- 1Stefanie Spielman Comprehensive Breast Cancer, The Ohio State University, Columbus, OH, United States
- 2Division of Medical Oncology, Comprehensive Cancer Center, The Ohio State University Medical Center, Columbus, OH, United States
- 3Department of Pathology, The Ohio State University, Columbus, OH, United States
- 4Department of Radiation Oncology, Comprehensive Cancer Center, The Ohio State University Medical Center, Columbus, OH, United States
- 5Department of Neurological Surgery, Comprehensive Cancer Center, The Ohio State University Medical Center, Columbus, OH, United States
Primary small cell carcinoma of the breast (SCCB) is a rare tumor subtype comprising <0.1% of all breast carcinomas. Here we present a case of thyroid transcription factor-1 (TTF-1) positive SCCB that recurred within 3 years of diagnosis in the lung and lymph nodes. Given the small number of cases, no clear guidelines exist on the appropriate management of patients with these aggressive tumors. We present a case study and review the current literature to highlight the knowledge gaps and needs of patients with these rare tumors. A 50-year-old premenopausal woman with no family history, presented with a palpable right breast mass. Biopsy was consistent with primary SCCB that was poorly differentiated, positive for synaptophysin and chromogranin and TTF-1 and presence of ductal carcinoma in situ component showing neuroendocrine differentiation. Imaging with PET, CT, and MRI brain excluded any other sites of primary disease. She underwent a right lumpectomy with axillary lymph node dissection and was treated with adjuvant cisplatin-based chemotherapy and concurrent radiation therapy. Thirty-four months later, routine scans showed a new right lower-lobe lung nodule and an enlarged sub-carinal node that was proven to be poorly differentiated neuroendocrine cancer. This case report sheds light on a rarely described disease and provides a comprehensive approach to diagnosis and management. Primary SCCB is an extremely rare, aggressive form of breast cancer that is molecularly and histologically similar to SCLC. However, a review of the literature highlights recent mutational analyses that show important differences between these two cancer types, including an increase in PIK3CA mutations in primary SCCB. Further studies, including genomic analyses are needed to better define this malignancy and to develop a standard treatment.
Introduction
Primary poorly differentiated neuroendocrine carcinoma/small cell carcinoma of the breast (SCCB) is a distinct subtype of breast cancer, accounting for <1% of cases of primary breast cancer. SCCB belongs to the class of tumors known as extra-pulmonary small cell carcinomas, which have also been described in the urinary bladder, prostate, esophagus, stomach, colon, rectum, gallbladder, larynx, salivary glands, cervix, and skin. The first primary case was described by Wade et al. in 1983 (1). Although it typically presents in women, there have been a few cases of primary SCCB in males reported in the literature (2). SCCB has been diagnosed in patients aged 29–81 years (3, 4) who present with a palpable breast mass. The histologic characteristics of mammary SCC are similar to SCC arising in other organs. The diagnosis of primary mammary SCC can be made if a non-mammary metastasis to the breast is excluded, or an in situ component is identified histologically.
Below we describe a case of a 50-year old postmenopausal women with early stage primary SCCB of the right breast without lymph node involvement. Following treatment, she remained disease-free for 3 years, but developed a right lower lobe nodule and enlarged sub-carinal lymph node, which was treated like limited small cell lung cancer (SCLC). This case is unique in that the invasive SCCB was associated with an in situ component which was also purely of neuroendocrine differentiation.
Case Presentation
A 50-year-old G4P3 premenopausal South Asian woman with a history of gastroesophageal reflux disease and polycystic ovarian syndrome initially presented with a painful, palpable mass in the upper outer quadrant of the right breast noticed on self-breast exam. The patient was a lifelong non-smoker and did not report use of oral contraceptives or hormone replacement therapy. She had no personal or family history of breast or ovarian cancers and had a normal screening mammogram 1 year prior to presentation.
Clinical examination revealed an irregular, firm 2.4 cm mass in the upper outer quadrant of the right breast, approximately 9.5 cm from the nipple. There were no changes of the overlying skin or nipple or palpable axillary adenopathy. Her lungs were clear to auscultation; abdominal exam was without masses and no inguinal, cervical or contralateral axillary adenopathy were palpated. Diagnostic mammogram and ultrasound (Figures 1A,B) indicated a 2.3 × 1.3 × 2.3 cm irregular mass in the upper outer quadrant of the right breast, highly suggestive of malignancy. Ultrasound guided core needle biopsy revealed poorly differentiated neuroendocrine carcinoma/small cell carcinoma (Figures 2A,B), estrogen receptor (ER) negative, progesterone receptor (PR) positive (10% weak intensity), and human epidermal growth receptor 2 (HER2) negative. Immuno-histochemical staining was positive for thyroid transcription factor 1 (TTF-1), synaptophysin and chromogranin A, and rare cells positive for GATA3, consistent with primary SCCB (Figures 2C,D). Importantly, and uniquely, an in situ component was identified (highlighted by the myoepithelial marker p40) which was also of purely neuroendocrine differentiation, which was conclusive evidence that this was a primary SCC of the breast. Due to the rarity of the case, these findings were confirmed at MD Anderson Cancer Center.
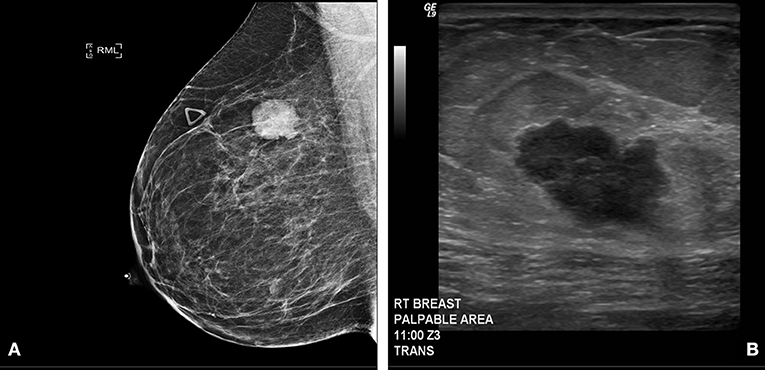
Figure 1. (A) Diagnostic Mammogram: Right medio-lateral oblique view showing an irregular high density mass in the superior central aspect of the right breast. (B) Ultrasonography of right breast and axilla: Irregular hypoechoic macro-lobulated mass in the superior central aspect of the right breast.
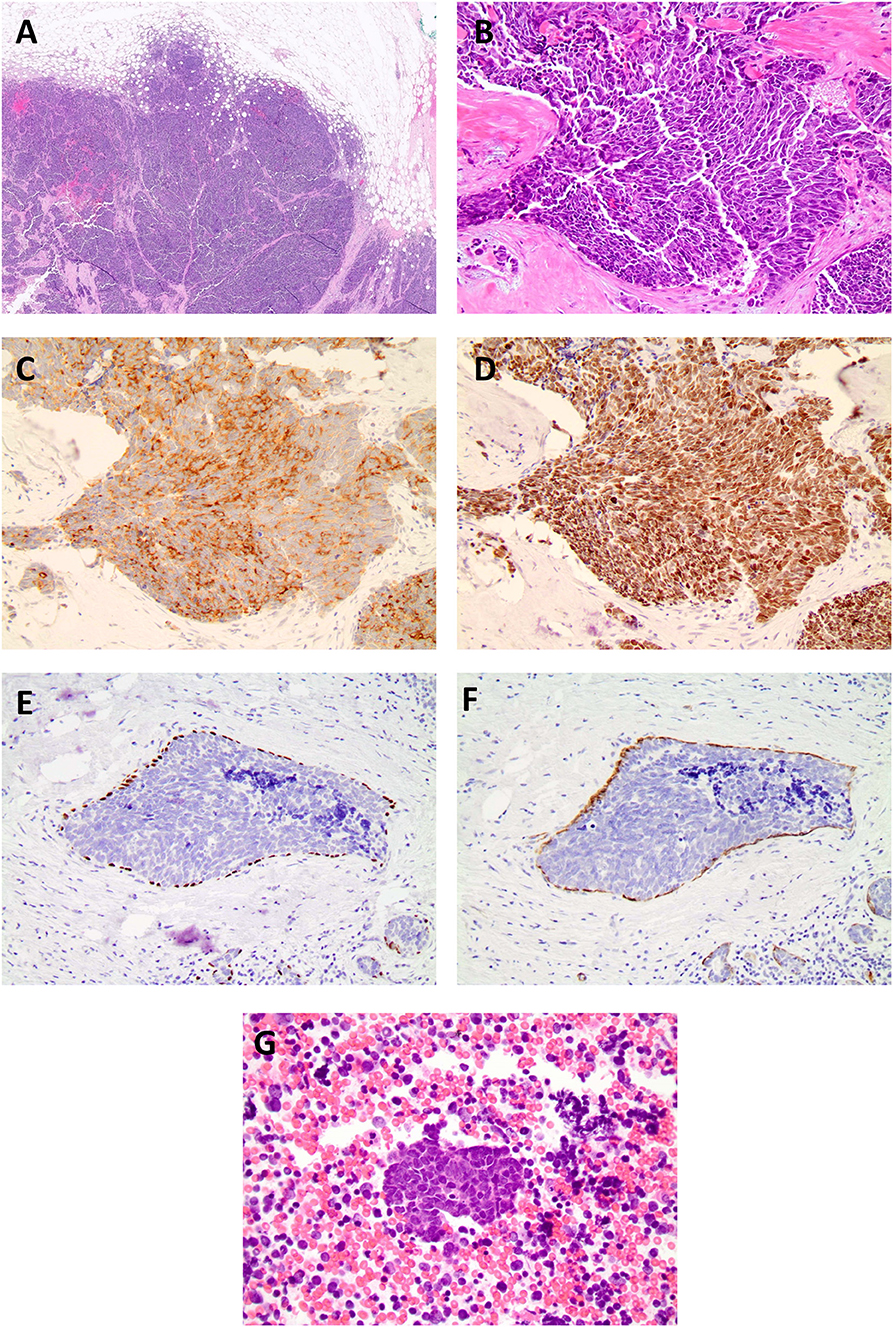
Figure 2. (A) Histopathological examination of the specimen stained on low (4x), (B) high power field (20x, 40x) showed neoplastic cells with high nuclear: cytoplasmic ratio, hyperchromatic nuclei, minimal cytoplasm, and indistinct nuclei, nuclear molding, high mitotic rate, consistent with the diagnosis of small cell carcinoma. Biopsy of breast cancer primary. Immuno-histochemical staining for TTF-1 synpatophysin, chromogranin, and p40 and smooth muscle myosin staining (SMMS) consistent with a diagnosis of primary SCCB, (C) small cell synaptophysin 20X. (D) small cell TTF-1 20X. (E) In situ component with neuroendocrine differentiation p40. (F) in situ component smooth muscle myosin stain. (G) Histopathological examination of the fine needle aspiration of the sub-carinal node revealed metastatic small cell carcinoma (Lymph node 40x).
Radiologic workup was conducted to exclude a non-mammary primary tumor origin and to complete staging for the breast cancer. Additionally, a positron emission tomography (PET) computerized tomography (CT) scans showed no evidence of lung, pancreatic, adrenal, or pelvic masses (Figures 3A,B). Plasma neuropeptide levels revealed elevations in gastrin (207 pg/ml; normal 0–125 pg/ml), substance P (382 pg/ml; normal 0–240 pg/ml) and neurotensin (306 pg/ml; normal <100 pg/ml). Plasma glucagon levels were also elevated (plasma neuropeptides or tumor markers are not required for the diagnosis). Germline genetic testing revealed a MUTYH E480 non-sense mutation which is associated with autosomal recessive MUTYH associated polyposis and would not be pathogenic when heterozygous with wild-type.
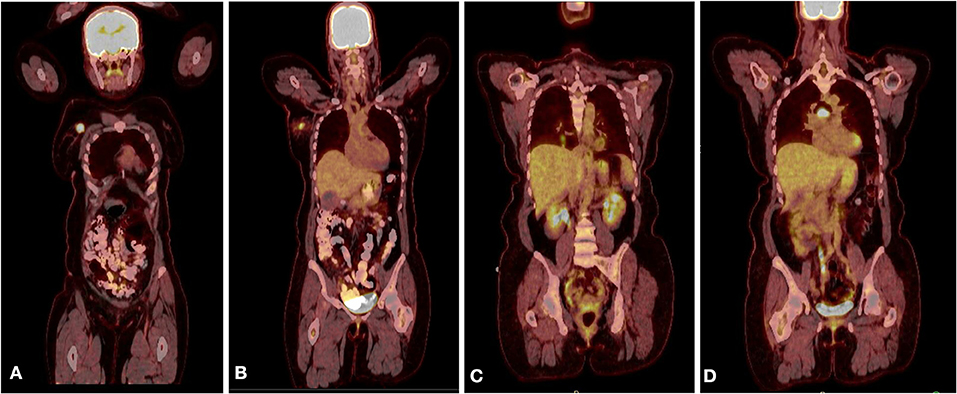
Figure 3. (A) PET CT scan showing the primary lesion in the right upper outer quadrant of the right breast. (B) A hyper metabolic right axillary lymph node, which is compatible with the patient's known right breast primary malignancy and a presumed metastatic right axillary lymph node. (C) PET scan after recurrence showing new lung nodules with FDG uptake. (D) PET scan after recurrence showing sub-carinal lymph node with new intense focus of FDG uptake.
Based on the early clinical stage, the patient underwent a right lumpectomy with axillary lymph node dissection. Pathology revealed a poorly differentiated neuroendocrine carcinoma/ small cell carcinoma, ER/PR and HER2, were repeated on the resection specimen and were all negative with Ki-67 proliferation index of 70%. In addition, a high grade neuroendocrine in situ component was identified (Figures 2E,F). Immuno-histochemical staining was positive for chromogranin A, CD56, synaptophysin and TTF-1 and, notably, the in-situ component was also positive for TTF-1. Tumor infiltrating lymphocytes (TILs) were low positive for PD-1 staining (1–24%) and tumor cells and TILs were negative for PD-L1 staining (antibody used for PD-L1 was clone 22C3 through Foundation One). The surgical margins were free of tumor and twelve lymph nodes were negative. The patient was diagnosed with primary SCCB (stage IIA).
Foundation One testing for mutations in thirty-five genes revealed the presence of a pathogenic variant in the MUTYH gene (c.1438G>T, p.Glu480*), loss of exons 19-27 in RB1, SMARCA4 P1975 mutation, and Tp53 R110_L111insL mutation as well as amplification in PIK3CA, FLT3, MYC, SOX2 and CDK8. She subsequently received adjuvant chemotherapy with four cycles of cisplatin 80 mg/m2 on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle. She received right whole breast radiation therapy in the form of 5000cGy in 25 fractions followed by a 1000cGy boost in 5 fractions (6000cGy cumulative) to the surgical site.
Following treatment, the patient remained disease-free for 3 years, as evidenced by lack of clinical evidence of recurrence and negative imaging. Routine follow-up CT scans of the thorax showed a right lower lobe nodule and enlarged sub-carinal lymph node, which showed FDG avidity by PET scan (Figures 3C,D). Bronchoscopy and trans-bronchial fine needle aspiration of the sub-carinal node revealed metastatic small cell carcinoma with Ki-67 staining in >90% of cells (Figure 2G). PD-L1 tumor proportion score was 0%. Repeat Foundation One testing performed on the slide from the sub-carinal lymph node revealed microsatellite stable status with a tumor mutation burden of 4 mutations/Mb, with amplifications noted in the CDK8, EPHB1, FLT3, MYC, PIK3CA, PIK3CB, RAD21 and SOX2 and loss of Rb and a MUTYH p E466 non-sense mutation.
She was treated with one cycle of cisplatin (80 mg/m2 on day 1) and etoposide 100mg/m2 on day 1-3) followed by one cycle concurrent with 45Gy external beam irradiation to the right lower lobe and mediastinum in 30 twice daily fractions. Follow-up CT scan of the chest revealed resolution of the right lower lobe nodule with residual scarring and significant decrease in size of the previously enlarged sub-carinal lymph node. She was then treated with two cycles of cisplatin-etoposide and two cycles of carboplatin-etoposide and concurrent atezolizumab, similar to the regimen used in the IMpower133 trial (5) for extensive stage small cell lung cancer but without maintenance atezolizumab, with radiologically and clinically stable disease and the MRI brain had no evidence of CNS metastases.
Discussion
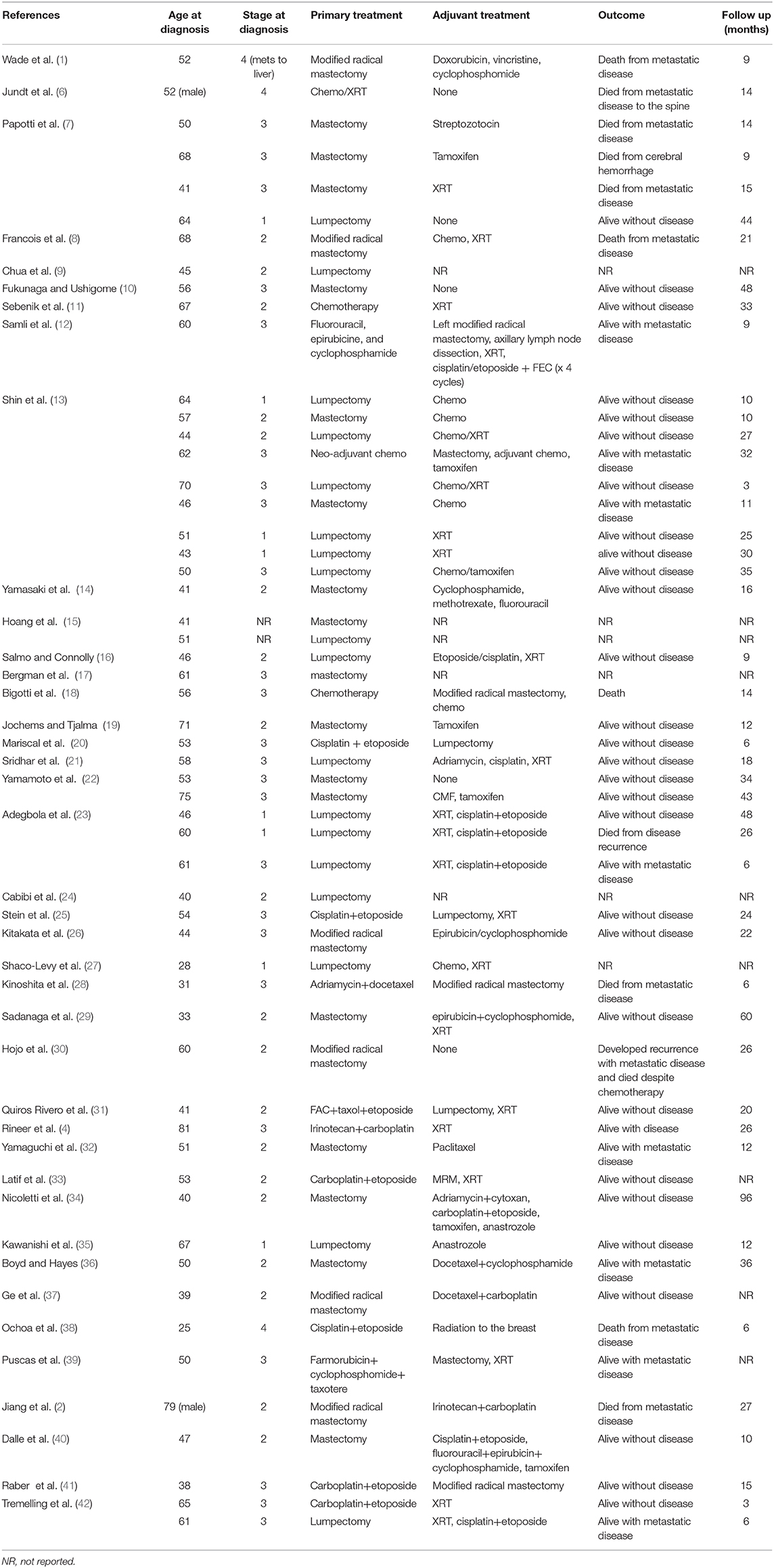
Primary SCCB is a rare, aggressive form of breast carcinoma, with only 56 cases reported in the literature (Table 1). It typically presents as a palpable breast or axillary mass in women over the age of 60, with lymph node involvement in 50–67% of cases at the time of diagnosis (33). Primary SCCB has similar histologic, morphologic and immune-histochemical features to SCLC, including the expression of TTF-1 in up to 50% of cases. The diagnosis of SCCB requires the clinical exclusion of a metastasis from a non-mammary primary site, or identification of an in situ component.
Due to the rarity and limited reports of primary SCCB, a standard approach to treatment is largely undefined. Treatment regimens in the literature include combinations of surgery, chemotherapy, radiation therapy, and endocrine therapy depending on tumor size and lymph node status. Hormonal therapy is added if the tumor expresses the appropriate receptors (13, 23, 43). More recent reports have described treatment with breast conservation therapy combined with either neo-adjuvant or adjuvant chemotherapy depending on the clinical scenario. Most adjuvant chemotherapy regimens include a platinum agent and etoposide, given that biologic markers of SCCB are similar to that of SCLC (see Appendix for discussion on characteristics of SCCB vs. SCLC and genomic studies in SCCB) (23, 37, 38, 44–46). However, some have reported using anthracycline and taxane based combinations typically used for invasive breast cancer (23, 33, 47, 48).
Our patient had a very high Ki-67, and she was treated with platinum based chemotherapy. The present report details a case of primary SCCB, as established with neuroendocrine markers on immuno-histochemical staining in combination with a solitary breast mass, lack of regional lymph node involvement and lack of evidence of any other masses in other organs on radiologic imaging, and importantly an in situ component which was of pure neuroendocrine differentiation, which is a rare finding. The patient was treated with breast conservation surgery, followed by systemic chemotherapy using carboplatin and etoposide along with radiation therapy. Unfortunately, the patient had a recurrence after 3 years with genomic studies indicating shared mutations consistent with a single primary and was treated with chemo-radiation similar to limited stage SCLC and is now in clinical remission. Her tumor also expressed an amplification of PIK3CA, which may serve as a therapeutic target in the future.
This case report sheds light on a rarely described disease and provides a comprehensive approach to diagnosis and management. Further studies, including genomic analyses are needed to better define this malignancy and to develop a standard treatment.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board, The Ohio State University, Wexner Medical Center. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HB, MK, EM, SF, and MC prepared the body of the manuscript. BR, GT, JW, MS, JV, SS, NW, DS, ML, RW, VP, TW, BK, and DC critically reviewed the publication. All authors endorsed the final form of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00228/full#supplementary-material
Abbreviations
CT, Computerized Tomography; ER, Estrogen Receptor; HER2, Human Epidermal Growth Factor Receptor 2; PET, Positron Emission Tomography; PR, Progesterone Receptor; SCCB, Small cell Carcinoma of the Breast; SCLC, Small Cell Lung Cancer; TTF1, Thyroid Transcription Factor 1.
References
1. Wade PM Jr, Mills SE, Read M, Cloud W, Lambert MJ 3rd, Smith RE. Small cell neuroendocrine (oat cell) carcinoma of the breast. Cancer. (1983) 52:121–5. doi: 10.1002/1097-0142(19830701)52:1<121::AID-CNCR2820520122>3.0.CO;2-F
2. Jiang J, Wang G, Lv L, Liu C, Liang X, Zhao H. et al. Primary small-cell neuroendocrine carcinoma of the male breast: a rare case report with review of the literature. Onco Targets Ther. (2014) 7:663. doi: 10.2147/OTT.S60782
3. Kanat O, Kilickap S, Korkmaz T, Ustaalioglu Oven BB, Canhoroz M, Cubukcu E., et al. Primary small cell carcinoma of the breast: report of seven cases and review of the literature. Tumori J. (2011) 97:473–8. doi: 10.1177/030089161109700410
4. Rineer Jm Choi K, Sanmugarajah J. Small cell carcinoma of the breast. J Natl Med Assoc. (2009) 101:290. doi: 10.1016/S0027-9684(15)31074-9
5. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
6. Jundt G, Schulz A, Heitz PU, Osborn M. Small cell neuroendocrine (oat cell) carcinoma of the male breast. Immunocytochemical and ultrastructural investigations. Virchows Arch A Pathol Anat Histopathol. (1984) 404:213–21. doi: 10.1007/BF00704065
7. Papotti M, Gherardi G, Eusebi V, Pagani A, Bussolati G. Primary oat cell (neuroendocrine) carcinoma of the breast. Report of four cases. Virchows Arch A Pathol Anat Histopathol. (1992) 420:103–8. doi: 10.1007/BF01605991
8. Francois A, Chatikhine VA, Chevallier B, Ren GS, Berry M, Chevrier A., et al. Neuroendocrine primary small cell carcinoma of the breast. Report of a case and review of the literature. Am J Clin Oncol. (1995) 18:133–8. doi: 10.1097/00000421-199504000-00008
9. Chua RS, Torno RB, Vuletin JC. Fine needle aspiration cytology of small cell neuroendocrine carcinoma of the breast. A case report. Acta Cytol. (1997) 41(4 Suppl):1341–4. doi: 10.1159/000333533
10. Fukunaga M, Ushigome S. Small cell (oat cell) carcinoma of the breast. Pathol Int. (1998) 48:744–8. doi: 10.1111/j.1440-1827.1998.tb03976.x
11. Sebenik M, Nair SG, Hamati HF. Primary small cell anaplastic carcinoma of the breast diagnosed by fine needle aspiration cytology: a case report. Acta Cytol. (1998) 42:1199–203. doi: 10.1159/000332114
12. Samli B, Celik S, Evrensel T, Orhan B, Tasdelen I. Primary neuroendocrine small cell carcinoma of the breast. Arch Pathol Lab Med. (2000) 124:296–8.
13. Shin SJ, DeLellis RA, Ying L, Rosen PP. Small cell carcinoma of the breast: a clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol. (2000) 24:1231–8. doi: 10.1097/00000478-200009000-00006
14. Yamasaki T, Shimazaki H, Aida S, Tamai S, Tamaki K, Hiraide H., et al. Primary small cell (oat cell) carcinoma of the breast: report of a case and review of the literature. Pathol Int. (2000) 50:914–8. doi: 10.1046/j.1440-1827.2000.01126.x
15. Hoang MP, Maitra A, Gazdar AF, Albores-Saavedra J. Primary mammary small-cell carcinoma: a molecular analysis of 2 cases. Hum Pathol. (2001) 32:753–7. doi: 10.1053/hupa.2001.25603
16. Salmo EN, Connolly CE. Primary small cell carcinoma of the breast: report of a case and review of the literature. Histopathology. (2001) 38:277–8. doi: 10.1046/j.1365-2559.2001.01068.x
17. Bergman S, Hoda SA, Geisinger KR, Creager AJ, Trupiano JK. E-cadherin-negative primary small cell carcinoma of the breast. Report of a case and review of the literature. Am J Clin Pathol. (2004) 121:117–21. doi: 10.1309/737Y07JNP8GQQJJY
18. Bigotti G, Coli A, Butti A, del Vecchio M, Tartaglione R, Massi G. Primary small cell neuroendocrine carcinoma of the breast. J Exp Clin Cancer Res. (2004) 23:691–6.
19. Jochems L, Tjalma WA. Primary small cell neuroendocrine tumour of the breast. Eur J Obstet Gynecol Reprod Biol. (2004) 115:231–3. doi: 10.1016/j.ejogrb.2003.12.013
20. Mariscal A, Balliu E, Díaz R, Casas JD, Gallart AM. Primary oat cell carcinoma of the breast: imaging features. AJR Am J Roentgenol. (2004) 183:1169–71. doi: 10.2214/ajr.183.4.1831169
21. Sridhar P, Matey P, Aluwihare N. Primary carcinoma of breast with small-cell differentiation. Breast. (2004) 13:149–51. doi: 10.1016/j.breast.2003.07.001
22. Yamamoto J, Ohshima K, Nabeshima K, Ikeda S, Iwasaki H, Kikuchi M. Comparative study of primary mammary small cell carcinoma, carcinoma with endocrine features and invasive ductal carcinoma. Oncol Rep. (2004) 11:825–31. doi: 10.3892/or.11.4.825
23. Adegbola T, Connolly C, Mortimer G. Small cell neuroendocrine carcinoma of the breast: a report of three cases and review of the literature. J Clin Pathol. (2005) 58:775–8. doi: 10.1136/jcp.2004.020792
24. Cabibi D, Cipolla C, Florena AM, Fricano S, Barresi E, Vieni S, et al. Solid variant of mammary “adenoid cystic carcinoma with basaloid features” merging with “small cell carcinoma”. Pathol Res Pract. (2005) 201:705–11. doi: 10.1016/j.prp.2005.04.012
25. Stein ME, Gershuny A, Abdach L, Quigley MM. Primary small-cell carcinoma of the breast. Clin Oncol. (2005) 17:201–2. doi: 10.1016/j.clon.2005.01.005
26. Kitakata H, Yasumoto K, Sudo Y, Minato H, Takahashi Y. A case of primary small cell carcinoma of the breast. Breast Cancer. (2007) 14:414–9. doi: 10.2325/jbcs.14.414
27. Shaco-Levy R, Dyomin V, Kachko L, Sion-Vardy N, Geffen DB, Koretz M. Small cell carcinoma of the breast: case report. Eur J Gynaecol Oncol. (2007) 28:142–4.
28. Kinoshita S, Hirano A, Komine K, Kobayashi S, Kyoda S, Takeyama H, et al. Primary small-cell neuroendocrine carcinoma of the breast: report of a case. Surg Today. (2008) 38:734–8. doi: 10.1007/s00595-007-3716-0
29. Sadanaga N, Okada S, Shiotani S, Morita M, Kakeji Y, Kitamura K, et al. Clinical characteristics of small cell carcinoma of the breast. Oncol Rep. (2008) 19:981–5. doi: 10.3892/or.19.4.981
30. Hojo T, Kinoshita T, Shien T, Terada K, Hirose S, Isobe Y, et al. Primary small cell carcinoma of the breast. Breast Cancer. (2009) 16:68–71. doi: 10.1007/s12282-008-0057-9
31. Quirós Rivero J, Muñoz García JL, Cabrera Rodríguez JJ, González Ferreira JA, Samper Ots P, Ríos Kavadoy Y. Extrapulmonary small cell carcinoma in breast and prostate. Clin Transl Oncol. (2009) 11:698–700. doi: 10.1007/s12094-009-0427-6
32. Yamaguchi R, Tanaka M, Otsuka H, Yamaguchi M, Kaneko Y, Fukushima T., et al. Neuroendocrine small cell carcinoma of the breast: report of a case. Med Mol Morphol. (2009) 42:58–61. doi: 10.1007/s00795-008-0432-9
33. Latif N, Rosa M, Samian L, Rana F. An unusual case of primary small cell neuroendocrine carcinoma of the breast. Breast J. (2010) 16:647–51. doi: 10.1111/j.1524-4741.2010.00974.x
34. Nicoletti S, Papi M, Drudi F, Fantini M, Canuti D, Tamburini E, et al. Small cell neuroendocrine tumor of the breast in a 40 year-old woman: a case report. J Med Case Rep. (2010) 4:201. doi: 10.1186/1752-1947-4-201
35. Kawanishi N, Norimatsu Y, Funakoshi M, Kamei T, Sonobe H, Kawano R, et al. Fine needle aspiration cytology of solid neuroendocrine carcinoma of the breast: a case report. Diagn Cytopathol. (2011) 39:527–30. doi: 10.1002/dc.21494
36. Boyd AS, Hayes BB. Metastatic small cell neuroendocrine carcinoma of the breast. J Cutan Pathol. (2012) 39:1042–6. doi: 10.1111/j.1600-0560.2012.01970.x
37. Ge QD, Lv N, Cao Y, Wang X, Tang J, Xie ZM, et al. A case report of primary small cell carcinoma of the breast and review of the literature. Chin J Cancer. (2012) 31:354. doi: 10.5732/cjc.012.10012
38. Ochoa R, Sudhindra A, Garcia-Buitrago M, Romilly AP, Cortes J, Gomez H, et al. Small-cell cancer of the breast: what is the optimal treatment? A report and review of outcomes. Clin Breast Cancer. (2012) 12:287–92. doi: 10.1016/j.clbc.2012.03.007
39. Puscas E, Lisencu C, Neagoe I. Case report of primary small cell neuroendocrine breast cancer. Clujul Med. (2013) 86:156–9.
40. Abou Dalle I, Abbas J, Boulos F, Salem Z, Assi HI. Primary small cell carcinoma of the breast: a case report. J Med Case Rep. (2017) 11:290. doi: 10.1186/s13256-017-1467-0
41. Raber B, Dao T, Howard E, Bredeweg A. Primary small-cell carcinoma of the breast. Proc Bayl Univ Med Cent. (2017) 30:200–2. doi: 10.1080/08998280.2017.11929586
42. Tremelling A, Samuel S, Murray M. Primary small cell neuroendocrine carcinoma of the breast – A case report and review of the literature. Int J Surg Case Rep. (2017) 38:29–31. doi: 10.1016/j.ijscr.2017.07.002
43. Adams R, Dyson P, Barthelmes L. Neuroendocrine breast tumours: breast cancer or neuroendocrine cancer presenting in the breast? Breast. (2014) 23:120–7. doi: 10.1016/j.breast.2013.11.005
44. Abbasi NZ, Zahur Z, Sheikh AS, Khan AA, Ali F, Memon KH, et al. Solid neuroendocrine carcinoma of the breast. J Coll Physicians Surg Pak. (2013) 23:820–2. 11.
45. Ali S, Desai G, Thomas S, Aggarwal L, Meena K, Kumar J, et al. Primary neuroendocrine carcinoma breast: our experience. Breast Disease. (2014) 34:95–9. doi: 10.3233/BD-130357
46. Sanguinetti A, Santoprete S, Lucchini R, Triola R, Loreti F, Avenia N. A rare breast tumor: solid neuroendocrine carcinoma. Ann Ital Chir. (2013) 84:81–5.
47. Inno A, Bogina G, Turazza M, Bortesi L, Duranti S, Massocco A. et al. Neuroendocrine carcinoma of the breast: current evidence and future perspectives. Oncologist. (2016) 21:28–32. doi: 10.1634/theoncologist.2015-0309
Keywords: TTF-1, small cell cancer, breast cancer, PDL-1, neuroendocrine cancer
Citation: Boutrid H, Kassem M, Tozbikian G, Morgan E, White J, Shah M, Vandeusen J, Sardesai S, Williams N, Stover DG, Lustberg M, Wesolowski R, Pudavalli V, Williams TM, Konda B, Fortier S, Carbone D, Ramaswamy B and Cherian MA (2020) TTF-1 Positive Primary Small Cell Carcinoma of the Breast: A Case Report and Review of the Literature. Front. Endocrinol. 11:228. doi: 10.3389/fendo.2020.00228
Received: 09 January 2020; Accepted: 30 March 2020;
Published: 29 April 2020.
Edited by:
Veronica Vella, University of Catania, ItalyReviewed by:
Dario Giuffrida, Mediterranean Institute of Oncology (IOM), ItalyEleonora Molinaro, University of Pisa, Italy
Copyright © 2020 Boutrid, Kassem, Tozbikian, Morgan, White, Shah, Vandeusen, Sardesai, Williams, Stover, Lustberg, Wesolowski, Pudavalli, Williams, Konda, Fortier, Carbone, Ramaswamy and Cherian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mathew A. Cherian, mathew.cherian@osumc.edu
 Hinda Boutrid1,2
Hinda Boutrid1,2 Mahmoud Kassem
Mahmoud Kassem Evan Morgan
Evan Morgan Julia White
Julia White Manisha Shah
Manisha Shah Nicole Williams
Nicole Williams Daniel G. Stover
Daniel G. Stover Maryam Lustberg
Maryam Lustberg Robert Wesolowski
Robert Wesolowski Terence M. Williams
Terence M. Williams Mathew A. Cherian
Mathew A. Cherian