- 1 Department of Neurology, University Hospital Zurich, Zurich, Switzerland
- 2 Department of Neurology, Case Western Reserve University, Cleveland, OH, USA
Transformation of head-fixed otolith signals into a space-fixed frame of reference is essential for perception of self-orientation and ocular motor control. In monkeys the nodulus and ventral uvula of the vestibulo-cerebellum facilitate this transformation by computing an internal estimate of direction of gravity. These experimental findings motivated the hypothesis that degeneration of the vestibulo-cerebellum in humans alter perceptual and ocular motor functions that rely on accurate estimates of gravity, such as subjective visual vertical (SVV), static ocular counterroll (OCR), and gravity-dependent modulation of vertical ocular drifts. We assessed the SVV, OCR, and spontaneous vertical ocular drifts in 12 patients with chronic vestibulo-cerebellar disease and in 10 controls. Substantially increased variability in estimated SVV was noted in the patients. Furthermore, gravity-dependent modulation of spontaneous vertical ocular drifts along the pitch plane was significantly (p < 0.05) larger in the patients. However, the gain and variability of static OCR and errors in SVV were not significantly different. In conclusion, in chronic vestibulo-cerebellar disease SVV and OCR remain intact except for an abnormal variability in the perception of verticality and impaired stabilization of gaze mediated by the otoliths. These findings suggest that OCR and perceived vertical are relatively independent from the cerebellum unless there is a cerebellar imbalance like an acute unilateral cerebellar stroke. The increased trial-to-trial SVV variability may be a general feature of cerebellar disease since a function of the cerebellum may be to compensate for such. SVV variability might be useful to monitor disease progression and treatment response in patients.
Introduction
The otolith organs sense self-motion in head-fixed coordinates, and therefore cannot discriminate relatively equivalent linear acceleration and gravity. However, accurate perception of self-orientation in space as well as optimal ocular motor and postural control require an estimate of motion in a space-fixed frame of reference. Self-motion is centrally transformed from head-fixed to space-fixed coordinates (Angelaki et al., 2004; Green et al., 2005; Shaikh et al., 2005) and is obtained by integrating multisensory (e.g., vestibular, visual, proprioceptive) input (Angelaki et al., 2009). By computing the internal estimate of gravity (based on otolith-input), the vestibulo-cerebellum (i.e., flocculus, nodulus, uvula), in particular the nodulus and the ventral uvula, facilitate this transformation as shown in non-human primates (Zhou et al., 2006; Yakusheva et al., 2007).
Acute unilateral damage of vestibulo-cerebellar structures, e.g., due to ischemia or hemorrhage, leads to typical signs of imbalance in otolith-mediated pathways, i.e., partial or complete ocular tilt reaction [OTR, i.e., a triad of head tilt, vertical ocular misalignment (also termed skew deviation), and ocular torsion; Westheimer and Blair, 1975] and tilts of the subjective visual vertical (SVV; Mossman and Halmagyi, 1997; Lee et al., 2005; Baier et al., 2008; Kim et al., 2009). In contrast, little is known on how diffuse bilateral cerebellar degeneration affects these otolith-mediated functions. Patients with chronic vestibulo-cerebellar disease typically demonstrate strong gravity-dependence of downbeat nystagmus (DBN) where there is an increase of drift in prone and a decrease in supine positions (Marti et al., 2002; Leigh and Zee, 2006). Similar modulation of spontaneous vertical ocular drift can be found in healthy human subjects, but to a much smaller extent, presumably because of the intact cerebellar inhibition of otolith-ocular reflexes (Marti et al., 2002). Several studies documented decreased function of the dynamic otolith-mediated translational vestibulo-ocular reflex (tVOR; Wiest et al., 2001; Liao et al., 2008), but static otolith-mediated functions, especially perception of the vertical (for instance measured by the SVV) and static ocular torsion in response to sustained head tilts (ocular counterroll, OCR), have not been analyzed in patients with diffuse bilateral cerebellar degeneration.
We hypothesize that due to the more balanced pattern of degeneration (Klockgether, 2008), diffuse and slowly progressive cerebellar disease results in more subtle changes in the accuracy of perceived vertical and torsional eye position as compared to changes in patients with acute unilateral cerebellar lesions. Whereas estimates in vertical likely will remain accurate, we predict increased trial-to-trial variability in perceptual estimates of verticality and ocular motor control of vertical and torsional eye position. We propose that an impaired transformation of head-fixed otolith signals to space-fixed signals resulting in more variable cerebellar estimates of direction of gravity could explain these findings. This hypothesis was examined in patients with degenerative vestibulo-cerebellar disease and compared findings with those from age-matched healthy control subjects. Preliminary results have been published in a conference proceedings (Tarnutzer et al., 2008).
Materials and Methods
Subjects
Twelve patients with chronic degeneration of the vestibulo-cerebellum due to hereditary or sporadic disease (Table 1; 5 females, mean age 56.8 years, range 32–81 years) and 10 age-matched healthy subjects (5 females; mean age 60.1 years, range 33–70 years) participated in this study. All patients presented with clinical findings consistent with midline cerebellar degeneration. Limb ataxia was found only in two patients and was rated as mild during clinical examination as it did not interfere with activities of daily life. Informed consent of all participants was obtained after full explanation of the experimental procedure. The protocol was approved by a local ethics committee, and was in accordance with the Declaration of Helsinki for research involving human subjects.
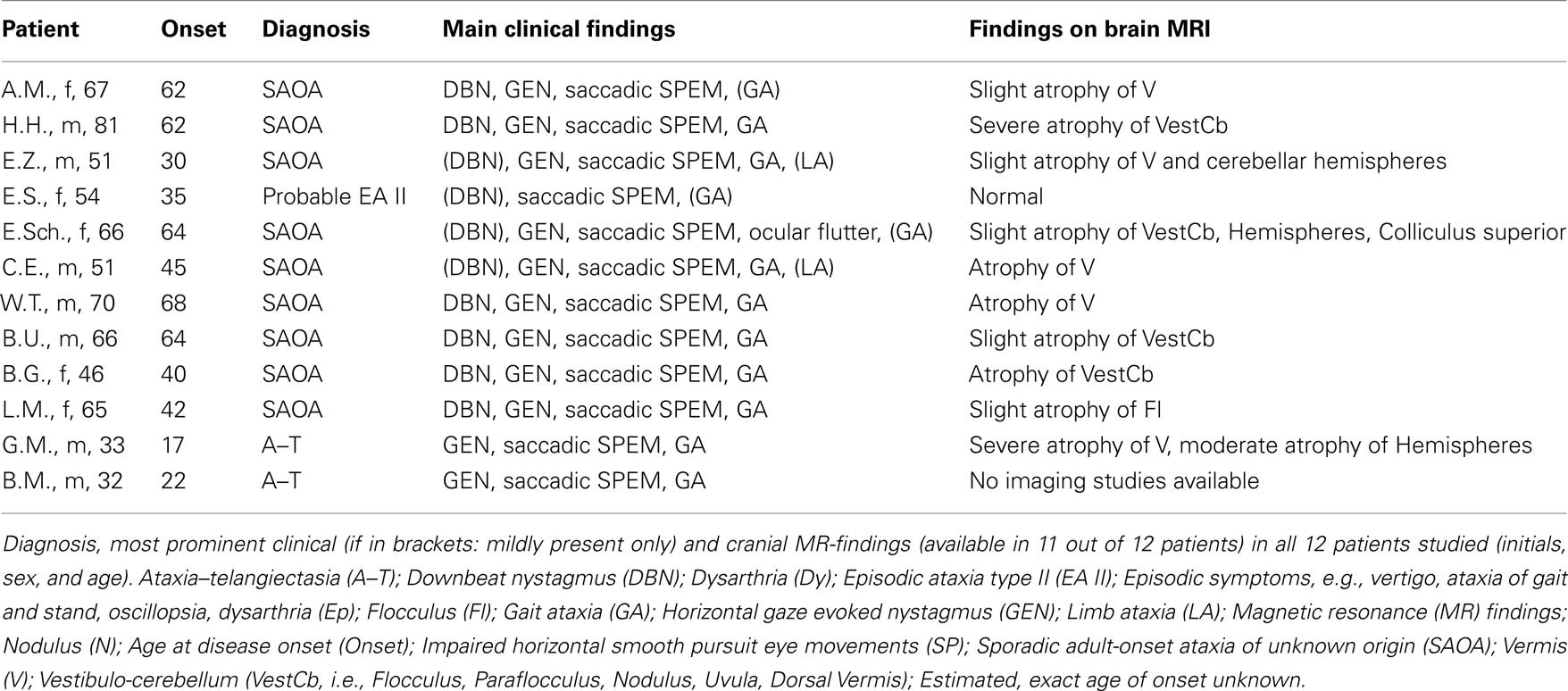
Table 1. Demographic and clinical data of all patients with diffuse, chronic vestibulo-cerebellar degeneration.
Experimental Setup
Subjects were seated upright on a motor-driven three axes turntable (Acutronic, Jona, Switzerland) with their head restrained looking straight-ahead for all paradigms. Subjects were positioned so that the roll axis of the turntable intersected the center of the inter-aural line. Pillows and safety belts minimized body movements.
Setup for assessment of the SVV
Turntable acceleration/deceleration during changes of body roll position was set to ±10°/s2. An arrow that extended over the central 9.5° of the visual field and that was projected onto a screen (distance to subject: 1.5 m) was used to indicate perceived vertical. Experiments were performed in otherwise complete darkness.
Recording of eye movements
Recording of eye movements were obtained monocularly (in 6/12 patients) or binocularly (in 6/12 patients and 10/10 controls) in three dimensions with a frequency of 1000 Hz using magnetic search coils (Skalar Instruments, Delft, The Netherlands). The search coil setup and the procedure was identical to the one described in Straumann et al., 1995 and Marti et al., 2002.
Experimental protocol for measuring the SVV
Subjects were asked to rapidly (<6 s) adjust the orientation of the arrow to perceived vertical with the arrow-head pointing up. Whenever completion was not confirmed within the time limit, the trial was repeated later. The presentation of the arrow started 10 s after the turntable came to a full stop and was offset clockwise (CW) or counter-clockwise (CCW) relative to vertical pseudo-randomly between 28° and 72°. Twenty-four adjustments were collected in each orientation (0° = upright; 75° = right-ear-down; -75° = left-ear-down) in pseudo-random order, resulting in a total of 72 trials, recorded in a single session. The direction of arrow rotation (CW vs. CCW) did not significantly affect its final orientation and its variability (p > 0.05, three-way ANOVA). We therefore pooled trials with CW and CCW arrow rotations for further analysis.
Experimental Protocol for Measuring Static Ocular Counterroll
Subjects were instructed to keep gaze steady upon a laser dot presented at 1.5 m distance straight-ahead in otherwise complete darkness. They were subsequently moved to 75°, 45°, 30°, 20°, and 10° left-ear-down (LED) roll positions and afterward back to upright position, then to 75°, 45°, 30°, 20°, and 10° right-ear-down (RED) roll positions and again back to upright position; every position was held for 30 s.
Experimental Protocol for Measuring Spontaneous Vertical Ocular Drift Velocity and Its Gravity-Dependent Modulation
For measurements of spontaneous vertical ocular drift subjects were asked to keep their eyes steady on a flashing laser dot (duration: 20 ms, interval: 2 s) projected straight-ahead on a turntable-fixed screen (distance: 0.59 m) in otherwise complete darkness. We used a flashing dot to ensure that visual following mechanisms could not aid fixation of the visual target. For the assessment of the gravity-dependent modulation of DBN, the paradigm of Marti was used (Marti et al., 2002).
Data Analysis
SVV data
Outliers >3 SDs from the mean were discarded, resulting in the removal of <0.1% of all trials. In the following, we will use the term “intra-individual variability” whenever we report trial-to-trial SD. If not stated otherwise, statistical analysis was done using ANOVA (Minitab, Minitab Inc., State College). Tukey’s correction was used to compensate for multiple comparisons. To assess for correlations between the distinct otolith – tests, generalized estimated equations (GEE) were used (SPSS 16, SPSS Inc., Chicago). Whenever t-tests were considered, Holm’s correction for multiple t-tests was applied (Holm, 1979; Aickin and Gensler, 1996).
Static ocular counterroll
Static OCR in the upright and in sustained 75°, 45°, 30°, 20°, and 10° roll positions to either side was analyzed as described elsewhere (Marti et al., 2008). For comparison with SVV intra-individual variability, we calculated the within-trial variability of static OCR in upright and 75° roll positions over the last 10 s in the respective position on a single trial basis. To determine spontaneous vertical ocular drift, we computed the median velocity for sections of 400 ms over a period of 10 s while subjects were fixating the flashing dot. Sections were analyzed interactively and discarded when the slow-phase velocity was inconstant. Such a procedure corresponds to implicit de-saccading (Straumann, 1991). To analyze the gravity-dependence of DBN, we used the same procedure as described in Marti et al., 2002 and computed amplitude and offsets based upon sinusoidal fits to the modulation of DBN slow-phase velocity.
Results
Subjective Visual Vertical
Figure 1A illustrates the summary of errors in SVV in cerebellar patients as compared to controls. In healthy controls, the errors in SVV during upright orientation (0.8 ± 2.4°; mean ± SD) were not significantly different from zero. When subjects were reoriented at 75° LED, the errors in SVV significantly deviated from zero (-10.9 ± 9.8°, p < 0.05, t-test). Latter is considered to be due to the phenomenon called roll under compensation. During body tilt in the opposition direction, i.e., during 75° RED the errors in SVV were 7.2 ± 10.6°. While this observation pointed toward roll under compensation again; the difference as compared to zero was not significant (p = 0.06). Within a given healthy subject (intra-individual) there was a minimal train-to-trial variability in the perception of SVV in upright orientation (1.6 ± 0.7°). However, there was an increase in SVV variability in both 75° LED (5.3 ± 1.2°) and 75° RED (5.2 ± 2.1°; see Figure 1B) position in healthy subjects.
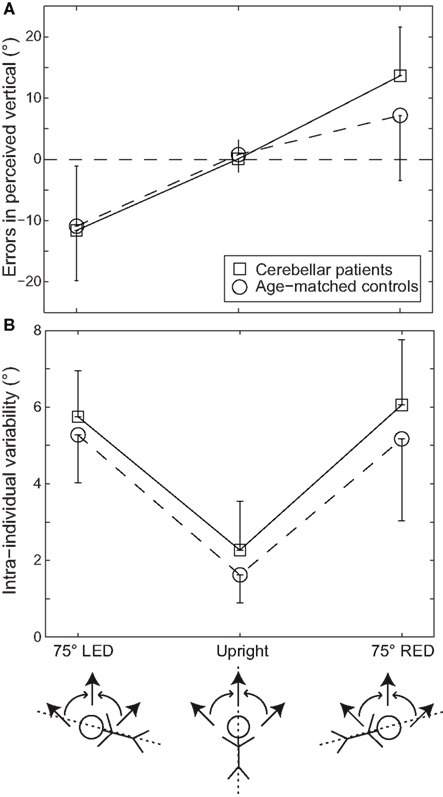
Figure 1. Average errors (±1 SD) in perceived vertical (A) and average intra-individual SVV variability (±1 SD) (B) are plotted against whole-body roll position both for the cerebellar patients (squares) and the age-matched healthy controls (circles). The direction (indicated by the curved arrows) of arrow adjustments (straight arrows: clockwise vs. counter-clockwise), as illustrated below the x-axis for all roll positions (upright, 75° left-ear-down or LED, and 75° right-ear-down or RED) tested was pseudo-randomized.
In the cerebellar patients, although SVV in upright orientation was accurate (0.1 ± 2.2°), the adjustments deviated significantly from zero (p < 0.005) both at 75° LED (-11.6 ± 8.2°) and 75° RED (13.7 ± 8.0°). Compared to the control group, SVV deviations in the patient group were not significantly larger (p > 0.05, three-way ANOVA) as shown in Figure 1A. Intra-individual variability, however, was significantly increased in both 75° LED (5.8 ± 1.2°) and 75° RED (6.1 ± 1.7°) compared to upright (2.3 ± 1.3°). Three-way ANOVA with groups, direction of arrow rotation, and whole-body roll orientation as factors yielded a significant main effect for the groups, indicating larger intra-individual variability for the patients (p < 0.01) as illustrated in Figure 1B. Furthermore, a significant (p < 0.001) main effect for whole-body roll orientation was noted. Meanwhile, no significant interactions between the whole-body orientation, direction of arrow rotation, and groups were noted. SVV adjustments were completed in both patients and healthy controls in slightly >3 s (3.30 ± 0.89 vs. 3.14 ± 0.73 s; patients vs. controls) with the patient group requiring on average about 5% more time than the controls; this difference was small but consistent and reached statistical significance (p = 0.03).
These results suggest that patients with chronic vestibulo-cerebellar disease are relatively more uncertain and tend to make more errors in perception of verticality.
Ocular Counterroll
Figure 2A depicts changes in static ocular torsion (torsional eye position) in response to head tilt to the sides. Latter response is also called OCR. As expected, static OCR systematically changed with whole-body tilt to the sides (roll tilts to the right or left; Collewijn et al., 1985; Bockisch and Haslwanter, 2001; Palla et al., 2006). No significant differences between left and right eye data at any roll position were noted in both patients and controls (p > 0.05, two-way ANOVA); therefore data from both eyes were pooled for further analysis. Differences between patients and controls in the amplitude of static OCR did not reach statistical significance in any roll position (p > 0.05, three-way ANOVA), as illustrated in Figure 2A. Furthermore, comparing LED and RED, absolute static OCR values in both groups were not significantly different (p > 0.05, three-way ANOVA). Differences in the within-trial variability of static OCR between patients and controls in 75° ear-down positions did not reach statistical significance either, as shown in Figure 2B.
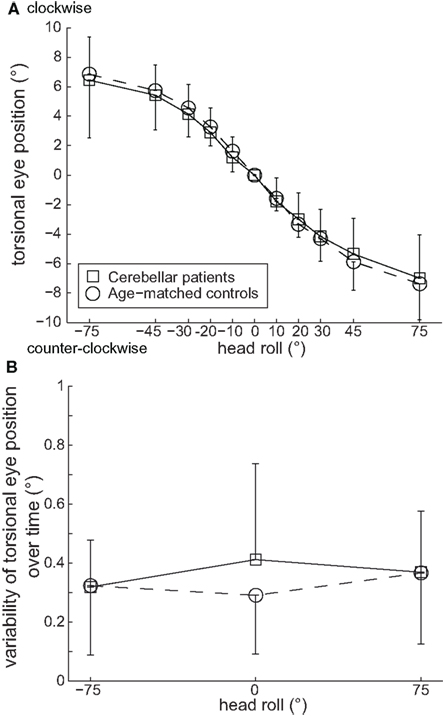
Figure 2. (A) Means of static torsional eye position (±1 SD) in 75°, 45°, 30°, 20°, and 10° whole-body roll tilt positions to either side (positive values corresponding to right-ear-down positions, i.e., clockwise roll tilts, as seen from the subject) did not differ significantly (p > 0.05) between patients (squares) and age-matched controls (circles). (B) Variability of static torsional eye position over time (10 s) in upright and 75° right- (positive values) and left-ear-down (negative values) positions to either side was not significantly different (p > 0.05) between patients (squares) and age-matched controls (circles).
Spontaneous Vertical Ocular Drift Velocity
The values of spontaneous vertical ocular drift in the left versus right eye data did not significantly differ in either patients or controls (p > 0.05, ANOVA); the data from both eyes were therefore pooled. Ten out of twelve patients demonstrated upward spontaneous vertical ocular drift, corresponding to DBN (drift velocity range: 0.27–9.92°/s). Two patients demonstrated subtle downward ocular drift, i.e., upbeat nystagmus (drift velocities: 0.23 and 0.80°/s, respectively). SVD was virtually absent in healthy subjects; the spontaneous vertical ocular drift velocities in the patients (1.92 ± 2.42°/s) were significantly larger than in controls (0.03 ± 0.4°/s; p = 0.029, ANOVA). There was a correlation between the amount of spontaneous vertical ocular drift in the patients and their corresponding SVV variability in 75° LED roll-tilted position (p = 0.007, GEE), and a trend toward significance for 75° RED (p = 0.075, GEE) orientation.
Gravity-Dependence of Spontaneous Vertical Ocular Drift
In the 10 patients, who had cerebellar degeneration due to the cause other than ataxia–telangiectasia (A–T), SVD exhibited the typical pattern of sinusoidal gravity-dependent modulation along the pitch plane: upward ocular drift increased in sustained prone positions and decreased in sustained supine positions as previously reported (Marti et al., 2002). The two patients with A–T, however, demonstrated an atypical pattern with non-sinusoidal exceedingly large amplitude modulation of their spontaneous vertical ocular drift. We therefore excluded the two A–T patients from this part of the analysis. ANOVA did not reveal any significant differences between the left and right eye data (p > 0.05) in patients (n = 10, five females, mean age 61.7 years, range 46–81 years) and in the adjusted control group (n = 8, three females, mean age 66.8, range 59–73 years); therefore data from both eyes were pooled. Both amplitude (ANOVA, p = 0.01, reflecting an approximately threefold increase) and offset (ANOVA, p < 0.001) of sinusoids fitted to the gravity-dependent modulation of DBN were significantly increased in patients (amplitude: 3.4 ± 2.6°/s; offset: 2.4 ± 2.0°/s upward drift) compared to controls (amplitude: 1.0 ± 0.6°; offset: 0.1 ± 0.9°/s downward drift).
Discussion
Precise computation of internal estimates of direction of gravity is needed for accurate spatial perception, ocular motor, and postural control and relies on various sensory signals, e.g., vestibular, proprioceptive, and visual inputs. The cerebellum is critically involved in the central processing of vestibular signals. Our results demonstrate that patients with diffuse vestibulo-cerebellar degeneration exhibit increased variability in internal estimates of verticality of modest but significant size with respect to self-orientation both on the perceptual and on the ocular motor levels. However, besides the increased variability of SVV noted, the patients did surprisingly well in these tasks with normal accuracy of SVV, preserved OCR gains reflecting an intact rotational VOR (rVOR) and no increased drift of torsional eye position over time. Our findings underline the robustness of the static otolith-mediated functions.
Mechanisms Contributing to Impaired Internal Estimates of Vertical in Vestibulo-Cerebellar Degenerative Disease
The increased SVV variability in the patient group may be best explained by a disturbance in the generation of a central estimate of direction of gravity, and thus the transformation of otolith-input from a head-fixed to a space-fixed reference frame (Angelaki et al., 2004; Green et al., 2005; Shaikh et al., 2005), which is a function of the vestibulo-cerebellum (Zhou et al., 2006; Yakusheva et al., 2007). The increased variability of vertical eye position in straight-ahead fixation over time adds further evidence to the hypothesis that the cerebellum is crucial in controlling for variability in motor commands as previously shown for the initiation of saccades (Xu-Wilson et al., 2009), reaching movements (Werner et al., 2009), and limb-coordination (Ilg et al., 2009) in patients with various degenerative cerebellar diseases. In analogy, we noted the trial-to-trial variability of SVV to be increased in patients with vestibulo-cerebellar degeneration. We hypothesize that the cerebellum not only is in charge of optimizing motor commands, but also of minimizing variability of internal estimates of direction of vertical based on otolith-input for conscious percept.
Theoretically, visual blurring due to vertical ocular drift may result in a more variable visual feedback of SVV adjustments and explain the patients’ impaired ability to perform precise line adjustments. However, no correlation between the amount of spontaneous vertical ocular drift and SVV variability was seen in upright position. Moreover, the patients’ ability to hold a certain torsional eye position over a 10-s period of time while roll-tilted was not impaired. Given the unchanged within-trial variability of OCR, we think that the decreased SVV precision in the patients cannot be attributed to less stable visual fixation while setting the SVV. Finally, the increased SVV variability could also be caused by limb ataxia, but only two patients showed mild limb ataxia and variability values observed in these two patients were either close to (EZ) or below (CE) the mean values observed for the patient population; therefore, limb ataxia is unlikely to play a major role in this context.
OCR and SVV Accuracy are Preserved in Vestibulo-Cerebellar Degenerative Disease
The preserved SVV accuracy and static OCR gains in our patients with diffuse vestibulo-cerebellar degeneration reflect an intact static rVOR in slowly progressive cerebellar disease and markedly contrasts to the pattern observed in patients with acute lateralized cerebellar lesions, where the nodulus (Mossman and Halmagyi, 1997; Kim et al., 2009) and the dentate nucleus (Baier et al., 2008) were identified to play a critical role in the transmission and processing of otolith-input. Contraversive SVV tilts and ocular tilt reaction were observed when lesions included the nodulus (Kim et al., 2009) and the dentate nucleus (Baier et al., 2008). Acute lateralized lesions sparing the nodulus led to isolated ipsilesional SVV tilt with torsional eye position and vertical ocular alignment being intact (Kim et al., 2009). One may therefore hypothesize that a clearly asymmetric, focal and acute cerebellar lesion is needed for a directional bias of the internal estimate of vertical. Further support for this assumption comes from a case report from a patient with an acute symmetrical bilateral infarction of the cerebellar nodulus that showed no SVV tilts or signs of ocular tilt reaction (Kim et al., 2009). Unfortunately, in this patient the trial-to-trial variability of SVV was not reported.
With regard to the clinical presentation of our patients, it seems reasonable to assume that the degenerative process has involved the nodulus, uvula, and the deep cerebellar nuclei on both sides to a similar extent. Thus, our data supports earlier reports that SVV tilts and ocular tilt reaction in cerebellar disease are suggestive of focal lesions disrupting otolith-input asymmetrically, while at least bilateral lesions of the nodulus seem not to give rise to ocular tilt reaction (Kim et al., 2009).
In our patients, static rVOR was preserved, indicated by static OCR values not being significantly impaired both in terms of gain and in terms of torsional gaze holding over time in comparison to age-matched controls. Wong and Sharpe, 2005 reported asymmetric static OCR gains in patients presenting with skew deviation and chronic focal cerebellar lesions. They hypothesized that these gain changes might be caused by a deficient cerebellar control of vertical vergence (Wong and Sharpe, 2005). Bilateral ablation of the vestibulo-cerebellum in cats yielded increases in dynamic OCR gains of approximately 30% (Robinson, 1976), however, static OCR gains were not determined. On the other hand, static OCR gains remained intact after ablation of the nodulus and uvula in non-human primates (Walker et al., 2008). These results suggest that cerebellar mechanisms involved in the control of static OCR are impaired in focal, asymmetric cerebellar lesions, however, may be preserved in patients with diffuse cerebellar degeneration.
In contrast to preserved static OCR in degenerative cerebellar disease, the tVOR has been found to be severely impaired (Wiest et al., 2001; Liao et al., 2008). Likely these marked differences are related to the involved neuronal networks in generating either rVOR or tVOR. Whereas the rVOR is driven by a phylogenetically old three-neuron arc, the tVOR relies on structures related to smooth pursuit, which previously has been found to be impaired in cerebellar degeneration (Zee et al., 1976).
Furthermore, binocular control of torsional eye position, i.e., cycloversion, in the cerebellar patients was preserved, suggesting no major involvement of the cerebellum in the control of cycloversion under static conditions. However, due to the small number of cerebellar patients where binocular torsional eye movements had been obtained (n = 6/12), conclusions on the state of cycloversion in degenerative vestibulo-cerebellar disease have to be made with caution and further investigations in a larger group of cerebellar patients are required to give a more definitive answer to question to which extent the cerebellum is involved in conjugate vertical and torsional eye movements.
Our data confirm that DBN, which is typical for chronic vestibulo-cerebellar disease (Leigh and Zee, 2006), shows prominent gravity-dependent modulation along the pitch plane (Marti et al., 2002). In the context of the present study, we suggest the overshooting gravity-dependent modulation of DBN can be best explained by deficient input–output coupling of gravitational otolith-input related to control of vertical eye position during low-frequency pitch (Paige and Seidman, 1999; Marti et al., 2002). Changes in the internal estimate of gravity over time may be crucial in the context of vertical ocular drift. When the orientation of the head changes with gravity, the estimate of the gravity vector would remain consistent since an intact vestibulo-cerebellum had transformed otolith derived, gravito-inertial acceleration to the space-fixed coordinates. As a result, there would be minimal gravitational estimate dependent modulation of spontaneous vertical drift. In patients with vestibulo-cerebellar disease on the other hand, the estimate of the true gravity vector by the brain does not stay consistent, since there is impaired transformation of head-fixed gravito-inertial acceleration (which changes with head orientation) to the space-fixed coordinates. This could explain the increased gravity-dependent modulation of DBN.
Limitations
The increase in trial-to-trial variability for the SVV task found in the patient group at a single roll angle (±75° ear-down) – although being statistically significant – was of small size, prompting interpretation of this finding with caution and demanding further studies with a larger and ideally more homogeneous sample size and additional roll-angles. Furthermore, the patients included clinically all demonstrated typical vestibulo-cerebellar disease, while magnetic resonance (MR) findings varied considerably and no volumetric analyses were performed. Therefore, structure-specific correlations of our findings remain limited. The patients with A–T demonstrated an atypical pattern of gravity-dependent modulation of vertical ocular drift. As we studied only two A–T patients, it remains unclear whether this pattern is generally found in A–T or not. Further studies in a larger number of A–T patients are needed to clarify on this point. Marked cerebellar atrophy – being most profound in the vermis – is considered a hallmark sign in A–T (Farina et al., 1994). Clinically, the two A–T patients studied fit this picture well; however, imaging was obtained in one only and dates back >10 years, not reflecting the progressive clinical course since then. Clearly, up-to-date MR-imaging would help correlating the clinical findings with the presumed cerebellar structural changes. However, due to head tremor MR-imaging in A–T is often complicated and of unsatisfactory quality.
Conclusion
Despite these limitations, we see important implications of this study both with regards to a better understanding of self-orientation relative to gravity in degenerative cerebellar disease and to the clinical evaluation of patients with vestibulo-cerebellar disease. Compared to patients with acute unilateral cerebellar lesions, patients with slowly progressive diffuse degenerative vestibulo-cerebellar disease do not present with offsets of their internal estimates of direction of gravity, however, as a surrogate of impaired cerebellar function, their ability to control for variability of motor commands and otolith-based internal estimates is impaired. Considering the relatively intact performance in various paradigms tested here and the slowly progressive nature of the patients underlying cerebellar diseases, central adaptation, and reweighting (Angelaki et al., 2009) of multisensory (e.g., vestibular, visual, proprioceptive) input used to obtain internal estimates of direction of gravity may have – at least partially – compensated for the deficient vestibulo-cerebellar processing. Our findings propagate an assessment of the SVV in patients with gait ataxia or other clinical signs suggestive for vestibulo-cerebellar disease with a focus on determining the patient’s ability to precisely estimate vertical. Measuring SVV is implemented widely and can be even performed at the bedside (Zwergal et al., 2009). Therefore the SVV seems a promising tool to monitor disease progression and could also be used as an outcome parameter in treatment.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank David S. Zee for critically reading the manuscript and Albert Züger for technical assistance. This work was supported by the Swiss National Science Foundation (3200B0-105434); the Betty and David Koetser Foundation for Brain Research, Zurich, Switzerland; the Boehringer Ingelheim Fonds Foundation; the Human Frontier’s Science Program; the Ataxia–Telangiectasia Society (UK); the Ataxia–Telangiectasia Children’s Project; the Bonizzi-Theler Foundation; and the Center of Integrative Human Physiology, University of Zurich, Switzerland. None of these sponsors was involved in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
References
Aickin, M., and Gensler, H. (1996). Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am. J. Public Health 86, 726–728.
Angelaki, D. E., Gu, Y., and DeAngelis, G. C. (2009). Multisensory integration: psychophysics, neurophysiology, and computation. Curr. Opin. Neurobiol. 19, 452–458.
Angelaki, D. E., Shaikh, A. G., Green, A. M., and Dickman, J. D. (2004). Neurons compute internal models of the physical laws of motion. Nature 430, 560–564.
Baier, B., Bense, S., and Dieterich, M. (2008). Are signs of ocular tilt reaction in patients with cerebellar lesions mediated by the dentate nucleus? Brain 131, 1445–1454.
Bockisch, C. J., and Haslwanter, T. (2001). Three-dimensional eye position during static roll and pitch in humans. Vision Res. 41, 2127–2137.
Collewijn, H., Van der Steen, J., Ferman, L., and Jansen, T. C. (1985). Human ocular counterroll: assessment of static and dynamic properties from electromagnetic scleral coil recordings. Exp. Brain Res. 59, 185–196.
Farina, L., Uggetti, C., Ottolini, A., Martelli, A., Bergamaschi, R., Sibilla, L., Zappoli, F., Egitto, M. G., and Lanzi, G. (1994). Ataxia–telangiectasia: MR and CT findings. J. Comput. Assist. Tomogr. 18, 724–727.
Green, A. M., Shaikh, A. G., and Angelaki, D. E. (2005). Sensory vestibular contributions to constructing internal models of self-motion. J. Neural Eng. 2, S164–S179.
Ilg, W., Synofzik, M., Brotz, D., Burkard, S., Giese, M. A., and Schols, L. (2009). Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology 73, 1823–1830.
Kim, H. A., Lee, H., Yi, H. A., Lee, S. R., Lee, S. Y., and Baloh, R. W. (2009). Pattern of otolith dysfunction in posterior inferior cerebellar artery territory cerebellar infarction. J. Neurol. Sci. 280, 65–70.
Klockgether, T. (2008). The clinical diagnosis of autosomal dominant spinocerebellar ataxias. Cerebellum 7, 101–105.
Lee, H., Lee, S. Y., Lee, S. R., Park, B. R., and Baloh, R. W. (2005). Ocular tilt reaction and anterior inferior cerebellar artery syndrome. J. Neurol. Neurosurg. Psychiatr. 76, 1742–1743.
Leigh, R. J., and Zee, D. S. (2006). The Neurology of Eye Movements, 4th Edn. New York: Oxford University Press.
Liao, K., Walker, M. F., and Leigh, R. J. (2008). Abnormal vestibular responses to vertical head motion in cerebellar ataxia. Ann. Neurol. 64, 224–227.
Marti, S., Palla, A., and Straumann, D. (2002). Gravity dependence of ocular drift in patients with cerebellar downbeat nystagmus. Ann. Neurol. 52, 712–721.
Marti, S., Tarnutzer, A. A., Schuknecht, B., and Straumann, D. (2008). Dissociation between canal- and otolithfunction in cerebellar atrophy. J. Neurol. 255, 769–771.
Mossman, S., and Halmagyi, G. M. (1997). Partial ocular tilt reaction due to unilateral cerebellar lesion. Neurology 49, 491–493.
Paige, G. D., and Seidman, S. H. (1999). Characteristics of the VOR in response to linear acceleration. Ann. N. Y. Acad. Sci. 871, 123–135.
Palla, A., Bockisch, C. J., Bergamin, O., and Straumann, D. (2006). Dissociated hysteresis of static ocular counterroll in humans. J. Neurophysiol. 95, 2222–2232.
Robinson, D. A. (1976). Adaptive gain control of vestibuloocular reflex by the cerebellum. J. Neurophysiol. 39, 954–969.
Shaikh, A. G., Green, A. M., Ghasia, F. F., Newlands, S. D., Dickman, J. D., and Angelaki, D. E. (2005). Sensory convergence solves a motion ambiguity problem. Curr. Biol. 15, 1657–1662.
Straumann, D. (1991). Off-line computing of slow-phase eye velocity profiles evoked by velocity steps or caloric stimulation. Int. J. Biomed. Comput. 29, 61–65.
Straumann, D., Zee, D. S., Solomon, D., Lasker, A. G., and Roberts, D. C. (1995). Transient torsion during and after saccades. Vision Res. 35, 3321–3334.
Tarnutzer, A. A., Marti, S., and Straumann, D. (2008). Gravity perception in cerebellar patients. Prog. Brain Res. 171, 369–372.
Walker, M. F., Tian, J., Shan, X., Tamargo, R. J., Ying, H., and Zee, D. S. (2008). Lesions of the cerebellar nodulus and uvula in monkeys: effect on otolith-ocular reflexes. Prog. Brain Res. 171, 167–172.
Werner, S., Bock, O., and Timmann, D. (2009). The effect of cerebellar cortical degeneration on adaptive plasticity and movement control. Exp. Brain Res. 193, 189–196.
Westheimer, G., and Blair, S. M. (1975). The ocular tilt reaction – a brainstem oculomotor routine. Invest. Ophthalmol. 14, 833–839.
Wiest, G., Tian, J. R., Baloh, R. W., Crane, B. T., and Demer, J. L. (2001). Otolith function in cerebellar ataxia due to mutations in the calcium channel gene CACNA1A. Brain 124, 2407–2416.
Wong, A. M., and Sharpe, J. A. (2005). Cerebellar skew deviation and the torsional vestibuloocular reflex. Neurology 65, 412–419.
Xu-Wilson, M., Chen-Harris, H., Zee, D. S., and Shadmehr, R. (2009). Cerebellar contributions to adaptive control of saccades in humans. J. Neurosci. 29, 12930–12939.
Yakusheva, T. A., Shaikh, A. G., Green, A. M., Blazquez, P. M., Dickman, J. D., and Angelaki, D. E. (2007). Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron 54, 973–985.
Zee, D. S., Yee, R. D., Cogan, D. G., Robinson, D. A., and Engel, W. K. (1976). Ocular motor abnormalities in hereditary cerebellar ataxia. Brain 99, 207–234.
Zhou, W., Tang, B. F., Newlands, S. D., and King, W. M. (2006). Responses of monkey vestibular-only neurons to translation and angular rotation. J. Neurophysiol. 96, 2915–2930.
Keywords: cerebellar degeneration, subjective visual vertical, ocular counterroll, spontaneous vertical deviation, otoliths
Citation: Tarnutzer AA, Shaikh AG, Palla A, Straumann D and Marti S (2011) Vestibulo-cerebellar disease impairs the central representation of self-orientation. Front. Neur. 2:11. doi:10.3389/fneur.2011.00011
Received: 18 November 2010;
Accepted: 13 February 2011;
Published online: 28 February 2011.
Edited by:
Herman Kingma, Eindhoven University of Technology, NetherlandsReviewed by:
Bernhard Baier, University Hospital of Mainz, GermanyBarry M. Seemungal, Imperial College London, UK
Copyright: © 2011 Tarnutzer, Shaikh, Palla, Straumann and Marti. This is an open-access article subject to an exclusive license agreement between the authors and Frontiers Media SA, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Alexander A. Tarnutzer, Vestibulo-Ocularmotor Lab, Department of Neurology, University Hospital Zurich, Frauenklinikstr. 26, 8091 Zurich, Switzerland. e-mail: alexander. tarnutzer@access.uzh.ch
