function (Bonnelle et al., 2012; Jilka et al., 2014; Menon, 2015). In other words, we did not find a significant impact of acetaminophen on the normal ebb-and-flow of off-task attentional states, or what would be predicted if acetaminophen were directly affecting DMN activation per se. Instead, using affectively neutral stimuli, we just found that acetaminophen’s oft-replicated propensity to attenuate the evaluative analysis of affectively-valenced events was more reliably present during off-task attentional states.
Ethics Statement
This study was carried out in accordance with the recommendations of UBC Clinical Research Ethics Board with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the UBC Clinical Research Ethics Board.
Author Contributions
JK, DR, SH, and TH contributed to the conception and design of the study. SJ and JGS acquired data for the work. JK helped with interpretation of data for the work. SJ drafted the manuscript and performed data analysis for the work. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
The funder (NSERC – RGPIN-2014-04495) had no role in the research, other than providing funds.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Andrews-Hanna, J. R. (2012). The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18, 251–270. doi: 10.1177/1073858411403316
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R., and Buckner, R. L. (2010). Article of the brain’s default network. Neuron 65, 550–562. doi: 10.1016/j.neuron.2010.02.005
Baird, B., Smallwood, J., Lutz, A., and Schooler, J. W. (2014). The decoupled mind: mind-wandering disrupts cortical phase-locking to perceptual events. J. Cogn. Neurosci. 26, 2596–2607. doi: 10.1162/jocn
Barron, E., Riby, L. M., Greer, J., and Smallwood, J. (2011). Absorbed in thought: the effect of mind wandering on the processing of relevant and irrelevant events. Psychol. Sci. 22, 596–601. doi: 10.1177/0956797611404083
Bertolini, A., Ferrari, A., Ottani, A., Guerzoni, S., Tacchi, R., and Leone, S. (2006). Paracetamol: new vistas of an old drug. CNS Drug Rev. 12, 250–275. doi: 10.1111/j.1527-3458.2006.00250.x
Blair, K., Marsh, A. A., Morton, J., Vythilingam, M., Jones, M., Mondillo, K., et al. (2006). Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J. Neurosci. 26, 11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006
Bonnelle, V., Ham, T. E., Leech, R., Kinnunen, K. M., Mehta, M. A., Greenwood, R. J., et al. (2012). Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. U.S.A. 109, 4690–4695. doi: 10.1073/pnas.1113455109
Brown, J. W., and Braver, T. S. (2005). Learned predictions of error likelihood in the anterior cingulate cortex. Science 307, 1118–1121. doi: 10.1126/science.1105783
Brown, S., van Steenbergen, H., Band, G., de Rover, M., and Nieuwenhuis, S. (2012). Functional significance of the emotion-related late positive potential. Front. Hum. Neurosci. 6:33. doi: 10.3389/fnhum.2012.00033
Bush, G., Vogt, B. A., Holmes, J., Dale, A. M., Greve, D., Jenike, M. A., et al. (2002). Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. U.S.A. 99, 523–528. doi: 10.1073/pnas.012470999
Cacioppo, J. T., and Berntson, G. G. (1994). Relationship between attitudes and evaluative space: a critical review, with emphasis on the separability of positive and negative substrates. Psychol. Bull. 115, 401–423. doi: 10.1037//0033-2909.115.3.401
Cacioppo, J. T., Crites, S. L., and Gardner, W. L. (1996). Attitudes to the right: evaluative processing is associated with lateralized late positive event-related brain potentials. Pers. Soc. Psychol. Bull. 22, 1205–1219. doi: 10.1177/01461672962212002
Cai, X., and Padoa-Schioppa, C. (2012). Neuronal encoding of subjective value in dorsal and ventral anterior cingulate cortex. J. Neurosci. 32, 3791–3808. doi: 10.1523/JNEUROSCI.3864-11.2012
Christoff, K., Gordon, A. M., Smallwood, J., Smith, R., and Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. U.S.A. 106, 8719–8724. doi: 10.1073/pnas.0900234106
Christoff, K., Irving, Z. C., Fox, K. C. R., and Spreng, R. N. (2016). Mind-wandering as spontaneous thought: a dynamic framework. Nat. Publish. Group 17, 718–731. doi: 10.1038/nrn.2016.113
Clissold, S. P. (1986). Paracetamol and Phenacetin. Drugs 32(Suppl. 4), 46–59. doi: 10.2165/00003495-198600324-00005
Crites, S. L., Cacioppo, J. T., Gardner, W. L., and Berntson, G. G. (1995). Bioelectrical echoes from evaluative categorization: II. A late positive brain potential that varies as a function of attitude registration rather than attitude report. J. Pers. Soc. Psychol. 68, 997–1013. doi: 10.1037/0022-3514.68.6.997
Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., and Lang, P. J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 52, 95–111. doi: 10.1016/S0301-0511(99)00044-7
DeWall, C. N., Chester, D. S., and White, D. S. (2015). Can acetaminophen reduce the pain of decision-making? J. Exp. Soc. Psychol. 56, 117–120. doi: 10.1016/j.jesp.2014.09.006
DeWall, C. N., MacDonald, G., Webster, G. D., Masten, C. L., Baumeister, R. F., Powell, C., et al. (2010). Acetaminophen reduces social pain. Psychol. Sci. 21, 931–937. doi: 10.1177/0956797610374741
Dixon, M. L., Andrews-hanna, J. R., Spreng, R. N., Irving, Z. C., Mills, C., Girn, M., et al. (2017). Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage 147, 632–649. doi: 10.1016/j.neuroimage.2016.12.073
Durso, G. R. O., Luttrell, A., and Way, B. M. (2015). Over-the-counter relief from pains and pleasures alike: acetaminophen blunts evaluation sensitivity to both negative and positive stimuli. Psychol. Sci. 26, 750–758. doi: 10.1177/0956797615570366
Forrest, J. A. H., Clements, J. A., and Prescott, L. F. (1982). Clinical pharmacokinetics of paracetamol. Clin. Pharmacokinet. 7, 93–107. doi: 10.2165/00003088-198207020-00001
Fung, K., and Alden, L. E. (2017). Once hurt, twice shy: social pain contributes to social anxiety. Emotion 17, 231–239. doi: 10.1037/emo0000223
Graham, G. G., and Scott, K. F. (2005). Mechanism of action of paracetamol. Am. J. Ther. 12, 46–55. doi: 10.1097/00045391-200501000-00008
Handy, T. C., and Kam, J. W. Y. (2015). Mind wandering and selective attention to the external world. Can. J. Exp. Psychol. 69, 183–189. doi: 10.1037/cep0000051
Handy, T. C., Smilek, D., Geiger, L., Liu, C., and Schooler, J. W. (2010). ERP evidence for rapid hedonic evaluation of logos. J. Cogn. Neurosci. 22, 124–138. doi: 10.1162/jocn.2008.21180
Heilbronner, S. R., and Hayden, B. Y. (2016). Dorsal anterior cingulate cortex: a bottom-up view. Annu. Rev. Neurosci. 39, 149–170. doi: 10.1146/annurev-neuro-070815-013952
Högestätt, E. D., Jönsson, B. A. G., Ermund, A., Andersson, D. A., Björk, H., Alexander, J. P., et al. (2005). Conversion of acetaminophen to the Bioactive N-Acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J. Biol. Chem. 280, 31405–31412. doi: 10.1074/jbc.M501489200
Holroyd, C. B., Nieuwenhuis, S., Yeung, N., Nystrom, L., Mars, R. B., Coles, M. G. H., et al. (2004). Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat. Neurosci. 7, 497–498. doi: 10.1038/nn1238
Ito, T. A., and Cacioppo, J. T. (2000). Electrophysiological evidence of implicit and explicit categorization processes. J. Exp. Soc. Psychol. 36, 660–676. doi: 10.1006/jesp.2000.1430
Jilka, S. R., Scott, G., Ham, T., Pickering, A., Bonnelle, V., Braga, R. M., et al. (2014). Damage to the salience network and interactions with the default mode network. J. Neurosci. 34, 10798–10807. doi: 10.1523/JNEUROSCI.0518-14.2014
Jóźwiak-Bebenista, M., and Nowak, J. (2014). Paracetamol: mechanism of action, applications and safety concern. Acta Pol. Pharm. 71, 11–23.
Kam, J. W. Y., Dao, E., Blinn, P., Krigolson, O. E., Boyd, L. A., and Handy, T. C. (2012). Mind wandering and motor control: off-task thinking disrupts the online adjustment of behavior. Front. Hum. Neurosci. 6:329. doi: 10.3389/fnhum.2012.00329
Kam, J. W. Y., Dao, E., Farley, J., Fitzpatrick, K., Smallwood, J., Schooler, J. W., et al. (2011). Slow fluctuations in attentional control of sensory cortex. J. Cogn. Neurosci. 23, 460–470. doi: 10.1162/jocn.2010.21443
Kam, J. W. Y., Dao, E., Stanciulescu, M., Tildesley, H., and Handy, T. C. (2013). Mind wandering and the adaptive control of attentional resources. J. Cogn. Neurosci. 25, 952–960. doi: 10.1162/jocn
Kam, J. W. Y., and Handy, T. C. (2013). The neurocognitive consequences of the wandering mind: a mechanistic account of sensory-motor decoupling. Front. Psychol. 4:725. doi: 10.3389/fpsyg.2013.00725
Kam, J. W. Y., Xu, J., and Handy, T. C. (2014). I don’t feel your pain (as much): the desensitizing effect of mind wandering on the perception of others’ discomfort. Cogn. Affect. Behav. Neurosci. 14, 286–296. doi: 10.3758/s13415-013-0197-z
Kirschner, A., Kam, J. W. Y., Handy, T. C., and Ward, L. M. (2012). Differential synchronization in default and task-specific networks of the human brain. Front. Hum. Neurosci. 6:139. doi: 10.3389/fnhum.2012.00139
Kolling, N., Behrens, T. E. J., Mars, R. B., and Rushworth, M. F. S. (2012). Neural mechanisms of foraging. Science 336, 95–98. doi: 10.1126/science.1216930
Mason, M. F., Norton, M. I., Van Horn, J. D., Wegner, D. M., Grafton, S. T., and Macrae, C. N. (2007). Wandering minds: the default network and stimulus-independent thought. Science 315, 393–396.
Menon, V. (2015). “Salience network,” in Brain Mapping: An Encyclopedic Reference, Vol. 2, ed. A. W. Toga (Amsterdam: Elsevier).
Menon, V., and Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. doi: 10.1007/s00429-010-0262-0
Miller, M. W., Rietschel, J. C., McDonald, C. G., and Hatfield, B. D. (2011). A novel approach to the physiological measurement of mental workload. Int. J. Psychophysiol. 80, 75–78. doi: 10.1016/j.ijpsycho.2011.02.003
Mischkowski, D., Crocker, J., and Way, B. M. (2016). From painkiller to empathy killer: acetaminophen (paracetamol) reduces empathy for pain. Soc. Cogn. Affect. Neurosci. 11, 1345–1353. doi: 10.1093/scan/nsw057
Nagamatsu, L. S., Chan, A., Davis, J. C., Beattie, B. L., Graf, P., Voss, M. W., et al. (2013). Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: a 6-month randomized controlled trial. J. Aging Res. 2013, 1–10. doi: 10.1155/2013/861893
Ouellet, M., and Percival, M. D. (2001). Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch. Biochem. Biophys. 387, 273–280. doi: 10.1006/abbi.2000.2232
Pickering, G., Estève, V., Loriot, M.-A., Eschalier, A., and Dubray, C. (2008). Acetaminophen reinforces descending inhibitory pain pathways. Clin. Pharmacol. Ther. 84, 47–51. doi: 10.1038/sj.clpt.6100403
Pickering, G., Kastler, A., Macian, N., Pereira, B., Valabregue, R., Lehericy, S., et al. (2015). The brain signature of paracetamol in healthy volunteers: a double-blind randomized trial. Drug Des. Dev. Ther. 9, 3853–3862. doi: 10.2147/DDDT.S81004
Polich, J. (2007). Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 118, 2128–2148. doi: 10.1016/j.clinph.2007.04.019
Randles, D., Heine, S. J., and Santos, N. (2013). The common pain of surrealism and death. Psychol. Sci. 24, 966–973. doi: 10.1177/0956797612464786
Randles, D., Kam, J. W. Y., Heine, S. J., Inzlicht, M., and Handy, T. C. (2016). Acetaminophen attenuates error evaluation in cortex. Soc. Cogn. Affect. Neurosci. 11, 899–906. doi: 10.1093/scan/nsw023
Roberts, I. D., Krajbich, I., Cheavens, J. S., Campo, J. V., and Way, B. M. (2018). Acetaminophen reduces distrust in individuals with borderline personality disorder features. Clin. Psychol. Sci. 6, 145–154. doi: 10.1177/2167702617731374
Sheth, R., Marcon, L., Bastida, M. F., Junco, M., Quintana, L., Dahn, R., et al. (2012). Hox genes regulate digit patterning by controlling the wavelength of a turing-type mechanism. Science 338, 1476–1480. doi: 10.1126/science.1226804
Smallwood, J., Beach, E., Schooler, J. W., and Handy, T. C. (2008). Going AWOL in the brain: mind wandering reduces cortical analysis of external events. J. Cogn. Neurosci. 20, 458–469. doi: 10.1162/jocn.2008.20037
Smallwood, J., Brown, K. S., Tipper, C., Giesbrecht, B., Franklin, M. S., Mrazek, M. D., et al. (2011). Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PLoS One 6:e0018298. doi: 10.1371/journal.pone.0018298
Sonuga-Barke, E. J. S., and Castellanos, F. X. (2007). Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 31, 977–986. doi: 10.1016/j.neubiorev.2007.02.005
Sridharan, D., Levitin, D. J., and Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U.S.A. 105, 12569–12574. doi: 10.1073/pnas.0800005105
Keywords: acetaminophen, mind wandering, ERP, attention, default mode network, salience network
Citation: Jaswal SM, Granados Samayoa JA, Kam JWY, Randles D, Heine SJ and Handy TC (2019) The Influence of Acetaminophen on Task Related Attention. Front. Neurosci. 13:444. doi: 10.3389/fnins.2019.00444
Received: 29 December 2018; Accepted: 17 April 2019;
Published: 03 May 2019.
Edited by:
Paul Newhouse, Vanderbilt University School of Medicine, United StatesReviewed by:
Christiane Thiel, University of Oldenburg, GermanyAlexandra Key, Vanderbilt University Medical Center, United States
Copyright © 2019 Jaswal, Granados Samayoa, Kam, Randles, Heine and Handy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Todd C. Handy, todd@psych.ubc.ca
 Sumeet Mutti Jaswal
Sumeet Mutti Jaswal Javier A. Granados Samayoa
Javier A. Granados Samayoa Julia W. Y. Kam
Julia W. Y. Kam Daniel Randles4
Daniel Randles4 Steven J. Heine
Steven J. Heine Todd C. Handy
Todd C. Handy