Metal and complementary molecular bioimaging in Alzheimer's disease
- 1Faculty of Medicine, Centre for Healthy Brain Ageing, School of Psychiatry, University of New South Wales, Sydney, NSW, Australia
- 2Bioanalytical Mass Spectrometry Facility, Mark Wainwright Analytical Centre, University of New South Wales, Sydney, NSW, Australia
- 3Faculty of Medicine, School of Medical Sciences, University of New South Wales, Sydney, NSW, Australia
- 4Solid State and Elemental Analysis Unit, Mark Wainwright Analytical Centre, University of New South Wales, Sydney, NSW, Australia
- 5Faculty of Biological Sciences, Centre for Ageing and Regeneration, P. Catholic University of Chile, Santiago, Chile
- 6Euroa Centre, Neuropsychiatric Institute, Prince of Wales Hospital, Sydney, NSW, Australia
Alzheimer's disease (AD) is the leading cause of dementia in the elderly, affecting over 27 million people worldwide. AD represents a complex neurological disorder which is best understood as the consequence of a number of interconnected genetic and lifestyle variables, which culminate in multiple changes to brain structure and function. These can be observed on a gross anatomical level in brain atrophy, microscopically in extracellular amyloid plaque and neurofibrillary tangle formation, and at a functional level as alterations of metabolic activity. At a molecular level, metal dyshomeostasis is frequently observed in AD due to anomalous binding of metals such as Iron (Fe), Copper (Cu), and Zinc (Zn), or impaired regulation of redox-active metals which can induce the formation of cytotoxic reactive oxygen species and neuronal damage. Metal chelators have been administered therapeutically in transgenic mice models for AD and in clinical human AD studies, with positive outcomes. As a result, neuroimaging of metals in a variety of intact brain cells and tissues is emerging as an important tool for increasing our understanding of the role of metal dysregulation in AD. Several imaging techniques have been used to study the cerebral metallo-architecture in biological specimens to obtain spatially resolved data on chemical elements present in a sample. Hyperspectral techniques, such as particle-induced X-ray emission (PIXE), energy dispersive X-ray spectroscopy (EDS), X-ray fluorescence microscopy (XFM), synchrotron X-ray fluorescence (SXRF), secondary ion mass spectrometry (SIMS), and laser ablation inductively coupled mass spectrometry (LA-ICPMS) can reveal relative intensities and even semi-quantitative concentrations of a large set of elements with differing spatial resolution and detection sensitivities. Other mass spectrometric and spectroscopy imaging techniques such as laser ablation electrospray ionization mass spectrometry (LA ESI-MS), MALDI imaging mass spectrometry (MALDI-IMS), and Fourier transform infrared spectroscopy (FTIR) can be used to correlate changes in elemental distribution with the underlying pathology in AD brain specimens. Taken together, these techniques provide new techniques to probe the pathobiology of AD and pave the way for identifying new therapeutic targets. The current review aims to discuss the advantages and challenges of using these emerging elemental and molecular imaging techniques, and highlight clinical achievements in AD research using bioimaging techniques.
Introduction
Alzheimer's disease (AD) is the most common progressive age-related neurodegenerative disorder, affecting about 2% of the population in the developed world (Mattson, 2004). Clinically, AD is characterized by devastating effects such as memory loss and decline in other cognitive abilities resulting in loss of independent functioning (Teri et al., 1989; Baddeley et al., 1991; Terry et al., 1991). Pathologically, AD is characterized by two main pathological hallmarks. These include extracellular amyloid plaques composed of insoluble amyloid beta (Aβ) protein produced by irregular cleavage of the amyloid precursor protein (APP), and intra-neuronal neurofibrillary tangles (NFTs) containing hyperphosphorylated tau protein (Khachaturian, 1985; Joachim et al., 1987; Selkoe et al., 1987; Mirra et al., 1991; Brun and Englund, 2002). Although the exact function of Aβ and APP remains unclear, recent studies suggest that APP may play a crucial role in modulating neuronal survival, neurite outgrowth, synaptic plasticity and cell adhesion (Mattson, 1997). NFTs are not restricted to AD, and are also present in other neurodegenerative diseases such as fronto-temporal dementia (Filley et al., 1994).
AD is a complex multifactorial disorder associated with irregular protein aggregation (Pimplikar et al., 2010). Interestingly, accumulation of Aβ protein has been observed in cognitively normal brain, and sometimes an absence of Aβ deposits has been noted in some postmortem in patients who had been clinically diagnosed with AD (Edison et al., 2007). Moreover, various pathobiological mechanisms that are un-related to amyloid accumulation have been associated with the development and progression of AD. For instance, familial mutations in APP and presenilin-1 have been shown to induce autophagic dysfunction and impaired lysosomal proteolysis, cerebral hypoperfusion, and AD (Lee et al., 2010; Pimplikar et al., 2010; Wong and Cuervo, 2010). Furthermore, excess or deficiency in several nutritional, environmental or genetic factors may also potentiate AD-like pathology, making the etiology of this debilitating disorder difficult to elucidate (Russ et al., 2012).
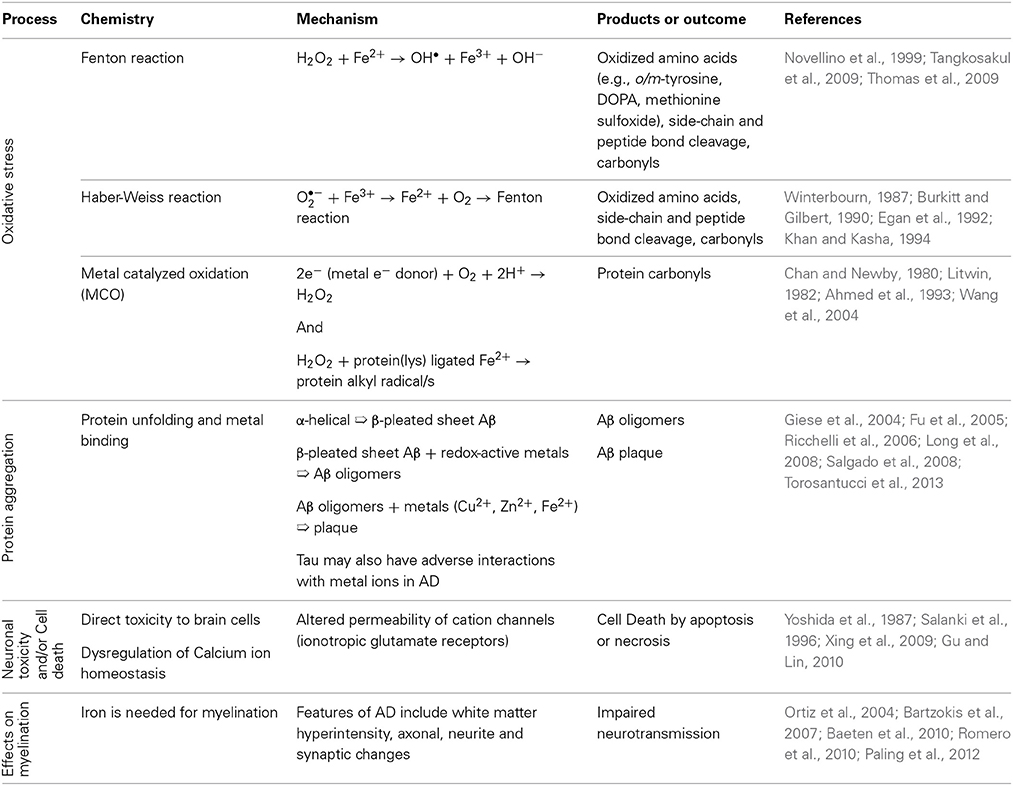
Metals have a diversity of roles in medical biology encompassing both health and disease states (Olanow and Arendash, 1994; Oteiza et al., 2004; Farina et al., 2013; Jellinger, 2013; Grubman et al., 2014). Metals such as lead and mercury cause well established neuropathologies. By contrast several types of metal ions, such as potassium, sodium and calcium are vital for normal nerve cell function. Several other metals (copper, zinc, iron, magnesium, manganese, cobalt) have functional roles in enzymes and proteins (Yokel, 2006; Molina-Holgado et al., 2007; Farina et al., 2013). For example, brain iron is used by lipid and cholesterol synthesizing enzymes (Bartzokis, 2004) and up to 70% of brain iron is found in association with myelin (de los Monteros et al., 2000; Bartzokis, 2004). However, the careful control of metal ion compartmentalization and usage in the brain is critical, so that metal associated toxicity is avoided. The etiology of several neuropathologies includes a dysfunctional association between otherwise important trace elements and particular proteins or peptides (Table 1). Consequently the pathophysiology of metal-protein interactions in neurodegenerative diseases generally and in AD specifically is an area of growing interest. Divalent metal cations accumulate in plaque deposits and the inflammatory and oxidative processes which are well documented in AD may be mediated through chemistries involving metals (Table 2). However, the biochemistry of metal-protein interactions, sources of accumulating metals and chelation mechanisms are yet to be fully explored in AD.
The toxicity of Aβ is linked to changes in its structure from the soluble α-helical form to the insoluble β-pleated sheet form with consequent plaque formation, in which metals such as copper, zinc and iron are sequestered (Lovell et al., 1998a). It is not clear what molecular events trigger plaque formation, a process which may begin much earlier in life than the clinical symptoms of AD (Almkvist and Winblad, 1999). However, dissolution of plaque with metal chelating agents such as clioquinol is a potential new treatment (Cherny et al., 2000, 2001), highlighting the significant role that metals play in the etiology of this disease (Richardson, 2004).
Metal ions, such as those sequestered in plaques, also participate in oxidation and free radical production (Figure 1) (Multhaup et al., 1996). These processes are well documented in AD as are inflammatory processes, mediated by the presence of activated microglia and astrocytes, which generate high levels of Aβ (Busciglio et al., 1993). Metals such as copper, zinc, iron and aluminum have been implicated as possible contributors to neurodegenerative processes. In a few cases, well established links between metals and the function of specific proteins have been demonstrated (Table 1). However, as a subset of all the proteins studied in neuropathology, the metalloproteins are under-represented (Dobson, 2001). Since metal containing active sites of proteins are often involved in oxidation reactions and/or free radical generation, alterations to their biochemistry may be of particular interest in neurodegenerative conditions. Links between protein dysfunction and the role of metals in AD are emerging; (i) divalent metal cations are sequestered in Aβ plaques, (ii) oxidative processes are well documented in AD and metal cations, particularly iron, are a potential source of reactive species. Though metals are likely to play a significant role in AD and other inflammatory diseases, relatively little is known about their sources, mechanisms of transport and chelation, biochemistry and interactions with proteins.
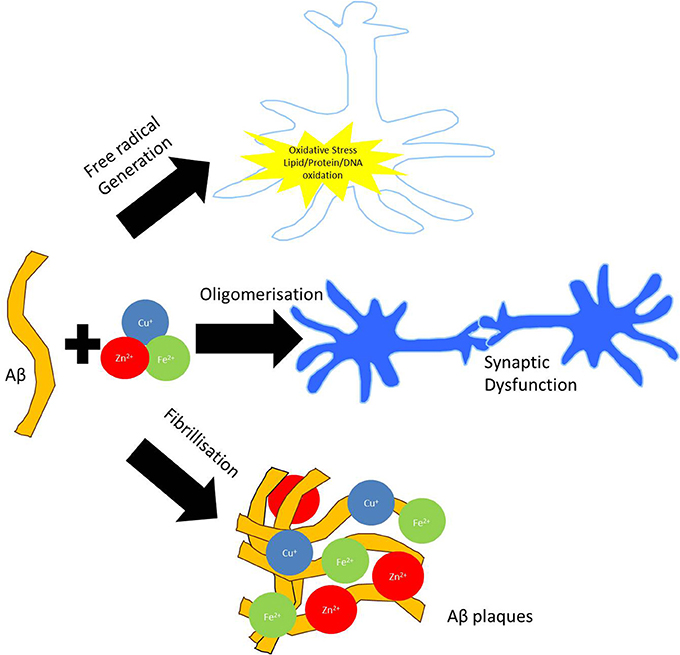
Figure 1. Involvement of metal dyshomeostasis in AD pathology. Aggregation of Aβ can bind redox active metals such as copper, iron, and zinc in amyloid plaques. Sequestration of these biometals on Aβ fibrils and oligomers can potentiate synaptic dysfunction. Redox cycling of Cu2+/Cu+ and Fe3+/Fe2+ in the amyloid plaques are capable of producing hydrogen peroxide (H2O2), which can enter the cell. Through Fenton chemistry this can lead to the production of hydroxyl radical (OH•) capable of inducing oxidative modifications to both extracellular (i.e., proteins and lipids) as well as intracellular (DNA) macromolecules.
Apart from redox active metals associated with the pathological hallmarks of AD, the presence of other trace metals may also be related to impaired cognitive function in AD. Several toxic heavy metals, including arsenic, lead, mercury, and cadmium are present in the environment due to their high industrial demand (Park et al., 2014). These metals serve no biological function, and their accumulation in the brain is attributed to contact between humans and the environment (Chowdhury and Chandra, 1987). Exposure to arsenic induces neuropathological and behavioral abnormalities similar to clinical features reported in AD and other related neurodegenerative disorders (Gong and O'Bryant, 2010). Lead, which is a well-established neurotoxic pollutant, can induce tau hyperphosphorylation, white matter degeneration, cellular apoptosis, and changes in cellular morphology, and impaired neuronal function (Yun and Hoyer, 2000; Rahman et al., 2012). While cadmium can induce hepatic and renal toxicity, cadmium and lead can also disrupt cholinergic transmission by reducing the turnover of the essential neurotransmitter, acetylcholine (Webster and Valois, 1981; Costa and Fox, 1983; Patra et al., 1999; Singh et al., 2012b). Inorganic mercury can mimic all the pathological hallmarks of AD in animal models (Saxe et al., 1999; Rusina et al., 2006; Mutter et al., 2010). Under normal physiological conditions, sequestration of arsenic, lead, cadmium, and mercury by the lateral choroid plexus represents a protective mechanism to prevent the influx of heavy metals from the blood and into the brain. However, elevated levels of cadmium and mercury can directly damage the choroid plexus, thus limiting the function of this endogenous defense mechanism (Gerhardsson et al., 2011). The toxicity of these metals in human neurodegenerative disorders is dependent on the concentration of the environmental contaminant, and chronic exposure to heavy metals can induce toxicity at relatively low levels (Llobett et al., 2003).
The presence of sequestered biometals such as copper, zinc, and iron in β-amyloid plaques of AD-affected brain tissue, and the presence of toxicological metals as potential pathological cofactors in AD, has led to a focus on metal imaging (Hutchinson et al., 2005; Lelie et al., 2011; Pithadia and Lim, 2012; Stavitski et al., 2013). We should note that not only metals, but a wide range of elements may be imaged, down to ultratrace levels, and at length scales from micron to tens of nanometers. In certain cases isotopes, and even oxidation state and the coordination environment around specific elements can be imaged, potentially increasing the scope of trace element research in neurological disease beyond what has been studied to date. Visualizing changes in element concentration and matching them to anatomical and pathological features enhances our traditional approach to exploring the role of metal ions in neurological disease. Reviews on metal imaging in neurobiology have been presented recently (Bourassa and Miller, 2012) and a comprehensive range of instrumental techniques is available from McRae et al. (2009). However, the field continues to expand rapidly as spatially resolved elemental analysis is now a well-recognized method to investigate chemical changes associated with pathology in biological tissues. The experimental techniques used to obtain elemental information from tissues are quite diverse, with a range of different capabilities in spatial resolution, sensitivity and quantification. This review provides an overview of common instrumental techniques and examples of biological imaging with an emphasis on Alzheimer's studies. Elemental imaging is the main topic of this review; although a selection of molecular imaging examples are presented to demonstrate how these techniques can supplement the elemental bioimaging. Selective colorimetric and fluorescent staining is not covered here, but has been recently reviewed with a focus on neurobiology (Que et al., 2008).
General Overview of Elemental Imaging Techniques for Biological Tissues
A significant challenge in this field is measuring a specific area on a sample that is small enough to remain biologically relevant, but large enough to enable the elements to be detected. When visual imaging is required to match anatomical features with the elemental distribution, the measurement needs to be carefully planned to leave any destructive analysis as the last step. Most of these techniques will damage the sample to an extent, for example radiation damage in the case of synchrotron techniques, or ablation of the sample surface into gas or ions in the case of laser and ion beam sampling.
It can be instructive to present the most common methods for elemental imaging by their sampling modes, as this will influence the achievable spatial resolution and detection limit. The most common techniques can be classified as (a) ablation of material off the surface that is then directed into an elemental analyzer, (b) ion generation within the sample, and (c) ion generation and ablation from the sample surface. Most of these techniques create spatially resolved elemental data by moving a flat specimen on a stage in precise intervals under the incident beam, and recording the change in analyte flux (ion, electron, photon) that is associated with a specific element.
Ablation Techniques
A pulsed laser can be used to ablate material from a selected area of the sample surface and the gaseous plume swept into another instrument for elemental analysis. A laser ablation (LA) sampling accessory can be integrated with a more traditional atomic spectroscopy system for sensitive multi-elemental analysis. These are usually inductively coupled plasma (ICP) systems using mass spectrometry (LA-ICPMS), or optical emission spectroscopy (LA-ICPOES) for detection of the elements (Qin et al., 2011). Mass spectrometry has the advantage of higher detection limits than optical emission spectroscopy, however there are drawbacks with mass interferences, and the time taken to sweep through the selected ion set, resulting in fewer available ions in order to create an image within a practical length of time. For example, imaging 6 metals across a 4 × 4 mm tissue section with a step size of 30 micron might take 12–24 h (Ketola and Mauriala, 2012). Nevertheless, LA-ICPMS is by far the more common technique for elemental imaging of biometals and toxicological metals in tissues than LA-ICPOES. Another variation on laser sampling is to detect the atomic excitation spectrum directly from the ablated plume, a technique known as laser induced breakdown spectroscopy (LIBS) (Pareja et al., 2013). LA techniques provide excellent analytical sensitivity in atmospheric or relatively low vacuum conditions. However, it is a destructive technique, and delivering sufficient energy to the sample to allow detection tends to limit the spatial resolution. As a result, LA techniques are well suited for analysis of whole tissue sections, but individual cells or pathological features such as amyloid plaques ~20 micron are represented in an image as a single measured point (Hare et al., 2010, 2011; Lear et al., 2012; Chou et al., 2014). Metal imaging of an individual cell or plaque requires the higher resolution available from some of the techniques described below.
Sample Ionization Techniques
Highly focused X-rays, electrons, or proton ion beams in a high-vacuum chamber can be used to eject an electron from the core shell of an atom in the sample (Fahrni, 2007). The energy of the ejected electron can be measured using X-ray photoelectron spectroscopy (XPS) to determine the element from which it originated. In certain cases, XPS is able to provide information on oxidation states and the chemical environment around an element, although spatial resolution is limited to 5–50 micron, and detection limits are relatively poor (around 0.1 atomic%), virtually ruling out the technique for trace metal studies (Paunesku et al., 2006). The majority of sample ionization techniques utilize the secondary process where outer shell electrons fill the core shell hole in the ion fluorescing X-rays with a characteristic wavelength for each element in the sample. When atoms are ionized using an electron beam, usually in an electron microscope, the technique is known as energy dispersive X-ray spectroscopy (EDX or EDS), sometimes referred to as electron photon micro analysis (EPMA). If ionization is achieved using an X-ray beam, the technique is X-ray fluorescence microscopy (XFM) or synchrotron radiation micro-X-ray fluorescence (SR-μXRF) (Paunesku et al., 2006; Ralle and Lutsenko, 2009). Ionization can also be performed using a focused beam of protons in a technique called particle induced X-ray emission (PIXE). All of these techniques are performed in high-vacuum environments, so steps such as cryopreservation or careful drying must be taken to protect biological samples or specimen degassing that can reduce the performance of the instrument (de Silva et al., 2006; George et al., 2011; Ramsay et al., 2011; Weekley et al., 2013).
Secondary Ionization Techniques
Ablation-ionization directs a highly focused beam of ions, such as oxygen or cesium in the case of secondary ionization mass spectrometry (SIMS), onto a tissue surface under vacuum (Altelaar and Piersma, 2010). This is a destructive process that results in ions being ejected from the surface. The ions are usually detected with a magnetic sector (NanoSIMS) or time-of-flight (TOF SIMS) mass spectrometer (Pacholski and Winograd, 1999; Eller et al., 2013; Fernandez-Lima et al., 2013). A recent review is available detailing the general capabilities of mass spectrometry-ablation techniques such as SIMS (Amstalden van Hove et al., 2010). The ability to focus ion beams down to very small spot sizes enables excellent spatial resolution, with features of 50 nanometers having been reported in the case of the NanoSIMS. However, micron to submicron imaging is more common since, in order to generate sufficient secondary ions for detection with a very small spot size, the ablation depth needs to increase. Submicron imaging at hundreds of nanometers is more common, and is sufficient for cellular differentiation or observing small pathological features (Quintana et al., 2007; Musat et al., 2012). It is notable that the mass spectrometry techniques also enable more specialized imaging of isotopes across a surface, as well as providing more general elemental imaging.
Other Techniques
Electron energy loss spectroscopy (EELS) measures the energy loss due to scattering processes when a low energy, monoenergetic electron beam interacts with a sample. When used in a transmission electron microscope, EELS can provide atomic-scale resolution with excellent detection limits although biological applications are limited (Quintana et al., 2000; Terada et al., 2002). There are a variety of X-ray techniques that have evolved as a result of the high-intensity X-ray sources available at numerous synchrotron facilities around the world. X-ray Absorption Near Edge Structure (XANES), also known as Near edge X-ray absorption fine structure (NEXAFS), is a technique where the element composition change the absorption spectrum of the X-ray beam, providing information on elemental oxidation state and coordination geometry around metal ions (Bourassa and Miller, 2012). Although potentially powerful, imaging of biological materials using this technique is still in development.
Magnetic resonance imaging (MRI) remains the most widely used metal imaging technique in the clinical setting (Helpern et al., 2004). Although recent advances in MRI have made it possible to detect the levels of iron at physiological concentrations, copper and manganese are still not widely detectable, since they are present in low concentrations in the brain (Schenck and Zimmerman, 2004). Current MRI techniques exhibit lower spatial resolution compared to elemental imaging techniques mentioned above, but demonstrate the advantage of imaging live patients rather than cryo-cut postmortem tissue sections (Schenck and Zimmerman, 2004).
Positron emission tomography (PET) is another technique which facilitates in vivo medical imaging, usually of small molecules including glucose and more recently Aβ plaques using Pittsburg Compound B (PiB PET). More recently a novel metal imaging PET approach has been developed, using radioactive coordination bis(thiosemicarbazonato)copper complex of 64Cu. This targets copper homeostasis and has been designed to bind selectively to amyloid plaques (Hickey et al., 2013). Copper radiolabels are essential for increasing our understanding on of the mechanisms of copper dyshomeostasis in AD.
Combined Bioimaging Techniques in Tissue Sections
Complementary information regarding the role, uptake, transport, and storage of redox active metals associated with irregular protein abnormalities can be obtained using a combination of elemental imaging techniques, such as LA-ICPMS, and other biomolecular mass spectrometry imaging techniques such as laser ablation coupled with electrospray ionization mass spectrometry (LA-ESI-MS) or MALDI-IMS. While LA-ICPMS can be employed to identify the specific protein-bound metals, ESI-MS/MALDI enables the identification of the structure, dynamics and biological function of metal-protein complexes (Becker et al., 2008; Dobrowolska et al., 2008; Jakubowski et al., 2008; Wu et al., 2009).
ESI-MS is an ionization technique that is employed to detect polar compounds within a biological specimen (Fenn et al., 1989). This method is used to identify molecules that do not contain an intrinsic ionizable site through formation of adduct ions. Molecules which exhibit sufficient dipole potential to interact with a small anion or cation can be readily ionized and detected using ESI-MS. It is useful for the detection of triacylglycerols (TAGs) which contain long chain fatty acids. These molecules can be ionized and quantified with sensitivity in the low picomole range due to the formation of lithiated adducts which are formed when chelated lithium ions non-covalently bond with the carbonyl structures that are present in the infused solution (Han et al., 2000; Han and Gross, 2001). The benefits of using ESI-MS include more accurate quantification of lipid classes and subclasses, a greater signal-to-noise ratio in comparison to other mass spectrometry techniques, and an almost linear relationship between the relative intensities of molecular ions and the mass of individual lipids (Han and Gross, 1994).
MALDI-IMS allows the analysis of a diversity of biopolymers with a variety of mass ranges. This approach has a lower spatial resolution but much higher mass range than TOF-SIMS, which is limited to identification of analytes with a molecular mass of less than 1 kDa (McArthur et al., 2004). A variety of analytes can be examined using MALDI-IMS, including metabolites, lipids, proteins, peptides, carbohydrates, and drugs. However, this method is limited by signal suppression effects. For instance, some analytes are more efficiently ionized during MALDI-IMS. These artifacts are not only due to their unique chemical structure, but also to relative amounts present in the biological tissue (Knochenmuss et al., 1998). Alternatively, proteins can be extracted from the tissue section using hydrophobic materials, while preserving their specific location (Chaurand et al., 2004). Adaptation of MALDI-TOF to 2D and even 3D tissue imaging applications has necessitated use of rapid fire long lived lasers, such as the 2 kHz Nd-YAG, to accommodate the need to acquire 1000s of spectra across a tissue section. High end MALDI imaging mass spectrometers currently combine high mass resolution of 40,000 (1 ppm mass accuracy), wider mass range (50–300,000 Da), spatial resolution down to 10 μm, and TOFTOF capabilities for peptide sequencing. This combination of features allows detailed characterization of a diversity of tissue constituents, top-down sequencing of proteins as well as the more commonly used bottom-up techniques involving enzymatic/tryptic digestion and peptide sequencing, analysis of posttranslational modifications such as glycosylation. A growing body of literature recognizes the power of this approach (Cornett et al., 2007; Schuerenberg et al., 2007). A combination of mass spectrometry imaging techniques using LA-ICPMS and detailed proteomics analysis can be performed using thin cryo-cut sections of brain. MALDI-IMS is a relatively non-destructive technique so the tissue remaining after initial proteomic, metabolomic or lipidomic analysis can then be analyzed for elemental composition using LA-ICPMS.
Fourier transform infrared spectroscopy (FTIR) is another molecular imaging tool that can be combined with LA-ICPMS. These tools have been used to image the secondary structure of metal-protein complexes (Haris and Severcan, 1999). FTIR is a non-destructive technique, allowing further analyses to provide complementary information and to show spatial relationships between diverse analytes and/or functional groups, which may provide insight into biological/functional relatedness. The protein's FTIR consists of two main features: the Amide I band (~1650 cm−1) which arises from the C=O stretching vibration, and the Amide II band (~1540 cm−1) which is due to the N-H bending and C-N stretching vibrations of the peptide backbone. The vibrational frequency of an aggregated protein is about 1620–1625 cm−1, owing to its hydrophobic environment (Goormaghtigh et al., 2006; Miller et al., 2006). Apart from examining the protein structure in vitro, FTIR can also be used to directly investigate irregular protein misfolding and aggregation both in vitro and in vivo. Protein aggregates are generally small, ranging from nanometers, to 20–30 μm for larger aggregates. As well, the spectral differences related to changes to protein conformation are subtle, requiring spectra with high signal to noise ratio (Choo et al., 1996; Miller et al., 2006). These difficulties have been resolved using the greater brightness of a synchrotron infrared source to directly assess protein aggregation and misfolding in AD tissue.
Recent Applications of Bioimaging in Alzheimer's Research
Metals have been shown to be associated with the pathogenesis of AD for over 50 years since the discovery of significant iron deposition in postmortem AD brain tissue using Prussian blue stain (Goodman, 1953). Since then, other redox active metals have been implicated in AD, including copper, zinc, and aluminum. Several metal bioimaging strategies have been utilized to examine the distribution of metals in human clinical AD brain tissue and AD mouse models to better understand the relationship between metal dyshomeostasis and the etiology and progression of AD.
Metals and Aβ Plaques
It has been well established that Aβ plaques are rich in metal ions (Opazo et al., 2002). These relatively high concentrations of metals within the plaques compared to adjacent tissue have been reaffirmed using a variety of bioimaging techniques. PIXE and XFM has been used to show that both the outer and central regions of the Aβ plaques contain elevated levels of iron, copper and zinc in human AD brain specimens (Lovell et al., 1998a,b). Although copper and zinc binding sites are present on the Aβ peptide, iron does not appear to directly interact with Aβ (Atwood et al., 2000; Bush, 2003; Roberts et al., 2012). Recently, synchrotron X-ray absorption, diffraction, and tomography techniques have been used to identify the presence of biogenic magnetite and/or maghematite in the plaque cores, implicating the likely role of a novel biomineralization process to account for the accumulation of iron in Aβ plaques (Collingwood et al., 2005, 2008).
Transgenic mouse models have provided additional advantages over postmortem human clinical AD specimens in the control of both genetics and onset of AD-like symptoms. Using XFM, no abnormal increase in copper or iron were reported in with disease progression in the PSAPP double transgenic mouse which expresses a chimeric mouse/human amyloid precursor protein (Mo/HuAPP695swe) and a mutant human presenilin 1 (PS1-dE9) both directed to CNS neurons. This mouse model develops amyloid pathology as well as learning and memory deficits by 6 months of age, independent of signs of neurodegeneration (Leskovjan et al., 2011). Moreover, only a slight upregulation in zinc concentrations was reported at the late stages of the disease. By contrast, the CRND mouse which expresses two familial mutations in the human Swedish (K595N/M596L) and Indiana (V717F) APP gene exhibited a 2–3-fold increase in the concentration of iron, copper, and zinc in the plaques after 6 months of age using PIXE. This unique mouse model develops diffuse and compact plaques by 10 weeks of age and Aβ deposition continues with advanced age (Rajendran et al., 2009). Similar findings have been reported using LA-ICPMS analysis of plaques present in the brains of TASTPM mice, which carry both the APP K670N/M671L and PS1M146V mutation and develop plaques by 4 months of age (Hutchinson et al., 2005).
Metal Dyshomeostasis in Aging and AD
Since ageing is a major risk factor for the development of AD, examining the age-related changes in metal distribution is critical for understanding the role that metals play during pathological and physiological conditions. Using LA-ICPMS, one study showed that iron levels were increased in the “physiologically” aged brain of a non-transgenic mice (14 months) compared to a young (2 month) mice (Becker et al., 2010). These increases were observed in the substantia nigra, thalamus, and the CA1 region of the hippocampus which are associated with development of neuropathologies. Remarkably, zinc levels remained unchanged and zinc-enrichment in the CA3 of the hippocampus was already detected in young mice. This may be associated with the important role of zinc as an essential neuro-co-transmitter that is released from synaptic vesicles (Becker et al., 2010).
Evidence of metal dyshomeostasis has also been reported in AD. Studies using PIXE have shown increased levels of zinc in the amygdala, hippocampus and neuropils of human AD brains (Danscher et al., 1997; Lovell et al., 1998a,b). This is likely to be associated with the increased distribution of zinc enriched neurons (ZEN) which are located in these regions. ZENs maintain intracellular pools of zinc which is necessary as a neuromodulator and neuro-co-transmitter. One hypothesis suggests that zinc released from these neurons can interact with Aβ and promote aggregation (Bush et al., 1994; Frederickson et al., 2005). Zinc deficiency can also lead to excitotoxicity and neurodegeneration (Sensi et al., 2009). Moreover, zinc reuptake is an energy dependent process, and mitochondrial dysfunction can lead to increased free zinc which can interact with Aβ and lead to further neurotoxicity (Mony et al., 2009).
Altered iron levels have also been suggested to play a prominent role in ageing and AD. Iron levels have been shown to increase in the substantia nigra, motor rotex, hippocampus, basal ganglia, putamen, cerebellum and cortex of human normal subjects during ageing (Connor et al., 1992; Deibel et al., 1996; Bartzokis et al., 2000). A similar increase was also reported iron, copper and zinc content was also reported in the PSAPP mouse model in the cortex and hippocampus, and coincided with increased plaque formation using XFM (Leskovjan et al., 2011). Ferritin, the main protein responsible for iron storage, has been shown to increase in the coronal region of human AD plaques using TEM and NanoSIMS (Quintana et al., 2006). It is likely that ferritin, which stores inactive iron (III) under normal physiological conditions may bind redox active iron (II) in the AD brain leading to cell death via oxidative stress.
Metals and NFTs
Metal dyshomeostasis may also play a role in the formation of NFTs. A 10-fold increase in iron and a 6-fold increase in copper, with a smaller increase in zinc, have been previously reported in NFTs (Morawski et al., 2005). Furthermore, hyperphosphorylated tau, which forms paired helical filaments (PHFs) that lead to NFTs, contains several binding domains which demonstrate some affinity to copper, and the presence of copper can enhance the formation of NFTs (Ma et al., 2006). Iron (III) can also induce NFT formation similar to copper (Yamamoto et al., 2003). Apart from copper, iron and zinc, aluminum has also been associated with the development of AD since it was first identified in neurons with NFTs (Perl and Brody, 1980). However, increased aluminum is also present in non-diseased brain tissue fixed with osmium tetroxide, which contains aluminum (Tokutake et al., 1995; Makjanic et al., 1997). Further work is warranted to validate the involvement of aluminum in AD.
Lipidomic Studies Using ESI/MS
ESI-MS techniques have been used to investigate the lipidome in patients with dementia. These studies have demonstrated specific changes to the lipidome in the postmortem gray and white matter in the frontal, temporal and parietal cortex at the earliest clinically-recognizable stage of AD compared to cognitively normal control (Han et al., 2001, 2002). Specifically, plasmenylethanolamine (PlsEtn) mass was reduced by up to 40 mol% of total plasmalogens, in white matter in early AD subjects compared to age-matched controls. PlsEtn mass levels were depleted by 10% in the gray matter in patients with severe AD. Sulfatides, which form specialized components in the myelin sheath which encapsulate neurons, were depleted by 93 and 58 mol% in gray and white matter, respectively, in AD patients in all brain regions that were investigated (Han et al., 2001, 2002). Additionally, a significant increase (>3 fold) in ceramide content was observed in the white matter of all investigated brain regions during early AD. No significant changes have been observed in the levels of other lipid classes, including phosphatidylglycerols, phosphatidylinositols, phosphatidylserines, and phosphatidic acids in early stages of AD although significant reduction (~ 15 mol%) of these lipids occurred in severe AD cases (Han et al., 2001, 2002). Taken together, these results suggest that changes to the lipidome may play a vital role in the pathogenesis of AD and may be associated with early molecular and cellular events which occur in the development of AD, such as neurodegeneration and synaptic dysfunction.
Maldi-MS Imaging in AD
Recently, MALDI-MS has been used to examine the spatial distribution and molecular contents of Aβ plaques. One study showed that Aβ-(1–40) and Aβ-(1–42) are the most abundant amyloid peptides in APP23 transgenic mice encoding the hAPP751 with Swedish mutation (Rohner et al., 2005). In support of this work, other studies have shown that vascular amyloid is primarily composed of Aβ-(1–40) and Aβ-(1–42) (Miller et al., 1993). Additionally, Aβ-(1–40) is the major peptide that is found in aqueous cerebral cortical extracts from AD brains. By contrast, the insoluble amyloid Aβ-(1–42) peptide is primarily localized in the senile plaque cores. Therefore, MALDI-MS can not only be used to identify known targets, but also facilitates mapping of the different peptide targets with high precision and accuracy, that is otherwise not possible when examining whole-brain extracts (Rohner et al., 2005).
FTIR Spectroscopic Imaging in AD
In AD, Aβ plaques are formed by the transformation of Aβ from a soluble form through to an oligomeric intermediate, culminating in the formation of an aggregated, fibrillary structure (Ii, 1995). The molecular mechanism which mediates the structural changes and cytotoxicity of Aβ during the aggregative process has been previously investigated in several in vitro studies using dichroism (CD) and nuclear magnetic resonance (NMR) to show the structural conversion of Aβ from a soluble α-helical protein to a fibrillar β-sheet protein (Barrow et al., 1992; Zhang and Rich, 1997). FTIR spectroscopy has been essential to examine the specific alignment of β-sheet strands within Aβ fibrils. A study by Petty and Decatur (2005) showed that β-sheets are antiparallel and in alignment across all strands (Petty and Decatur, 2005). Recently, it has been suggested that oligomeric Aβ is more neurotoxic than Aβ fibrils and can form pore-like structures in lipid membranes that can disrupt ion homeostasis, culminating in cell death. FTIR spectroscopy has shown that Aβ oligomers exhibit an antiparallel β-sheet structure, which is closely related to that of bacterial outer membrane porins (Komatsu et al., 2007).
Apart from the Aβ protein, FTIR spectroscopy has also been used to gain a greater understanding of the structural conformation of the tau protein, which is hyperphosphorylated in AD, leading to the formation of NFTs. In vitro FTIR analysis provided confirmatory evidence that soluble tau protein is natively unfolded and composed of random coil structures, whilst PHFs which are present in the AD brain have a greater level of β-structure (Berriman et al., 2003). These results provide evidence to support the hypothesis that the repeat domain of tau (which is located within the core of PHFs) displays an enhanced level of β-structure during aggregation, while the N- and C-terminal domains which venture away from the central PHF core are largely random coils (Barghorn et al., 2004).
Conclusion
Metal imaging techniques are currently primed to facilitate an understanding of the pathobiology of AD, as well as identifying novel diagnostics and therapeutics. Bioimaging techniques are important for elucidating the role of metals in neurodegenerative diseases generally and AD in particular. Advancements in methodology and improved spatial resolution and detection sensitivities are essential for greater insight into the localization and distribution of metal ions at the cellular and tissue level, and their role in disease development and progression. A combination of other imaging techniques such as ESI- and MALDI-IMS, FTIR spectroscopy, and clinical techniques allowing in vivo analysis, such as MRI and PET, are invaluable in obtaining further understanding on the molecular mechanisms involved in the pathogenesis of AD and to confirm the diagnosis of AD through the identification of unique biomarkers present in the metabolome, lipidome and/or proteome. Additionally, the techniques described in this review have the potential to follow disease progression in AD patients from early to severe stages and assess the effect of novel therapeutic interventions which may retard, stop or reverse progressive neurodegeneration, the ultimate goal being a cure for this debilitating neurodegenerative disorder.
Author Contributions
Nady Braidy, Christopher Marjo, Anne Poljak, Tharusha Jayasena, Nibaldo C. Inestrosa, and Perminder Sachdev wrote the draft, reviewed and interpreted the bioimages. Helen Rutlidge and Anne Rich processed the images. Nibaldo C. Inestrosa and Perminder Sachdev provided the conceptual foundation of the review, writing of drafts and interpretation of data.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a National Health and Medical Research Council of Australia Capacity Building Grant and a UNSW Faculty of Medicine Research Grant. Nady Braidy is the recipient of an Alzheimer's Australia Viertel Foundation Postdoctoral Research Fellowship, and the NHMRC Early Career Postdoctoral Research Fellowship at the University of New South Wales. Tharusha Jayasena is a recipient of the University of New South Wales Postgraduate Award (UPA). The authors thank the Rebecca Cooper Medical Research Foundation for their ongoing financial support.
References
Ahmed, N., Liggins, J., and Furth, A. J. (1993). Metal-catalysed oxidation and post-Amadori reactions of serum albumin and other model proteins. Biochem. Soc. Trans. 21:93S.
Almkvist, O., and Winblad, B. (1999). Early diagnosis of Alzheimer dementia based on clinical and biological factors. Eur. Arch. Psychiatry Clin. Neurosci. 249(Suppl. 3), 3–9. doi: 10.1007/PL00014171
Altelaar, A. F., and Piersma, S. R. (2010). Cellular imaging using matrix-enhanced and metal-assisted SIMS. Methods Mol. Biol. 656, 197–208. doi: 10.1007/978-1-60761-746-4_11
Amstalden van Hove, E. R., Smith, D. F., and Heeren, R. M. (2010). A concise review of mass spectrometry imaging. J. Chromatogr. A 1217, 3946–3954. doi: 10.1016/j.chroma.2010.01.033
Atwood, C. S., Scarpa, R. C., Huang, X. D., Moir, R. D., Jones, W. D., Fairlie, D. P., et al. (2000). Characterization of copper interactions with Alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta 1-42. J. Neurochem. 75, 1219–1233. doi: 10.1046/j.1471-4159.2000.0751219.x
Baddeley, A. D., Bressi, S., Della Sala, S., Logie, R., and Spinnler, H. (1991). The decline of working memory in Alzheimer's disease. A longitudinal study. Brain 114(pt 6), 2521–2542. doi: 10.1093/brain/114.6.2521
Baeten, K., Adriaensens, P., Hendriks, J., Theunissen, E., Gelan, J., Hellings, N., et al. (2010). Tracking of myelin-reactive T cells in Experimental Autoimmune Encephalomyelitis (EAE) animals using small particles of iron oxide and MRI. NMR Biomed. 23, 601–609. doi: 10.1002/nbm.1501
Barghorn, S., Davies, P., and Mandelkow, E. (2004). Tau paired helical filaments from Alzheimer's disease brain and assembled in vitro are based on beta-structure in the core domain. Biochemistry 43, 1694–1703. doi: 10.1021/bi0357006
Barrow, C. J., Yasuda, A., Kenny, P. T., and Zagorski, M. G. (1992). Solution conformations and aggregational properties of synthetic amyloid beta-peptides of Alzheimer's disease. Analysis of circular dichroism spectra. J. Mol. Biol. 225, 1075–1093. doi: 10.1016/0022-2836(92)90106-T
Bartzokis, G. (2004). Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol. Aging 25, 5–18; author reply 49–62. doi: 10.1016/j.neurobiolaging.2003.03.001
Bartzokis, G., Lu, P. H., Tishler, T. A., Fong, S. M., Oluwadara, B., Finn, J. P., et al. (2007). Myelin breakdown and iron changes in Huntington's disease: pathogenesis and treatment implications. Neurochem. Res. 32, 1655–1664. doi: 10.1007/s11064-007-9352-7
Bartzokis, G., Sultzer, D., Cummings, J., Holt, L. E., Hance, D. B., Henderson, V. W., et al. (2000). In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Arch. Gen. Psychiatry 57, 47–53. doi: 10.1001/archpsyc.57.1.47
Basu, S., Mohan, M. L., Luo, X., Kundu, B., Kong, Q. Z., and Singh, N. (2007). Modulation of proteinase K-resistant prion protein in cells and infectious brain homogenate by redox iron: implications for prion replication and disease pathogenesis. Mol. Biol. Cell 18, 3302–3312. doi: 10.1091/mbc.E07-04-0317
Becker, J. S., Dobrowolska, J., Zoriy, M., and Matusch, A. (2008). Imaging of uranium on rat brain sections using laser ablation inductively coupled plasma mass spectrometry: a new tool for the study of critical substructures affined to heavy metals in tissues. Rapid Commun. Mass Spectrom. 22, 2768–2772. doi: 10.1002/rcm.3673
Becker, J. S., Matusch, A., Palm, C., Salber, D., Morton, K. A., and Becker, S. (2010). Bioimaging of metals in brain tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and metallomics. Metallomics 2, 104–111. doi: 10.1039/b916722f
Berriman, J., Serpell, L. C., Oberg, K. A., Fink, A. L., Goedert, M., and Crowther, R. A. (2003). Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure. Proc. Natl. Acad. Sci. U.S.A. 100, 9034–9038. doi: 10.1073/pnas.1530287100
Binolfi, A., and Fernandez, C. O. (2013). “Interactions of alpha-Synuclein with metal ions: new insights into the structural biology and bioinorganic chemistry of Parkinson's disease,” in Brain Diseases and Metalloproteins, ed D. R. Brown (Pan Stanford Publishing), 327–366. doi: 10.4032/9789814364072
Björkblom, B., Adilbayeva, A., Maple-Grødem, J., Piston, D., Ökvist, M., Xu, X. M., et al. (2013). Parkinson disease protein DJ-1 binds metals and protects against metal-induced cytotoxicity. J. Biol. Chem. 288, 22809–22820. doi: 10.1074/jbc.M113.482091
Bourassa, M. W., and Miller, L. M. (2012). Metal imaging in neurodegenerative diseases. Metallomics 4, 721–738. doi: 10.1039/c2mt20052j
Bowman, A. B., Kwakye, G. F., Hernandez, E. H., and Aschner, M. (2011). Role of manganese in neurodegenerative diseases. J. Trace Elem. Med. Biol. 25, 191–203. doi: 10.1016/j.jtemb.2011.08.144
Brun, A., and Englund, E. (2002). Regional pattern of degeneration in Alzheimer's disease: neuronal loss and histopathological grading. Histopathology 41, 40–55. doi: 10.1046/j.1365-2559.2002.148910.x
Burkitt, M. J., and Gilbert, B. C. (1990). Model studies of the iron-catalysed Haber-Weiss cycle and the ascorbate-driven Fenton reaction. Free Radic. Res. Commun. 10, 265–280. doi: 10.3109/10715769009149895
Busciglio, J., Gabuzda, D. H., Matsudaira, P., and Yankner, B. A. (1993). Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc. Natl. Acad. Sci. U.S.A. 90, 2092–2096. doi: 10.1073/pnas.90.5.2092
Bush, A. I. (2003). The metallobiology of Alzheimer's disease. Trends Neurosci. 26, 207–214. doi: 10.1016/S0166-2236(03)00067-5
Bush, A. I., Pettingell, W. H., Multhaup, G., Paradis, M. D., Vonsattel, J. P., Gusella, J. F., et al. (1994). Rapid induction of Alzheimer a-beta amyloid formation by zinc. Science 265, 1464–1467. doi: 10.1126/science.8073293
Chan, H. W., and Newby, V. K. (1980). Haemoprotein- and transition metal ion-catalysed oxidation of linoleic acid. Selectivity of the position of oxygenation. Biochim. Biophys. Acta 617, 353–362. doi: 10.1016/0005-2760(80)90001-6
Chaurand, P., Schwartz, S. A., Billheimer, D., Xu, B. G. J., Crecelius, A., and Caprioli, R. M. (2004). Integrating histology and imaging mass spectrometry. Anal. Chem. 76, 1145–1155. doi: 10.1021/ac0351264
Cherny, R. A., Atwood, C. S., Xilinas, M. E., Gray, D. N., Jones, W. D., McLean, C. A., et al. (2001). Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30, 665–676. doi: 10.1016/S0896-6273(01)00317-8
Cherny, R. A., Barnham, K. J., Lynch, T., Volitakis, I., Li, Q. X., McLean, C. A., et al. (2000). Chelation and intercalation: complementary properties in a compound for the treatment of Alzheimer's disease. J. Struct. Biol. 130, 209–216. doi: 10.1006/jsbi.2000.4285
Choo, L. P., Wetzel, D. L., Halliday, W. C., Jackson, M., LeVine, S. M., and Mantsch, H. H. (1996). In situ characterization of beta-amyloid in Alzheimer's diseased tissue by synchrotron Fourier transform infrared Microspectroscopy. Biophys. J. 71, 1672–1679. doi: 10.1016/S0006-3495(96)79411-0
Chou, J., Austin, C., Doble, P., Ben-Nissan, B., and Milthorpe, B. (2014). Trace elemental imaging of coralline hydroxyapatite by laser-ablation inductively coupled plasma-mass spectroscopy. J. Tissue Eng. Regen. Med. 8, 515–520. doi: 10.1002/term.1544
Chowdhury, B. A., and Chandra, R. K. (1987). Biological and health implications of toxic heavy metal and essential trace element interactions. Prog. Food Nutr. Sci. 11, 55–113.
Collingwood, J. F., Chong, R. K. K., Kasama, T., Cervera-Gontard, L., Dunin-Borkowski, R. E., Perry, G., et al. (2008). Three-dimensional tomographic imaging and characterization of iron compounds within Alzheimer's plaque core material. J. Alzheimers Dis. 14, 235–245.
Collingwood, J. F., Mikhaylova, A., Davidson, M. R., Batich, C., Streit, W. J., Eskin, T., et al. (2005). High-resolution x-ray absorption spectroscopy studies of metal compounds in neurodegenerative brain tissue. J. Phys. Conf. Ser. 17, 54–60. doi: 10.1088/1742-6596/17/1/009
Connor, J. R., Snyder, B. S., Beard, J. L., Fine, R. E., and Mufson, E. J. (1992). Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimers-disease. J. Neurosci. Res. 31, 327–335. doi: 10.1002/jnr.490310214
Cornett, D. S., Reyzer, M. L., Chaurand, P., and Caprioli, R. M. (2007). MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat. Methods 4, 828–833. doi: 10.1038/nmeth1094
Costa, L. G., and Fox, D. A. (1983). A selective decrease of cholinergic muscarinic receptors in the visual cortex of adult rats following developmental lead exposure. Brain Res. 276, 259–266. doi: 10.1016/0006-8993(83)90733-3
Danscher, G., Jensen, K. B., Frederickson, C. J., Kemp, K., Andreasen, A., Juhl, S., et al. (1997). Increased amount of zinc in the hippocampus and amygdala of Alzheimer's diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material. J. Neurosci. Methods 76, 53–59. doi: 10.1016/S0165-0270(97)00079-4
Dashdorj, U., Tserensodnom, B., Bold, B., Chimedregzen, U., Komatsu, F., and Kagawa, Y. (2012). Association of cumulative some heavy metal exposure with Parkinson's disease. Mov. Disord. 27, S1. doi: 10.1002/mds.25052
Deibel, M. A., Ehmann, W. D., and Markesbery, W. R. (1996). Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer's disease: possible relation to oxidative stress. J. Neurol. Sci. 143, 137–142. doi: 10.1016/S0022-510X(96)00203-1
de los Monteros, A. E., Korsak, R. A., Tran, T., Vu, D., de Vellis, J., and Edmond, J. (2000). Dietary iron and the integrity of the developing rat brain: a study with the artificially-reared rat pup. Cell. Mol. Biol. (Noisy-le-grand) 46, 501–515.
de Silva, R., Gutierrez, L. F., Raval, A. N., McVeigh, E. R., Ozturk, C., and Lederman, R. J. (2006). X-ray fused with magnetic resonance imaging (XFM) to target endomyocardial injections: validation in a swine model of myocardial infarction. Circulation 114, 2342–2350. doi: 10.1161/CIRCULATIONAHA.105.598524
Divers, T. J., Cummings, J. E., de Lahunta, A., Hintz, H. F., and Mohammed, H. O. (2006). Evaluation of the risk of motor neuron disease in horses fed a diet low in vitamin E and high in copper and iron. Am. J. Vet. Res. 67, 120–126. doi: 10.2460/ajvr.67.1.120
Dobrowolska, J., Dehnhardt, M., Matusch, A., Zoriy, M., Palomero-Gallagher, N., Koscielniak, P., et al. (2008). Quantitative imaging of zinc, copper and lead in three distinct regions of the human brain by laser ablation inductively coupled plasma mass spectrometry. Talanta 74, 717–723. doi: 10.1016/j.talanta.2007.06.051
Dobson, J. (2001). Nanoscale biogenic iron oxides and neurodegenerative disease. FEBS Lett. 496, 1–5. doi: 10.1016/S0014-5793(01)02386-9
Dunaief, J. L., Richa, C., Franks, E. P., Schultze, R. L., Aleman, T. S., Schenck, J. F., et al. (2005). Macular degeneration in a patient with aceruloplasminemia, a disease associated with retinal iron overload. Ophthalmology 112, 1062–1065. doi: 10.1016/j.ophtha.2004.12.029
Edison, P., Archer, H. A., Hinz, R., Hammers, A., Pavese, N., Tai, Y. F., et al. (2007). Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology 68, 501–508. doi: 10.1212/01.wnl.0000244749.20056.d4
Egan, T. J., Barthakur, S. R., and Aisen, P. (1992). Catalysis of the Haber-Weiss reaction by iron-diethylenetriaminepentaacetate. J. Inorg. Biochem. 48, 241–249. doi: 10.1016/0162-0134(92)84051-N
Eller, M. J., Verkhoturov, S. V., Della-Negra, S., and Schweikert, E. A. (2013). SIMS instrumentation and methodology for mapping of co-localized molecules. Rev. Sci. Instrum. 84, 103706. doi: 10.1063/1.4824199
Fahrni, C. J. (2007). Biological applications of X-ray fluorescence microscopy: exploring the subcellular topography and speciation of transition metals. Curr. Opin. Chem. Biol. 11, 121–127. doi: 10.1016/j.cbpa.2007.02.039
Farina, M., Avila, D. S., da Rocha, J. B., and Aschner, M. (2013). Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem. Int. 62, 575–594. doi: 10.1016/j.neuint.2012.12.006
Fenn, J. B., Mann, M., Meng, C. K., Wong, S. F., and Whitehouse, C. M. (1989). Electrospray ionization for mass-spectrometry of large biomolecules. Science 246, 64–71. doi: 10.1126/science.2675315
Fernandez-Lima, F. A., Debord, J. D., Schweikert, E. A., Della-Negra, S., Kellersberger, K. A., and Smotherman, M. (2013). Surface characterization of biological nanodomains using NP-ToF-SIMS. Surf. Interface Anal. 45, 1–8. doi: 10.1002/sia.4901
Filley, C. M., Kleinschmidt-De Masters, B. K., and Gross, K. F. (1994). Non-Alzheimer fronto-temporal degenerative dementia. A neurobehavioral and pathologic study. Clin. Neuropathol. 13, 109–116.
Frederickson, C. J., Koh, J. Y., and Bush, A. I. (2005). The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 6, 449–462. doi: 10.1038/nrn1671
Fu, L., Blakely, E., Marcus, M., Bjornstad, K., Due, B., Rehrmann, M., et al. (2005). Alzheimer's disease β-amyloid (Aβ) mediates metal-dependent human lens protein aggregation and light scattering. Investig. Ophthalmol. Vis. Sci. 46, 2906–B459.
George, A. K., Sonmez, M., Lederman, R. J., and Faranesh, A. Z. (2011). Robust automatic rigid registration of MRI and X-ray using external fiducial markers for XFM-guided interventional procedures. Med. Phys. 38, 125–141. doi: 10.1118/1.3523621
Gerhardsson, L., Lundh, T., Londos, E., and Minthon, L. (2011). Cerebrospinal fluid/plasma quotients of essential and non-essential metals in patients with Alzheimer's disease. J. Neural Transm. 118, 957–962. doi: 10.1007/s00702-011-0605-x
Giese, A., Levin, J., Bertsch, U., and Kretzschmar, H. (2004). Effect of metal ions on de novo aggregation of full-length prion protein. Biochem. Biophys. Res. Commun. 320, 1240–1246. doi: 10.1016/j.bbrc.2004.06.075
Gong, G., and O'Bryant, S. E. (2010). The arsenic exposure hypothesis for Alzheimer disease. Alzheimer Dis. Assoc. Disord. 24, 311–316. doi: 10.1097/WAD.0b013e3181d71bc7
Gonzalez-Cuyar, L. F., Perry, G., Miyajima, H., Atwood, C. S., Riveros-Angel, M., Lyons, P. F., et al. (2008). Redox active iron accumulation in aceruloplasminemia. Neuropathology 28, 466–471. doi: 10.1111/j.1440-1789.2008.00901.x
Goodman, L. (1953). Alzheimer's disease; a clinico-pathologic analysis of twenty-three cases with a theory on pathogenesis. J. Nerv. Ment. Dis. 118, 97–130. doi: 10.1097/00005053-195308000-00001
Goormaghtigh, E., Ruysschaert, J. M., and Raussens, V. (2006). Evaluation of the information content in infrared spectra for protein secondary structure determination. Biophys. J. 90, 2946–2957. doi: 10.1529/biophysj.105.072017
Grubman, A., White, A. R., and Liddell, J. R. (2014). Mitochondrial metals as a potential therapeutic target in neurodegeneration. Br. J. Pharmacol. 171, 2159–2173. doi: 10.1111/bph.12513
Gu, Q., and Lin, R. L. (2010). Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J. Appl. Physiol. 108, 891–897. doi: 10.1152/japplphysiol.01371.2009
Han, X. L., Abendschein, D. R., Kelley, J. G., and Gross, R. W. (2000). Diabetes-induced changes in specific lipid molecular species in rat myocardium. Biochem. J. 352, 79–89. doi: 10.1042/0264-6021:3520079
Han, X. L., and Gross, R. W. (1994). Electrospray-ionization mass spectroscopic analysis of human erythrocyte plasma-membrane phospholipids. Proc. Natl. Acad. Sci. U.S.A. 91, 10635–10639. doi: 10.1073/pnas.91.22.10635
Han, X. L., and Gross, R. W. (2001). Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal. Biochem. 295, 88–100. doi: 10.1006/abio.2001.5178
Han, X. L., Holtzman, D. M., and McKeel, D. W. (2001). Plasmalogen deficiency in early Alzheimer's disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 77, 1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x
Han, X. L., Holtzman, D. M., McKeel, D. W., Kelley, J., and Morris, J. C. (2002). Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J. Neurochem. 82, 809–818. doi: 10.1046/j.1471-4159.2002.00997.x
Hare, D., Austin, C., Doble, P., and Arora, M. (2011). Elemental bio-imaging of trace elements in teeth using laser ablation-inductively coupled plasma-mass spectrometry. J. Dent. 39, 397–403. doi: 10.1016/j.jdent.2011.03.004
Hare, D. J., George, J. L., Grimm, R., Wilkins, S., Adlard, P. A., Cherny, R. A., et al. (2010). Three-dimensional elemental bio-imaging of Fe, Zn, Cu, Mn and P in a 6-hydroxydopamine lesioned mouse brain. Metallomics 2, 745–753. doi: 10.1039/c0mt00039f
Haris, P. I., and Severcan, F. (1999). FTIR spectroscopic characterization of protein structure in aqueous and non-aqueous media. J. Mol. Catal. B Enzym. 7, 207–221. doi: 10.1016/S1381-1177(99)00030-2
Helpern, J. A., Jensen, J., Lee, S. P., and Falangola, M. F. (2004). Quantitative MRI assessment of Alzheimer's disease. J. Mol. Neurosci. 24, 45–48. doi: 10.1385/JMN:24:1:045
Hickey, J. L., Lim, S., Hayne, D. J., Paterson, B. M., White, J. M., Villemagne, V. L., et al. (2013). Diagnostic imaging agents for Alzheimer's disease: copper radiopharmaceuticals that target Aβ plaques. J. Am. Chem. Soc. 135, 16120–16132. doi: 10.1021/ja4057807
Hutchinson, R. W., Cox, A. G., McLeod, C. W., Marshall, P. S., Harper, A., Dawson, E. L., et al. (2005). Imaging and spatial distribution of beta-amyloid peptide and metal ions in Alzheimer's plaques by laser ablation-inductively coupled plasma-mass spectrometry. Anal. Biochem. 346, 225–233. doi: 10.1016/j.ab.2005.08.024
Ii, K. (1995). The role of beta-amyloid in the development of Alzheimer's disease. Drugs Aging 7, 97–109. doi: 10.2165/00002512-199507020-00004
Ince, P. G., Shaw, P. J., Candy, J. M., Mantle, D., Tandon, L., Ehmann, W. D., et al. (1994). Iron, selenium and glutathione-peroxidase activity are elevated in sporadic motor-neuron disease. Neurosci. Lett. 182, 87–90. doi: 10.1016/0304-3940(94)90213-5
Jakubowski, N., Waentig, L., Hayen, H., Venkatachalam, A., von Bohlen, A., Roos, P. H., et al. (2008). Labelling of proteins with 2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid and lanthanides and detection by ICP-MS. J. Anal. Atom. Spectrom. 23, 1497–1507. doi: 10.1039/b800346g
Jellinger, K. A. (2013). The relevance of metals in the pathophysiology of neurodegeneration, pathological considerations. Int. Rev. Neurobiol. 110, 1–47. doi: 10.1016/B978-0-12-410502-7.00002-8
Joachim, C. L., Morris, J. H., Selkoe, D. J., and Kosik, K. S. (1987). Tau epitopes are incorporated into a range of lesions in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 46, 611–622. doi: 10.1097/00005072-198711000-00001
Kedziora, J., Witas, H., Bartosz, G., Leyko, W., Jeske, J., and Rozynkowa, D. (1978). Down syndrome - transferrin parallels plasma iron changes. Experientia 34, 712–713. doi: 10.1007/BF01947275
Ketola, R. A., and Mauriala, T. (2012). Mass spectrometric tools for cell and tissue studies. Eur. J. Pharm. Sci. 46, 293–314. doi: 10.1016/j.ejps.2012.03.011
Khachaturian, Z. S. (1985). Diagnosis of Alzheimer's disease. Arch. Neurol. 42, 1097–1105. doi: 10.1001/archneur.1985.04060100083029
Khan, A. U., and Kasha, M. (1994). Singlet molecular oxygen in the Haber-Weiss reaction. Proc. Natl. Acad. Sci. U.S.A. 91, 12365–12367. doi: 10.1073/pnas.91.26.12365
Knochenmuss, R., Karbach, V., Wiesli, U., Breuker, K., and Zenobi, R. (1998). The matrix suppression effect in matrix-assisted laser desorption/ionization: application to negative ions and further characteristics. Rapid Commun. Mass Spectrom. 12, 529–534.
Koeppen, A. H., Michael, S. C., Knutson, M. D., Haile, D. J., Qian, J., Levi, S., et al. (2007). The dentate nucleus in Friedreich's ataxia: the role of iron-responsive proteins. Acta Neuropathol. 114, 163–173. doi: 10.1007/s00401-007-0220-y
Komatsu, H., Liu, L., Murray, I. V., and Axelsen, P. H. (2007). A mechanistic link between oxidative stress and membrane mediated amyloidogenesis revealed by infrared spectroscopy. Biochim. Biophys. Acta 1768, 1913–1922. doi: 10.1016/j.bbamem.2007.05.026
Kono, S., Suzuki, H., Takahashi, K., Takahashi, Y., Shirakawa, K., Murakawa, Y., et al. (2006). Hepatic iron overload associated with a decreased serum ceruloplasmin level in a novel clinical type of aceruloplasminemia. Gastroenterology 131, 240–245. doi: 10.1053/j.gastro.2006.04.017
Lear, J., Hare, D. J., Fryer, F., Adlard, P. A., Finkelstein, D. I., and Doble, P. A. (2012). High-resolution elemental bioimaging of Ca, Mn, Fe, Co, Cu, and Zn employing LA-ICP-MS and hydrogen reaction gas. Anal. Chem. 84, 6707–6714. doi: 10.1021/ac301156f
Lee, J. H., Yu, W. H., Kumar, A., Lee, S., Mohan, P. S., Peterhoff, C. M., et al. (2010). Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141, 1146–1158. doi: 10.1016/j.cell.2010.05.008
Lelie, H. L., Liba, A., Bourassa, M. W., Chattopadhyay, M., Chan, P. K., Gralla, E. B., et al. (2011). Copper and zinc metallation status of copper-zinc superoxide dismutase from amyotrophic lateral sclerosis transgenic mice. J. Biol. Chem. 286, 2795–2806. doi: 10.1074/jbc.M110.186999
Leskovjan, A. C., Kretlow, A., Lanzirotti, A., Barrea, R., Vogt, S., and Miller, L. M. (2011). Increased brain iron coincides with early plaque formation in a mouse model of Alzheimer's disease. Neuroimage 55, 32–38. doi: 10.1016/j.neuroimage.2010.11.073
Liggi, M., Murgia, D., Civolani, A., Demelia, E., Sorbello, O., and Demelia, L. (2013). The relationship between copper and steatosis in Wilson's disease. Clin. Res. Hepatol. Gastroenterol. 37, 36–40. doi: 10.1016/j.clinre.2012.03.038
Lim, C. K., Kalinowski, D. S., and Richardson, D. R. (2008). Protection against hydrogen peroxide-mediated cytotoxicity in Friedreich's ataxia fibroblasts using novel iron chelators of the 2-pyridylcarboxaldehyde isonicotinoyl hydrazone class. Mol. Pharmacol. 74, 225–235. doi: 10.1124/mol.108.046847
Litwin, J. A. (1982). Transition metal-catalysed oxidation of 3,3′-diaminobenzidine [DAB] in a model system. Acta Histochem. 71, 111–117. doi: 10.1016/S0065-1281(82)80023-8
Llobett, J. M., Falco, G., Casas, C., Teixido, A., and Domingo, J. L. (2003). Concentrations of arsenic, cadmium, mercury, and lead in common foods and estimated daily intake by children, adolescents, adults, and seniors of Catalonia, Spain. J. Agric. Food Chem. 51, 838–842. doi: 10.1021/jf020734q
Long, X. F., Zhang, C. H., Cheng, J. J., and Bi, S. P. (2008). A novel method for study of the aggregation of protein induced by metal ion aluminum(III) using resonance Rayleigh scattering technique. Spectrochim. Acta A 69, 71–77. doi: 10.1016/j.saa.2007.03.011
Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. L., and Markesbery, W. R. (1998a). Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 158, 47–52. doi: 10.1016/S0022-510X(98)00092-6
Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. L., and Markesbery, W. R. (1998b). Use of micro-pixe in the analysis of zinc, copper and iron in Alzheimer's disease senile plaques. Abstr. Pap. Am. Chem. Soc. 215, U969–U969.
Lucas, H. R. (2012). “Amyloids and copper biochemistry: effects of metal dyshomeostasis in Alzheimer's and Parkinson's disease,” in Abstract Paper Presented at the 244th ACS National Meeting and Exposition (Philadelphia, PA: Pennsylvania Convention Center).
Ma, Q. F., Li, Y. M., Du, J. T., Liu, H. D., Kanazawa, K., Nemoto, T., et al. (2006). Copper binding properties of a tau peptide associated with Alzheimer's disease studied by CD, NMR, and MALDI-TOF MS. Peptides 27, 841–849. doi: 10.1016/j.peptides.2005.09.002
Makjanic, J., McDonald, B., and Watt, F. (1997). Nuclear microscopy study of neurofibrillary tangles in Alzheimer's disease. Nucl. Instrum. Methods Phys. Res. Sect. B 130, 439–443. doi: 10.1016/S0168-583X(97)00236-X
Mattson, M. P. (1997). Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 77, 1081–1132.
Mattson, M. P. (2004). Pathways towards and away from Alzheimer's disease. Nature 430, 631–639. doi: 10.1038/nature02621
McArthur, S. L., Vendettuoli, M. C., Ratner, B. D., and Castner, D. G. (2004). Methods for generating protein molecular ions in ToF-SIMS. Langmuir 20, 3704–3709. doi: 10.1021/la0358419
McRae, R., Bagchi, P., Sumalekshmy, S., and Fahrni, C. J. (2009). In situ imaging of metals in cells and tissues. Chem. Rev. 109, 4780–4827. doi: 10.1021/cr900223a
Michael, S., Petrocine, S. V., Qian, J., Lamarche, J. B., Knutson, M. D., Garrick, M. D., et al. (2006). Iron and iron-responsive proteins in the cardiomyopathy of Friedreich's ataxia. Cerebellum 5, 257–267. doi: 10.1080/14734220600913246
Miller, D. L., Papayannopoulos, I. A., Styles, J., Bobin, S. A., Lin, Y. Y., Biemann, K., et al. (1993). Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimers-disease. Arch. Biochem. Biophys. 301, 41–52. doi: 10.1006/abbi.1993.1112
Miller, L. M., Wang, Q., Telivala, T. P., Smith, R. J., Lanzirotti, A., and Miklossy, J. (2006). Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer's disease. J. Struct. Biol. 155, 30–37. doi: 10.1016/j.jsb.2005.09.004
Mirra, S. S., Heyman, A., McKeel, D., Sumi, S. M., Crain, B. J., Brownlee, L. M., et al. (1991). The consortium to establish a registry for Alzheimer's disease (CERAD). Part II. standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 41, 479–486. doi: 10.1212/WNL.41.4.479
Molina-Holgado, F., Hider, R. C., Gaeta, A., Williams, R., and Francis, P. (2007). Metals ions and neurodegeneration. Biometals 20, 639–654. doi: 10.1007/s10534-006-9033-z
Mony, L., Kew, J. N. C., Gunthorpe, M. J., and Paoletti, P. (2009). Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br. J. Pharmacol. 157, 1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x
Morawski, M., Meinecke, C., Reinert, T., Dorffel, A. C., Riederer, P., Arendt, T., et al. (2005). Determination of trace elements in the human substantia nigra. Nucl. Instrum. Methods Phys. Res. Sect. B 231, 224–228. doi: 10.1016/j.nimb.2005.01.061
Multhaup, G., Schlicksupp, A., Hesse, L., Beher, D., Ruppert, T., Masters, C. L., et al. (1996). The amyloid precursor protein of Alzheimer's disease in the reduction of copper(II) to copper(I). Science 271, 1406–1409. doi: 10.1126/science.271.5254.1406
Musat, N., Foster, R., Vagner, T., Adam, B., and Kuypers, M. M. (2012). Detecting metabolic activities in single cells, with emphasis on nanoSIMS. FEMS Microbiol. Rev. 36, 486–511. doi: 10.1111/j.1574-6976.2011.00303.x
Mutter, J., Curth, A., Naumann, J., Deth, R., and Walach, H. (2010). Does inorganic mercury play a role in Alzheimer's disease? A systematic review and an integrated molecular mechanism. J. Alzheimers Dis. 22, 357–374. doi: 10.3233/JAD-2010-100705
Ni, W., Dong, Q. Y., Zhang, Y., and Wu, Z. Y. (2013). Zinc monotherapy and a low-copper diet are beneficial in patients with Wilson disease after liver transplantation. CNS Neurosci. Ther. 19, 905–907. doi: 10.1111/cns.12167
Novellino, L., Napolitano, A., and Prota, G. (1999). 5,6-dihydroxyindoles in the Fenton reaction: a model study of the role of melanin precursors in oxidative stress and hyperpigmentary processes. Chem. Res. Toxicol. 12, 985–992. doi: 10.1021/tx990020i
Oide, T., Yoshida, K., Kaneko, K., Ohta, M., and Arima, K. (2006). Iron overload and antioxidative role of perivascular astrocytes in aceruloplasminemia. Neuropathol. Appl. Neurobiol. 32, 170–176. doi: 10.1111/j.1365-2990.2006.00710.x
Olanow, C. W., and Arendash, G. W. (1994). Metals and free radicals in neurodegeneration. Curr. Opin. Neurol. 7, 548–558. doi: 10.1097/00019052-199412000-00013
Opazo, C., Huang, X., Cherny, R. A., Moir, R. D., Roher, A. E., White, A. R., et al. (2002). Metalloenzyme-like activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2). J. Biol. Chem. 277, 40302–40308. doi: 10.1074/jbc.M206428200
Ortiz, E., Pasquini, J. M., Thompson, K., Felt, B., Butkus, G., Beard, J., et al. (2004). Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J. Neurosci. Res. 77, 681–689. doi: 10.1002/jnr.20207
Oteiza, P. I., Mackenzie, G. G., and Verstraeten, S. V. (2004). Metals in neurodegeneration: involvement of oxidants and oxidant-sensitive transcription factors. Mol. Aspects Med. 25, 103–115. doi: 10.1016/j.mam.2004.02.012
Pacholski, M. L., and Winograd, N. (1999). Imaging with mass spectrometry. Chem. Rev. 99, 2977–3006. doi: 10.1021/cr980137w
Paling, D., Tozer, D., Wheeler-Kingshott, C., Kapoor, R., Miller, D. H., and Golay, X. (2012). Reduced R ‘(2) in multiple sclerosis normal appearing white matter and lesions may reflect decreased myelin and iron content. J. Neurol. Neurosurg. Psychiatry 83, 785–792. doi: 10.1136/jnnp-2012-302541
Pareja, J., Lopez, S., Jaramillo, D., Hahn, D. W., and Molina, A. (2013). Laser ablation-laser induced breakdown spectroscopy for the measurement of total elemental concentration in soils. Appl. Opt. 52, 2470–2477. doi: 10.1364/AO.52.002470
Park, J. H., Lee, D. W., Park, K. S., and Joung, H. (2014). Serum trace metal levels in Alzheimer's disease and normal control groups. Am. J. Alzheimer's Dis. Other Demen. 29, 76–83. doi: 10.1177/1533317513506778
Patra, R. C., Swarup, D., and Senapati, S. K. (1999). Effects of cadmium on lipid peroxides and superoxide dismutase in hepatic, renal and testicular tissue of rats. Vet. Hum. Toxicol. 41, 65–67.
Paunesku, T., Vogt, S., Maser, J., Lai, B., and Woloschak, G. (2006). X-ray fluorescence microprobe imaging in biology and medicine. J. Cell. Biochem. 99, 1489–1502. doi: 10.1002/jcb.21047
Peng, F. Y., Lutsenko, S., Sun, X. K., and Muzik, O. (2012). Imaging copper metabolism imbalance in Atp7b (-/-) knockout mouse model of Wilson's disease with PET-CT and orally administered (CuCl2)-Cu-64. Mol. Imaging Biol. 14, 600–607. doi: 10.1007/s11307-011-0532-0
Perl, D. P., and Brody, A. R. (1980). Alzheimers-disease - X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science 208, 297–299. doi: 10.1126/science.7367858
Petty, S. A., and Decatur, S. M. (2005). Experimental evidence for the reorganization of beta-strands within aggregates of the a beta(16-22) peptide. J. Am. Chem. Soc. 127, 13488–13489. doi: 10.1021/ja054663y
Pimplikar, S. W., Nixon, R. A., Robakis, N. K., Shen, J., and Tsai, L. H. (2010). Amyloid-independent mechanisms in Alzheimer's disease pathogenesis. J. Neurosci. 30, 14946–14954. doi: 10.1523/JNEUROSCI.4305-10.2010
Pithadia, A. S., and Lim, M. H. (2012). Metal-associated amyloid-beta species in Alzheimer's disease. Curr. Opin. Chem. Biol. 16, 67–73. doi: 10.1016/j.cbpa.2012.01.016
Popescu, B. F., Pickering, I. J., George, G. N., and Nichol, H. (2007). The chemical form of mitochondrial iron in Friedreich's ataxia. J. Inorg. Biochem. 101, 957–966. doi: 10.1016/j.jinorgbio.2007.03.004
Prasher, V. P., Gosling, P., and Blair, J. (1998). Role of iron in Alzheimer-type dementia in Down syndrome. Int. J. Geriatr. Psychiatry 13, 818–819.
Qin, Z., Caruso, J. A., Lai, B., Matusch, A., and Becker, J. S. (2011). Trace metal imaging with high spatial resolution: applications in biomedicine. Metallomics 3, 28–37. doi: 10.1039/c0mt00048e
Que, E. L., Domaille, D. W., and Chang, C. J. (2008). Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 108, 1517–1549. doi: 10.1021/cr078203u
Quintana, C., Bellefqih, S., Laval, J. Y., Guerquin-Kern, J. L., Wu, T. D., Avila, J., et al. (2006). Study of the localization of iron, ferritin, and hemosiderin in Alzheimer's disease hippocampus by analytical microscopy at the subcellular level. J. Struct. Biol. 153, 42–54. doi: 10.1016/j.jsb.2005.11.001
Quintana, C., Lancin, M., Marhic, C., Perez, M., Martin-Benito, J., Avila, J., et al. (2000). Initial studies with high resolution TEM and electron energy loss spectroscopy studies of ferritin cores extracted from brains of patients with progressive supranuclear palsy and Alzheimer disease. Cell Mol. Biol. 46, 807–820.
Quintana, C., Wu, T. D., Delatour, B., Dhenain, M., Guerquin-Kern, J. L., and Croisy, A. (2007). Morphological and chemical studies of pathological human and mice brain at the subcellular level: correlation between light, electron, and nanosims microscopies. Microsc. Res. Tech. 70, 281–295. doi: 10.1002/jemt.20403
Rahman, A., Khan, K. M., Al-Khaledi, G., Khan, I., and Attur, S. (2012). Early postnatal lead exposure induces tau phosphorylation in the brain of young rats. Acta Biol. Hung. 63, 411–425. doi: 10.1556/ABiol.63.2012.4.1
Rajendran, R., Ren, M. Q., Ynsa, M. D., Casadesus, G., Smith, M. A., Perry, G., et al. (2009). A novel approach to the identification and quantitative elemental analysis of amyloid deposits-Insights into the pathology of Alzheimer's disease. Biochem. Biophys. Res. Commun. 382, 91–95. doi: 10.1016/j.bbrc.2009.02.136
Ralle, M., and Lutsenko, S. (2009). Quantitative imaging of metals in tissues. Biometals 22, 197–205. doi: 10.1007/s10534-008-9200-5
Ramsay, S. C., Cassidy, N., de Jonge, M. D., Howard, D. L., Paterson, D., and Ketheesan, N. (2011). Examination of trafficking of phagocytosed colloid particles in neutrophils using synchrotron-based X-ray fluorescence microscopy (XFM). J. Biol. Phys. 37, 493–506. doi: 10.1007/s10867-011-9233-9
Ricchelli, F., Buggio, R., Drago, D., Salmona, M., Forloni, G., Negro, A., et al. (2006). Aggregation/fibrillogenesis of recombinant human prion protein and Gerstmann-Straussler-Scheinker disease peptides in the presence of metal ions. Biochemistry. 45, 6724–6732. doi: 10.1021/bi0601454
Richardson, D. R. (2004). Novel chelators for central nervous system disorders that involve alterations in the metabolism of iron and other metal ions. Ann. N.Y. Acad. Sci. 1012, 326–341. doi: 10.1196/annals.1306.026
Roberts, B. R., Ryan, T. M., Bush, A. I., Masters, C. L., and Duce, J. A. (2012). The role of metallobiology and amyloid-ss peptides in Alzheimer's disease. J. Neurochem. 120, 149–166. doi: 10.1111/j.1471-4159.2011.07500.x
Rodella, L. F., Ricci, F., Borsani, E., Stacchiotti, A., Foglio, E., Favero, G., et al. (2008). Aluminium exposure induces Alzheimer's disease-like histopathological alterations in mouse brain. Histol. Histopathol. 23, 433–439.
Rohner, T. C., Staab, D., and Stoeckli, M. (2005). MALDI mass spectrometric imaging of biological tissue sections. Mech. Ageing Dev. 126, 177–185. doi: 10.1016/j.mad.2004.09.032
Romero, M. D. C., Pliego-Rivero, F. B., Altamirano, B. M., and Otero, G. A. (2010). Effect of postlactation iron deficiency on the composition of fatty acids of whole brain myelin. Nutr. Neurosci. 13, 237–244. doi: 10.1179/147683010X12611460764606
Rusina, R., Matej, R., Urban, P., Cabelkova, Z., Nerudova, J., and Cikrt, M. (2006). Regional mercury concentration in Alzheimer's disease brains. Eur. J. Neurol. 13, 169–294. doi: 10.1111/j.1468-1331.2006.01622.x
Russ, T. C., Batty, G. D., Hearnshaw, G. F., Fenton, C., and Starr, J. M. (2012). Geographical variation in dementia: systematic review with meta-analysis. Int. J. Epidemiol. 41, 1012–1032. doi: 10.1093/ije/dys103
Salanki, J., Carpenter, D. O., Gyori, J., and Rubakhin, S. (1996). Toxic heavy metals potentiate ligand-gated C1-currents in gastropoda neurons. Neurobiology 4, 277–278.
Salgado, E. N., Lewis, R. A., Faraone-Mennella, J., and Tezcan, F. A. (2008). Metal-mediated self-assembly of protein superstructures: influence of secondary interactions on protein oligomerization and aggregation. J. Am. Chem. Soc. 130, 6082. doi: 10.1021/ja8012177
Savelieff, M. G., Lee, S., Liu, Y., and Lim, M. H. (2013). Untangling amyloid-beta, tau, and metals in Alzheimer's disease. ACS Chem. Biol. 8, 856–865. doi: 10.1021/cb400080f
Saxe, S. R., Wekstein, M. W., Kryscio, R. J., Henry, R. G., Cornett, C. R., Snowdon, D. A., et al. (1999). Alzheimer's disease, dental amalgam and mercury. J. Am. Dent. Assoc. 130, 191–199. doi: 10.14219/jada.archive.1999.0168
Schenck, J. F., and Zimmerman, E. A. (2004). High-field magnetic resonance imaging of brain iron: birth of a biomarker? NMR Biomed. 17, 433–445. doi: 10.1002/nbm.922
Schuerenberg, M., Luebbert, C., Deininger, S. O., Ketterlinus, R., and Suckau, D. (2007). MALDI tissue imaging: mass spectrometric localization of biomarkers in tissue slices. Nat. Methods 4, 3–4.
Selkoe, D. J., Bell, D. S., Podlisny, M. B., Price, D. L., and Cork, L. C. (1987). Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science 235, 873–877. doi: 10.1126/science.3544219
Sensi, S. L., Paoletti, P., Bush, A. I., and Sekler, I. (2009). Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 10, 780–791. doi: 10.1038/nrn2734
Singh, A., Mohan, M. L., Isaac, A. O., Luo, X., Petrak, J., Vyoral, D., et al. (2009). Prion protein modulates cellular iron uptake: a novel function with implications for prion disease pathogenesis. PLoS ONE 4:e4468. doi: 10.1371/journal.pone.0004468
Singh, A., Qing, L. T., Kong, Q. Z., and Singh, N. (2012a). Change in the characteristics of ferritin induces iron imbalance in prion disease affected brains. Neurobiol. Dis. 45, 930–938. doi: 10.1016/j.nbd.2011.12.012
Singh, N., and Singh, A. (2010). “Redox-iron and prion disease pathogenesis,” in Congress Abstract Presented at PRION 2010, Abstract Ppo5-18: Basic Mechanisms of Neurodegeneration and Pathology (Salzburg: Salzburg Congress Center).
Singh, P. K., Baxi, D., Diwedi, R., and Ramachandran, A. V. (2012b). Prior cadmium exposure improves glucoregulation in diabetic rats but exacerbates effects on metabolic dysregulation, oxidative stress, and hepatic and renal toxicity. Drug Chem. Toxicol. 35, 167–177. doi: 10.3109/01480545.2011.589450
Stavitski, E., Smith, R. J., Bourassa, M. W., Acerbo, A. S., Carr, G. L., and Miller, L. M. (2013). Dynamic full-field infrared imaging with multiple synchrotron beams. Anal. Chem. 85, 3599–3605. doi: 10.1021/ac3033849
Tangkosakul, T., Tantimongcolwat, T., Isarankura-Na-Ayudhya, C., Mejare, M., Bulow, L., and Prachayasittikul, V. (2009). Native and chimeric metal-binding lactate dehydrogenase as detection and protection tools for oxidative stress induced by Fenton's reaction. Excli J. 8, 1–11.
Terada, S., Aoyama, T., Yano, F., and Mitsui, Y. (2002). Time-resolved acquisition technique for spatially-resolved electron energy-loss spectroscopy by energy-filtering TEM. J. Electron Microsc. 51, 291–296. doi: 10.1093/jmicro/51.5.291
Teri, L., Borson, S., Kiyak, H. A., and Yamagishi, M. (1989). Behavioral disturbance, cognitive dysfunction, and functional skill. Prevalence and relationship in Alzheimer's disease. J. Am. Geriatr. Soc. 37, 109–116.
Terry, R. D., Masliah, E., Salmon, D. P., Butters, N., DeTeresa, R., Hill, R., et al. (1991). Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580. doi: 10.1002/ana.410300410
Thinnes, F. P. (2010). Opening cell membrane-standing type-1 VDAC/porin channels by trivalent aluminium-a factor in amyotrophic lateral sclerosis and Alzheimer's disease? Mol. Genet. Metab. 101, 299–300. doi: 10.1016/j.ymgme.2010.06.004
Thomas, C., Mackey, M. M., Diaz, A. A., and Cox, D. P. (2009). Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep. 14, 102–108. doi: 10.1179/135100009X392566
Tokutake, S., Nagase, H., Morisaki, S., and Oyanagi, S. (1995). Aluminum detected in senile plaques and neurofibrillary tangles is contained in lipofuscin granules with silicon, probably as aluminosilicate. Neurosci. Lett. 185, 99–102. doi: 10.1016/0304-3940(94)11234-A
Torosantucci, R., Sharov, V. S., van Beers, M., Brinks, V., Schoneich, C., and Jiskoot, W. (2013). Identification of oxidation sites and covalent cross-links in metal catalyzed oxidized interferon beta-1a: potential implications for protein aggregation and immunogenicity. Mol. Pharm. 10, 2311–2322. doi: 10.1021/mp300665u
Valentin, A., Lin, J., Samuel, M., Hulse, G., Alarcon, H., Dervos, H., et al. (2006). Micro-electrode recordings from Globus Pallidus Internus (GPi) using general anaesthesia in Neurodegeneration with Brain Iron Accumulation 1 (NBIA1). Mov. Disord. 21, S614–S708. doi: 10.1002/mds.21249
Walshe, J. M. (2012). Serum ‘free’ copper in Wilson disease. QJM 105, 419–423. doi: 10.1093/qjmed/hcr229
Wang, X., Zhang, Q., Guo, Q., Lou, Y., Yang, L., and Wang, Y. (2004). Iron-catalysed propylene epoxidation by nitrous oxide: dramatic shift of allylic oxidation to epoxidation by the modification with alkali metal salts. Chem. Commun. 1396–1397. doi: 10.1039/b402839b
Watt, A. D., Villemagne, V. L., and Barnham, K. J. (2013). Metals, membranes, and amyloid-beta oligomers: key pieces in the Alzheimer's disease puzzle? J. Alzheimer's Dis. 33(Suppl. 1), S283–S293. doi: 10.3233/JAD-2012-129017
Webster, W. S., and Valois, A. A. (1981). The toxic effects of cadmium on the neonatal mouse CNS. J. Neuropathol. Exp. Neurol. 40, 247–257. doi: 10.1097/00005072-198105000-00003
Weekley, C. M., Aitken, J. B., Finney, L., Vogt, S., Witting, P. K., and Harris, H. H. (2013). Selenium metabolism in cancer cells: the combined application of XAS and XFM techniques to the problem of selenium speciation in biological systems. Nutrients 5, 1734–1756. doi: 10.3390/nu5051734
Winterbourn, C. C. (1987). The ability of scavengers to distinguish OH. production in the iron-catalyzed Haber-Weiss reaction: comparison of four assays for OH. Free Rad. Biol. Med. 3, 33–39. doi: 10.1016/0891-5849(87)90037-2
Wong, E., and Cuervo, A. M. (2010). Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 13, 805–811. doi: 10.1038/nn.2575
Wu, B., Zoriy, M., Chen, Y., and Becker, J. S. (2009). Imaging of nutrient elements in the leaves of Elsholtzia splendens by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Talanta 78, 132–137. doi: 10.1016/j.talanta.2008.10.061
Xing, X., Du, M., Xu, X., Rui, Q., and Wang, D. (2009). Exposure to metals induces morphological and functional alteration of AFD neurons in nematode Caenorhabditis elegans. Environ. Toxicol. Pharm. 28, 104–110. doi: 10.1016/j.etap.2009.03.006
Yamamoto, A., Shin, R. W., Hasegawa, K., Naiki, H., Sato, H., Yoshimasu, F., et al. (2003). Iron(III) induces aggregation hyperphosphorylated tau and its of reduction to iron(II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer's disease (vol 82, pg 1137, 2002). J. Neurochem. 86, 1568–1568. doi: 10.1046/j.1471-4159.2002.01061.x
Yokel, R. A. (2006). Blood-brain barrier flux of aluminum, manganese, iron and other metals suspected to contribute to metal-induced neurodegeneration. J. Alzheimer's Dis. 10, 223–253.
Yoshida, S., Yase, Y., Iwata, S., Yoshida, H., and Mizumoto, Y. (1987). Trace-metals and its relationship to early pathological changes in motor neurons in amyotrophic lateral sclerosis. Rinsho Shinkeigaku 27, 518–527.
Yun, S. W., and Hoyer, S. (2000). Effects of low-level lead on glycolytic enzymes and pyruvate dehydrogenase of rat brain in vitro: relevance to sporadic Alzheimer's disease? J. Neural Transm. 107, 355–368. doi: 10.1007/s007020050030
Keywords: LA-ICPMS, metals, Alzheimer's disease, bioimaging, MALDI, FTIR
Citation: Braidy N, Poljak A, Marjo C, Rutlidge H, Rich A, Jayasena T, Inestrosa NC and Sachdev P (2014) Metal and complementary molecular bioimaging in Alzheimer's disease. Front. Aging Neurosci. 6:138. doi: 10.3389/fnagi.2014.00138
Received: 02 December 2013; Accepted: 09 June 2014;
Published online: 15 July 2014.
Edited by:
Roger S. Chung, Macquarie University, AustraliaReviewed by:
Junming Wang, University of Mississippi Medical Center, USACarlos Beas-Zarate, Universidad de Guadalajra Mexico, Mexico
Copyright © 2014 Braidy, Poljak, Marjo, Rutlidge, Rich, Jayasena, Inestrosa and Sachdev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Perminder Sachdev, NPI, Euroa Centre, UNSW School of Psychiatry, Prince of Wales Hospital, Barker Street, Randwick, Sydney, NSW 2031, Australia e-mail: p.sachdev@unsw.edu.au
 Nady Braidy
Nady Braidy Anne Poljak
Anne Poljak Christopher Marjo
Christopher Marjo Helen Rutlidge4
Helen Rutlidge4  Nibaldo C. Inestrosa
Nibaldo C. Inestrosa Perminder Sachdev
Perminder Sachdev