Effects of harvest time on functional compounds and fruit antioxidant capacity in ten strawberry cultivars
Abstract
BACKGROUND:
Huelva (Spain) is the main region for strawberry production in Europe. Most fruit production is exported for fresh consumption to European countries, where consumers demand high fruit quality and appreciate its healthy properties. Strawberry intake is a valuable source of antioxidants compounds with important health benefits. The higher the antioxidant capacity of a cultivar, the better the enhancement of human health.
OBJECTIVE:
The comparative knowledge of fruit composition on antioxidant compounds and its variation along the cropping season, in ten strawberry cultivars cropped at Huelva.
METHODS:
Fruit yield and citric acid, ascorbic acid, total phenolics, anthocyanins content as well as antioxidant capacity of fruits were evaluated in ten strawberry cultivars at three harvesting times during the 2014 field campaign.
RESULTS:
Yield and fruit parameters analyzed were strongly influenced by the genotype and by the time of harvesting. Strawberry fruit quality and antioxidant properties were greater when harvested from mid- to late- season and were not associated with higher yields.
CONCLUSIONS:
Healthy properties of strawberry fruits depend on cultivar and harvest time. Knowledge of the nutritional properties of these strawberry cultivars might translate into benefits to growers and enhancement of health for consumers.
1Introduction
Strawberry is among the most widely consumed fruits in the world. The Huelva region in Spain (south western coast of Spain), is the main strawberry cropping area in Europe [1], and its production, characterised by high quality fruits, is largely exported to most European countries [2]. Freshly consumed fruits produced in Spain belong to different strawberry cultivars (Fragaria × ananassa Duch). In 2014, more than 65% of the cropping area of Huelva was distributed among ‘Splendor’ (26%), ‘Sabrina’ (24%) and ‘Florida-Fortuna’ (16%), followed by ‘Primoris’ (7%), Candonga ® (6%) and others. Most of these cultivars were developed by different public and private strawberry breeding programs [3] aiming to achieve better agronomic traits (yield and fruit quality) and, recently, to improve healthy features of fruits.
Fruit healthy benefits have been related to the presence of different compounds with antioxidant properties and the capacity of neutralizing free radical activity [4, 5]. The chemical nature of these compounds is diverse but includes a group of enzymes (superoxide dismutase, catalase, peroxidase, etc.), phenolic acids (catechins, flavonols, anthocyanins, etc.) and vitamins (C, E and A) [6]. In addition of being an enriched source of vitamins and minerals, intake of fruits and vegetables enriched in these bioactive products has been associated with low incidence of proliferative [7, 8] and cardiovascular diseases [9, 10]. More concretely, berries, including strawberry, contain high amounts of flavonoids, phenolic acids and anthocyanins (responsible of red fruit color) [11–18]. The later has been directly associated with a large number of human health benefits ranging from antioxidant potential, anticancer activity, anti-inflammatory and anti-angiogenic properties [19–25].
The spectra of bioactive compounds vary in different berries according to species, cultivation conditions and production area [26]. In strawberry, it has been reported that genotype influences the concentration of these compounds and their relative amounts [13, 26, 27, 28]. These reports are pointing out that synthesis of phytochemicals in fruits and vegetables is the result of genotypic and environmental interactions [29]. In this sense, it is reasonable to hypothesize that changes in environmental conditions (i.e. light, temperature and relative humidity) along the cropping season might also influence the fruit concentration of bioactive compounds at different harvesting periods.
Therefore, assessment of strawberry nutritional quality has to take into account the whole chain of production, including cultivars and cropping conditions (i.e. crop management, cultivation techniques, variability of environmental conditions), which are quite variable among productive areas worldwide. However, little information is available about the content on bioactive compounds of the cultivars currently growing on the main strawberry productive area of Europe. In addition to the organoleptic features, knowledge of the fruit health properties would represent an extra-value for consumers and would increase benefits for growers.
To analyze, the influence of harvesting time on organic acids and antioxidants compounds on different genotypes, we evaluated 10 short-day strawberry cultivars well-adapted to the productive area of Huelva growing under the conventional cropping system.
2Material and Methods
2.1Plant material and experimental design
Ten short-day strawberry cultivars, ‘Antilla’, ‘Sabrina’, Candonga ®, ‘Fontanilla’, ‘Florida-Florida’ (‘F. Fortuna’), ‘Liberty’, ‘Primoris’, ‘Rabida’, ‘Sahara’ and ‘Splendor’, were planted in mid-October 2013 at the IFAPA experimental station “El Cebollar” (Huelva, Spain). All cultivars are well-adapted to Huelva agroclimatic conditions [30] and were grown following conventional cropping–practices. Planting was done in a double row mulched raised beds (35 cm high and 50 cm wide) of a sandy soil with 5.8% clay, 5% silt and 89.2% sand, 0.09% organic matter, previously biosolarizated [31]. Fruit set takes place from January (mid-winter) to end of May (late spring). Polyethylene-covered tunnel structures (macrotunnel; [32]) were installed in mid-November and removed at the end of the croppingseason.
In order to evaluate effects of cultivar and harvest time on the nutraceutic features of the fruits, a field experiment was setup in a complete randomized block design with three replicate plots per cultivar and 50 plants per plot spaced at 25×25 cm. Throughout the crop season (January-May 2014), all mature fruits per plot were harvested once to twice a week. Total yield (g plant - 1) for each cultivar was calculated for the whole season and in three harvesting periods: ‘extra-early’ (from January to February), ‘early’ (March) and ‘late’ (April to May). According to these harvesting periods, fruit quality analyses were done on three sampling dates: 19th February, 21st March and 9th April.
2.2Sample preparation for fruit quality determination
At each harvest date, ∼250 g of mature strawberry fruits per plot (8–10 fruits) were taken and homogenized with a blender immediately after harvesting. Pulp samples were stored at −20°C until processed accordingly to the following assays at the laboratory.
2.3Titratable acidity and ascorbic acid content
For titratable acidity (TA) determinations, the pulp was filtrated and diluted with distilled water (1g: 100 mL). Titration to pH 8.1 (end point the third pK value of citric acid; AOAC 22.058) with 0.01M NaOH was done at room temperature with Titroline Easy (Schott Instruments ®, GmbH) portable pH meter. Total acidity was expressed as grams of citric acid per 100 grams of fresh weight (FW) [33].
For ascorbic acid quantification (AA), pulp samples were diluted with distilled water (1g: 10 mL) and homogenized. Reagent test strips were used with the reflectometer set of Merck Co (Merck Rqflex 10). Results were expressed as milligrams of ascorbic acid per 100 grams of FW.
2.4Total phenolics and anthocyanins content
Two grams of pulp samples were diluted with 10 mL of extraction solvent: methanol (99.9%) and ClH (0.1%), stored at 4°C during 24 h, and centrifuged at 10000 rpm for 15 min at 4°C. The supernatant was diluted in extraction solvent (2:1) and stored at −20°C until analyzed.
Total phenolic content in extracts were determined by the Folin–Ciocalteu method [34, 35] modified by Tulipani et al. [36]. Appropriately diluted extracts (2 ml) were mixed with 0.2 mL Folin-Ciocalteu reagent and 0.4 mL sodium carbonate (35% w/v) was added. After 1 h at room temperature and darkness, absorbance at 725 nm was measured in a spectrophotometer. Gallic acid (Sigma) was used as standard, and results were expressed as milligrams of gallic acid equivalents (GAE) per 100 grams of FW.
Total anthocyanin content was measured with the pH differential absorbance method [37, 38]. The pH values of the diluted pulp juice were 1.0 (in 0.025 M potassium chloride buffer) and 4.5 (in 0.4 M sodium acetate buffer). Absorbance at 510 and 700 nm was measured in a UV–VIS spectrophotometer. The absorbance values of the diluted samples (A) were calculated as follows: A = [(A 510 – A 700) pH1.0 – (A 510 – A 700) pH4.5].
The total anthocyanin pigment was expressed as milligrams of pelargonidin-3-glucoside (Pg-glc) equivalent per 100 grams of FW. It was calculated as the product of A, the molecular weight of Pg-glc, and the dilution factor divided by the molar extinction coefficient of Pg-glc [39]. Total Anthocyanins = A×MW×df×1000/ɛ.
2.5Antioxidant capacity: ABTS assay
To determine antioxidant capacity, 2.8 g pulps were added to 10 ml methanol (60%) and homogenized. Afterwards, sample was centrifuged at 3000 rpm for 15 min at 4°C. The supernatant was stored at −20°C until analyzed.
Total antioxidant capacity was evaluated according to TEAC (Trolox Equivalent Antioxidant Capacity, [40]) assay. This assay employ ABTS molecules (a chromogen and colorless substance that changes into its colored monocationic radical form (ABTS) by an oxidative agent). The absorption peak of ABTS is at 734 nm. Addition of antioxidants reduces ABTS into its colorless form. Therefore, the extent of decolorization, as percentage of inhibition of ABTS, is determined as a function of concentration and calculated relative to the reactivity of Trolox. Antioxidant activity is expressed as μmol of Trolox equivalents (TE) per gram of fresh weight (FW).
2.6Statistical analysis
Data were subjected to analysis of variance (randomized complete block design; ANOVA) for means comparison, and differences between mean values were compared by Tukey honest significant difference (HSD) with the analytical software STATISTIX 9.0 (Analytical Software, Florida, USA) and reported as means ± standard error of the mean. Correlations between the levels of the different antioxidants were calculated by Pearson correlation.
3Results and discussion
3.1Cultivars effect on fruit quality and yield
The influence of cultivar on organic acids (citric and ascorbic acids) was significant (Table 1). Among the 10 cultivars studied, ‘Sabrina’, Candonga ® and ‘Sahara’ showed the highest values of citric acid (more than 0.8 g TA/100 g FW) whilst ‘Antilla’ had the lowest (0.65 g TA/100 g FW). Thus, the amount of citric acid depended on the cultivars as it was previously described by Sturm et al. [41]. Besides its importance in flavor, citric acid is also of great interest because promotes the antioxidant action and seem to contribute to the antioxidant activity [42]. In this sense, ‘Sabrina’, Candonga ® and ‘Sahara’ would be more appropriate in terms of health, whereas ‘Antilla’ would be better for taste, since a low amount of acid is associated with a higher sugar/acid ratio [43].
Regarding to ascorbic acid content, the highest value was shown by ‘Liberty’ (55.29 mg AA/100 g FW; 37.7% more ascorbic acid than the lowest one ‘Rabida’) followed by ‘Sahara’ and Candonga ® (Table 1). Differences in ascorbic acid among strawberry cultivars were also observed by several authors [36, 44, 45] working with cultivars adapted to Sweden, Italy, and Belgium; in general all seem to give ascorbic acid values in the same range. Likewise, ascorbic acid is an important antioxidant, involved in human health [11, 46] and, in this sense, the consumption of strawberry cultivars with high content in ascorbic acid (i.e. ‘Liberty’; Table 1) would lead to an improvement in health. Specifically this acid has repeatedly been associated with lowered risk of developing several diseases, as cancer [47].
Great variability existed among the examined strawberry fruits regarding to their phenolic compounds (Table 1). ‘Primoris’ and Candonga ® showed significantly higher contents than the others cultivars (up to 17%). The results found in this work are comparable and in accordance with those observed by Pincemail et al. [45] and Buendia et al. [48] who found important differences in the content of phenolic compounds among various cultivars of strawberry.
Differences among cultivars were also observed for anthocyanin content (Table 1). In this sense, the highest amounts of anthocyanin were shown by ‘Florida-Fortuna’ and ‘Sabrina’, which showed up to 49% more anthocyanins than ‘Antilla’ (the cultivar with the lowest value). High variability in anthocyanin content among strawberry cultivars was previously described by Tulipani et al. [36]. These type of cultivars are of great interest in a healthy diet, due to anthocyanins may play an important role in controlling oxidative reactions and exhibit antiinflammatory and anti-angiogenic properties [19–25].
As regards antioxidant capacity, significant differences among the 10 cultivars were shown (Table 1). The highest value (39.1μmol Teq/ g FW) was obtained by the cultivar Candonga ®, whilst ‘Primoris’ showed the lowest one. Variations in antioxidant capacity among strawberry cultivars were already observed in several studies [29, 49, 50, 51].
Differences in the amount of organic acids, phenolic and anthocyanin compounds in the cultivars analyzed, confirm that the genotype largely determines antioxidant capacity in strawberry. Therefore, the knowledge of antioxidant composition of cultivars would favor their consumption and might contribute to improving human health [7, 8, 9, 10]. Also, those cultivars with high antioxidant compounds can be used as parental in breeding programs to obtain new cultivars with increased antioxidant capacity.
A significant correlation (Table 3) was observed between total antioxidant capacity and anthocyanin content (R2 = 0.3613, p < 0.05, n = 90), ascorbic acid (R2 = 0.5044, p < 0.001, n = 90), and total phenolic compounds (R2 = 0.5176, p < 0.001, n = 90); indicating the important contribution of these compounds for the antioxidant capacity on the strawberry cultivars studied. These results are in agreement with Aaby et al. [46], who reported that ascorbic acid was the most important contributor to the antioxidant capacity in strawberry. In contrast, Pincemail et al. [45] only found those correlations in one out of the twelve cultivars they studied. Previous studies reported that citric acid contributed greatly to antioxidant capacity on strawberry [42], but our results are not consistent with those since no significant correlation between total antioxidant capacity and citric acid was found, despite citric acid was significantly correlated with anthocyanin content (R2 = 0.4668, p < 0.001, n = 90). Given that anthocyanins are pigments that confer colors from orange to purple, the significant relationship between anthocyanins and antioxidants capacity is pointing out that the more red is the fruit, the greater antioxidant capacity and health benefit.
All study cultivars displayed fruit yields comparable to commercial orchards along the whole season. Total fruit yield was above 900 g/plant but significant differences among cultivars were found (Fig. 1). ‘Sabrina’ and ‘Fontanilla’ displayed the highest values of total production (1240.4 ± 12.6 and 1225.4 ± 13.4 g/plant, respectively), followed by ‘Sahara’, ‘Rabida’ and ‘Florida-Fortuna’ (with a yield decrease of 5.69, 7.29 and 12.05% respect to ‘Sabrina’) being ‘Antilla’ the cultivar with the lowest fruit yield (with a decrease about 30%). ‘Splendor’, ‘Primoris’, ‘Liberty’ and Candonga ® displayed intermediate fruit yields. Among the most productive cultivars, ‘Rabida’ and ‘Sahara’, showed also high precocity (189.3 ± 9.6 and 161.7 ± 7.8 g/plant in ‘Extra-early’ period, respectively; Fig. 1) together with ‘Splendor’ and ‘Primoris’ (174.1 ± 14.6 and 148.6 ± 1.8 g/plant in ‘Extra-early’ period, respectively), but the two latter showed lower total yields (1020.6 ± 44.6 and 961.9 ± 30.9 g/plant). These results are indicating that high precocity do not necessarily translate into higher yields at the end of the cropping season and vice versa. Fruit production in ‘Extra-early’ (January to February), ‘Early’ (March) and ‘Late’ (from April to May), was 13 % , 24% and 63% , respectively. This is suggesting that fruit production is associated to the main flowering bloom that takes place in late winter and early spring since averaged ripening from anthesis is about 38 days [52].
3.2Harvest time influence on fruit quality
Previous studies on strawberry have shown that harvest time affected ascorbic acid, phenolic content and antioxidant capacity of fruits [45]. However, environmental conditions, cropping system (i.e. open field or greenhouse) and harvest dates (i.e. May-November) were quite different.
In this work, the three harvesting times tested (19th February, 21st March and 9th April) significantly affected the content of organic acids and antioxidant compounds of fruits (at the same ripening stage) of the 10 cultivars studied (Table 2). Thereby, at first harvest time (19th February) all cultivars displayed the lowest content of organic acids and antioxidant compounds. More concretely, citric acid, ascorbic acid, phenolics acid, anthocyanin and total antioxidant capacity were lower in extra-early period by 12.02% , 8.30% , 11.09% , 22.86% and 17.18% respectively, compared to early and late average value. This is indicating that fruits harvested from the middle to the end of season (early: 21st March and late period: 9th April; Table 2), which coincides with the peak of production in the Huelva area (Fig. 1), would have better healthy properties.
However, significant interaction of cultivars and harvest periods were found (p < 0.001) in the analyzed parameters, evidencing that cultivars behave differently across harvest times (Fig. 2). Although in most cultivars there was a tendency to increase citric acid content through the field campaign, in ’Sabrina’, with intermediate values, it did not change along the crop season (Fig. 2A). Regarding ascorbic acid, all cultivars achieved their highest values in the early harvest with the exception of ‘Fontanilla’, which displayed the highest ascorbic acid content in the extra-early (19th February) period, and ‘Liberty’, in which vitamin C content did not change along the field campaign, being significantly higherst than in any other cultivar (Fig. 2B). The highestr ascorbic acid content in extra-early period observed in ‘Fontanilla’ could be of great interest due to its precocity.
Regarding to phenolic compounds, most cultivars followed a similar bell-shaped pattern with the highest values in the early period (21st March). However in ‘Florida-Fortuna’, ‘Sabrina’, ‘Antilla’ and ‘Fontanilla’ values remained almost unchanged (Fig. 2C).
Pattern of variation of anthocyanin content was similar in most cultivars, which showed a marked increase between extra-early and early harvest times, except in ‘Antilla’, ‘Candonga’, and ‘Fontanilla’, in which anthocyanins did not change significantly (Fig. 2D). Regarding to total antioxidant capacity, patterns of variation were quite different among cultivars. ‘Primoris’, ‘Splendor’, ‘Rabida’, and ‘Candonga’ displayed a bell-shaped pattern (i.e. maximum values at early-harvest); ‘Fontanilla’ and ‘Florida-Fortuna’ remained unchanged; ‘Antilla’ increased progressively; and a drop was observed in ‘Liberty’ and ‘Sahara’ in the late harvest (Fig. 2E).
Such variability in the accumulation of antioxidant compounds in the fruits, is suggesting that cultivars are differently affected by the changes in the environmental conditions during the cropping season [53]. In this sense, it is known that high temperatures and light intensity influences the synthesis of organic and antioxidant compounds in growing fruits [53–55]. Therefore, selection of cultivars enriched in healthy compounds (i.e. antioxidants) and with high stability across changing environmental conditions of the growing areas might represent an advantage for human health and would translate into benefits for growers.
4Conclusion
Widely consumed strawberry cultivars produced on the main strawberry production area of Europe, differ largely in the fruit their fruit composition in of organic acid and antioxidant compounds. Differences among cultivars might not be kept along the cropping season since fruit quality and healthy properties vary at different harvest times. The range of variation depends on the cultivar; therefore harvest time has to be taken into account when comparing nutraceutic properties. Overall, fruit quality is substantially better when fruits are harvested from mid-March to early April in the season. Among cultivars, Candonga ®, ‘Sabrina’, ‘Fontanilla’ and ‘Sahara’ have very high content of citric acid and antioxidant compounds in conjunction with the highest yields. It is remarkable that ‘Fontanilla’ and ‘Sahara’ also displayed precocity, suggesting that their cultivation would increase the economic profit for growers by the early arrival of high quality fruits to the markets.
In addition to giving relevant information about the healthy properties of commercial strawberries, these results have importance for the choice and selection of parental in breeding programs.
Acknowledgments
This research was funded by the PP.AVA.AVA201301.6, RF2011-00016, TRA201300.6, and RTA2012-00001-00-00 projects, and co-financed by INIA and the European Union (FEDER and/or FSE funds). Dr. Ariza is supported by IFAPA. Junta de Andalucía (20%) and by the Programa Operativo Fondo Social Europeo (FSE) de Andalucía 2007-2013 (80%) under the topic “Andalucía se mueve con Europa”.
REFERENCES
1 | FAOSTAT Agricultural Data, 2014. http://www.faostat.fao.org |
2 | Romero F. Regional workshop on methyl bromide alternatives for North Africa and Southern European countries. Paris, France: United Nations Environment Program (UNEP); 2000. Alternatives for North African and Southern European Countries; pp. 185–190 |
3 | Soria C, Sánchez-Sevilla JF, Ariza MT, Gálvez J, López-Aranda JM, Medina-Mínguez JJ, Miranda L, Arjona A, Bartual R(2008) ‘Amiga’ StrawberryHortScience43: 3943944 |
4 | Cao G, Sofic E, Prior RL(1996) Antioxidant capacity of tea and common vegetablesJ Agric Food Chem44: 1134263431 |
5 | Wang H, Cao G, Prior RL(1996) Total antioxidant capacity of fruitsJ Agric Food Chem44: 701705 |
6 | Fang YZ, Yang S, Wu SG(2002) Free radicals, antioxidants, and nutritionNutrition18: 872879 |
7 | Yi-Fang C, Jie S, Xianzhong W, Rui HL(2002) Antioxidant and antiproliferative activities of common vegetablesJ Agric Food Chem50: 69106916 |
8 | Smith-Warner SA, Spiegelman D, Yaun SS, Albanes D, Beeson WL, van den Brandt PA, Feskanich D, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Kushi LH, Miller AB, Pietinen P, Rohan TE, Speizer FE, Willett WC, Hunter DJ(2003) Fruits, vegetables and lung cancer: A pooled analysis of cohort studiesInt J Cancer107: 10011011 |
9 | Heinonen IM, Meyer AS, Frankel EN(1998) Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidationJ Agric Food Chem46: 41074112 |
10 | Johnsen SP, Overvad K, Stripp C, Tjonneland A, Husted SE, Sorensen HT(2003) Intake of fruit and vegetables and the risk of ischaemic stroke in a cohort of Danish men and womenAm J Clin Nutr78: 5764 |
11 | Meyers KJ, Watkins CB, Pritts MP, Liu RH(2003) Antioxidant and antiproliferative activities of strawberriesJournal of Agricultural and Food Chemistry51: 2368876892 |
12 | Kevers C, Falkowski M, Tabart J, Defraigne JO, Dommes J, Pincemail J(2007) Evolution of antioxidant capacity during storage of selected fruits and vegetablesJournal of Agricultural and Food Chemistry55: 2185968603 |
13 | Hegedus A, Balogh E, Engel R, Sipos BZ, Papp J, Blazovics A(2008) Comparative nutrient element and antioxidant characterization of berry fruit species and cultivars grown in HungaryHortscience43: 617111715 |
14 | Chen L, Xin X, Yuan Q, Su D, Liu W(2014) Phytochemical properties and antioxidant capacities of various colored berriesJ Sci Food Agric94: 2180188 |
15 | Pinto MD, Lajolo FM, Genovese MI(2007) Bioactive compounds and antioxidant capacity of strawberry jamsPlant Food Hum Nutr62: 3127131 |
16 | Szajdek A, Borowska EJ(2008) Bioactive compounds and health-promoting properties of Berry fruits: A reviewPlant Food Hum Nutr63: 4147153 |
17 | Dujmović Purgar D, Duralija B, Voća S, Vokurka A, Ercisli SA(2012) Comparison of fruit chemical characteristics of two wild grown Rubus species from different locations of CroatiaMolecules17: 1039010398 |
18 | Terefe NS, Yang YH, Knoerzer K, Buckow R, Versteeg C(2010) High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry pureeInnov Food Sci Emerg Tech11: 5260 |
19 | Wang H, Nair MG, Strasburg GM, Chang YC, Booren AM, Gray JI, Dewitt DL(1999) Antioxidant and anti-inflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherriesJ Nat Prod62: 294296 |
20 | Clifford MN(2000) Anthocyanins— Nature, occurrence and dietary burdenJ Sci Food Agric80: 10631072 |
21 | Matsumoto H, Nakamura Y, Hirayama M, Yoshiki Y, Okubo K(2002) Antioxidant activity of black currant anthocyanin aglycons and their glycosides measured by chemiluminescence in a neutral pH region and in human plasmaJ Agric Food Chem50: 50345037 |
22 | Kong J, Chia L, Goh N, Chia T, Brouillard R(2003) Analysis and biological activities of anthocyaninsPhytochemisty64: 923933 |
23 | Rossi A, Serraino I, Dugo P, di Paola R, Mondello L, Genovese T, Morabito D, Dugo G, Sautebin L, Caputi AP(2003) Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammationFree Rad Res37: 891900 |
24 | Voæa S, Dobrièeviæ N, Dragoviæ-Uzelac V, Duralija B, Družiæ J, Èmelik Z, Skendroviæ-Babojeliæ M(2008) Fruit quality of new early ripening strawberry cultivars in CroatiaFood Tech Biotech46: 292298 |
25 | Sadilova E, Stintzing FC, Kammerer DR, Carle R(2009) Matrix dependent impact of sugar and ascorbic acid addition on color and anthocyanin stability of black carrot, elderberry and strawberry single strength and from concentrate juices upon thermal treatmentFood Res Int42: 10231033 |
26 | Jiménez-Garcia SN, Guevara-González RG, Miranda-López R, Feregrino-Perez AA, Torres-Pacheco I, Vázquez-Cruz MA(2013) Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology and genomicsFood Res Int54: 111951207 |
27 | Viskelis P, Rubinskiene M, Jasutiene I, Sarkinas A, Daubaras R, Cesoniene L(2009) Anthocyanins, antioxidative, and antimicrobial properties of american cranberry (Vaccinium macrocarpon Ait) and their press cakes. J Food Sci74: 2157161 |
28 | Beekwilder J, Jonker H, Meesters P, Hall RD, van der Meer IM, de Vos CHR(2005) Antioxidants in raspberry: On-line analysis links antioxidant activity to a diversity of individual metabolitesJournal of Agricultural and Food Chemistry53: 933133320 |
29 | Wang SY, Millner P(2009) Effect of different cultural systems on antioxidant capacity, phenolic content, and fruit quality of strawberries (Fragaria x ananassa Duch.)J Agric Food Chem57: 96519657 |
30 | López Aranda, JM. The cultivation of the strawberry in Huelva. In: Junta de Andalucía (Ed.). The strawberry crop at Huelva. Ideas, Exclusivas y Publicidad S.L., Sevilla (Spain); 2008. pp. 101–174 |
31 | Medina JJ, Miranda L, Soria C, Palencia P, López-Aranda JM(2009) Non-chemical alternatives to methyl bromide for strawberry: biosolarization as a case-study in HuelvaActa Hortic842: 961964 |
32 | Ariza MT, Soria C, Medina-Mínguez JJ, Martínez-Ferri E(2012) Incidence of Misshapen Fruits in Strawberry Plants Grown under Tunnels Is Affected by Cultivar, Planting Date, Pollination, and Low TemperaturesHortscience47: 15691573 |
33 | Hancock JFStrawberries1999WallingfordCAB International |
34 | Slinkard K, Singleton VL(1977) Total phenol analysis: Automation and comparison with manual methodsAm J Enol Vitic28: 4955 |
35 | Singleton VL, Orthofer R, Lamuela-Raventos RM(1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagentMethods Enzymol299: 152178 |
36 | Tulipani S, Mezzetti B, Capocasa F, Bompadre S, Battino M(2008) Antioxidants in strawberry: From the genotype to the fruit compositionProg Nutr10: 224229 |
37 | Cheng GW, Breen PJ(1991) Activity of phenylalanine ammonialyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruitJ Am Soc Hortic Sci116: 865869 |
38 | Giusti MM, Wrolstad RE. Unit F1.2.1-13. Anthocyanins. Characterization and measurement with Uvvisible spectroscopy. In: R.E. Wrosltad (Ed.), Current Protocols in Food Analytical Chemistry. New York: Willey; 2001 |
39 | Cerezo AB, Cuevas EP, Winterhalter MC, Garcia-Parrilla Troncoso AM(2010) Isolation, identification, and antioxidant activity of anthocyanin compounds in ‘Camarosa’ strawberryFood Chem123: 574582 |
40 | Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C(1999) Antioxidant activity applying an improved ABTS radical cation decolorization assayFree Radical Biology and Medicine26: 9-1012311237 |
41 | Sturm K, Koron D, Stampar F(2003) The composition of fruit of different strawberry varieties depending on maturity stageFood Chemistry83: 3417422 |
42 | Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA(2000) Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processingJ Agric Food Chem48: 45814589 |
43 | Alavoine F, Crocho M(1989) Taste quality of strawberryActa Hort265: 449452 |
44 | Olsson ME, Ekvall J, Gustavsson KE, Nilsson J, Pillai D, Sjoholm I, Svensson U, Akesson B, Nyman MGL(2004) Antioxidants, low molecular weight carbohydrates, and total antioxidant capacity in strawberries (Fragaria x ananassa): Effects of cultivar, ripening, and storageJ Agric Food Chem52: 24902498 |
45 | Pincemail J, Kevers C, Tabart J, Defraigne JO, Dommes J(2012) Cultivars, culture conditions and harvest time influence phenolic and ascorbic acid contents and antioxidant capacity of strawberry (Fragaria x ananassa)J Food Sci Chem77: 205210 |
46 | Aaby K, Ekeberg D, Skrede G(2007) Characterization of phenolic compounds in strawberry (Fragaria x ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacityJ Agric Food Chem5: 43954406 |
47 | Leong LP, Shui G(2002) An investigation of antioxidant capacity of fruits in Singapore marketsFood Chemistry76: 6975 |
48 | Buendia B, Gil MI, Tudela JA, Gady AL, Medina JJ, Soria C, Lopez JM, Tomas-Barberan FA(2010) HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivarsJ Agric Food Chem58: 39163926 |
49 | Cheplick S, Kwon YI, Bhowmik P, Shetty K(2010) Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertensionBiores Technol101: 404413 |
50 | Wang QL, Tury E, Rekika D, Charles MT, Tsao R, Hao YJ, Dube C, Khanizadeh S(2010) Agronomic characteristics and chemical composition of newly developed dayneutral strawberry lines by agriculture and agri-food canadaInt J Food Proper13: 12341243 |
51 | Tulipani S, Marzban G, Herndl A, Laimer M, Mezzetti B, Battino M(2011) Influence of environmental and genetic factors on health-related compounds in strawberryFood Chem124: 906913 |
52 | Ariza MT, Soria C, Medina JJ, Martínez-Ferri E(2011) Fruit misshapen in strawberry cultivars (Fragaria×ananassa) is related to achenes functionalityAnnals of Applied Biology158: 1130138 |
53 | Wang SY, Zheng W(2001) Effect of plant growth temperature on antioxidant capacity in strawberryJ Agric Food Chem49: 49774982 |
54 | Ayala-Zavala FJ, Wang SY, Wang CY, González-Aguilar GA(2004) Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruitLebensm.-Wiss. u.-Technol37: 687695 |
55 | Lee SK, Kader AA(2000) Preharvest and postharvest factors influencing vitamin C content of horticultural cropsPostharvest Biol. Technol20: 207220 |
Figures and Tables
Fig.1
Accumulated Extra-early (January to February), Early (March) and Total (January to May) fruit yield (g/plant) on the ten strawberry cultivars under standard cropping conditions at Huelva during the 2014 cropping-season. Means within the same period followed by different letters were significantly different (p < 0.01). Vertical bars are mean ± SE (n = 3 blocks of 50 plants).
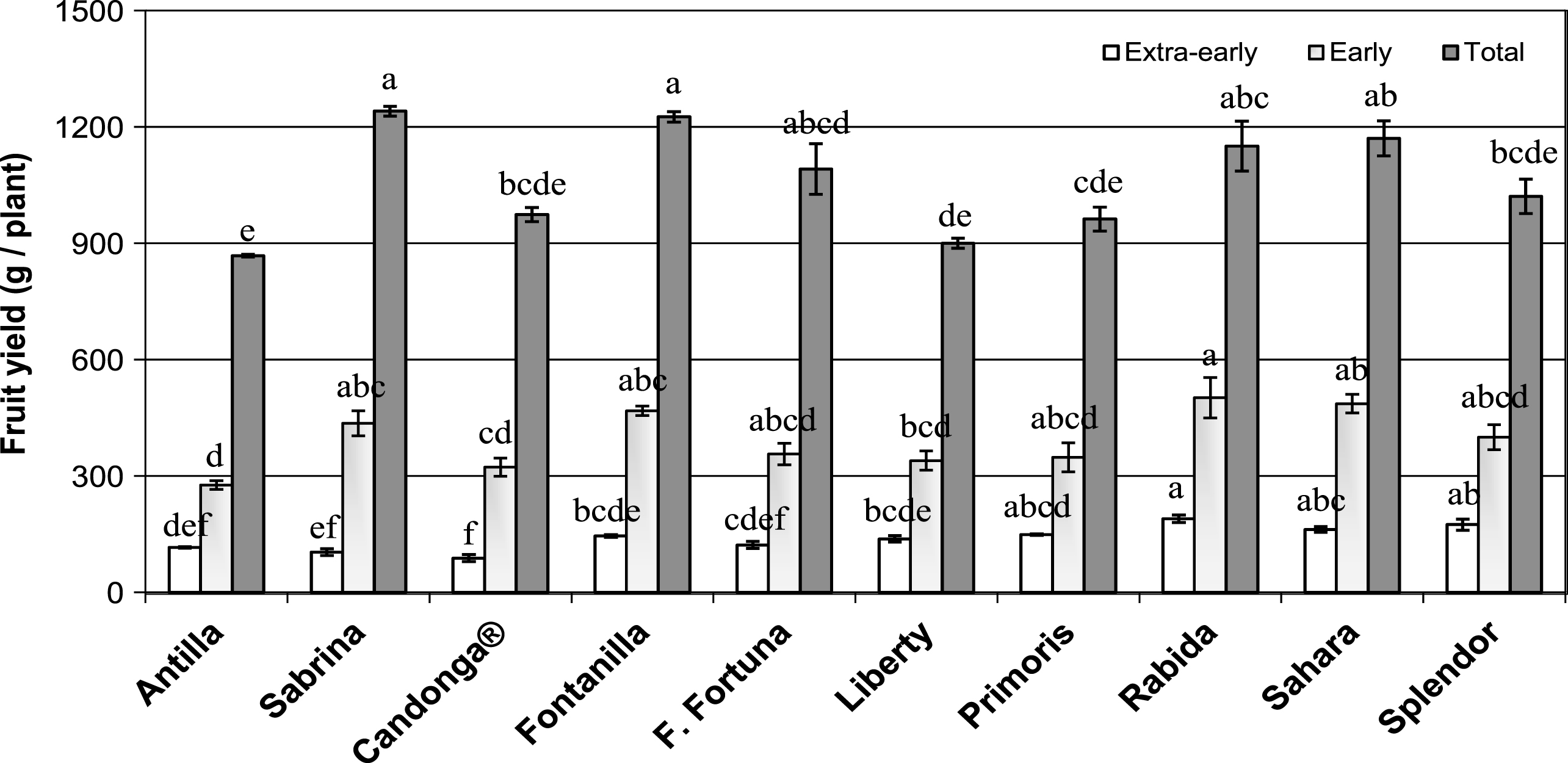
Fig.2
Fruit content of citric acid (A), ascorbic acid (B), total phenolics (C), anthocyanins (D) and fruit antioxidant capacity (TEAC; E) of the 10 strawberry cultivars during the 2014 cropping-season. Fruits were analyzed at the three harvest times (Extra-early: 19th February; Early: 21st March; Late: 9th April).
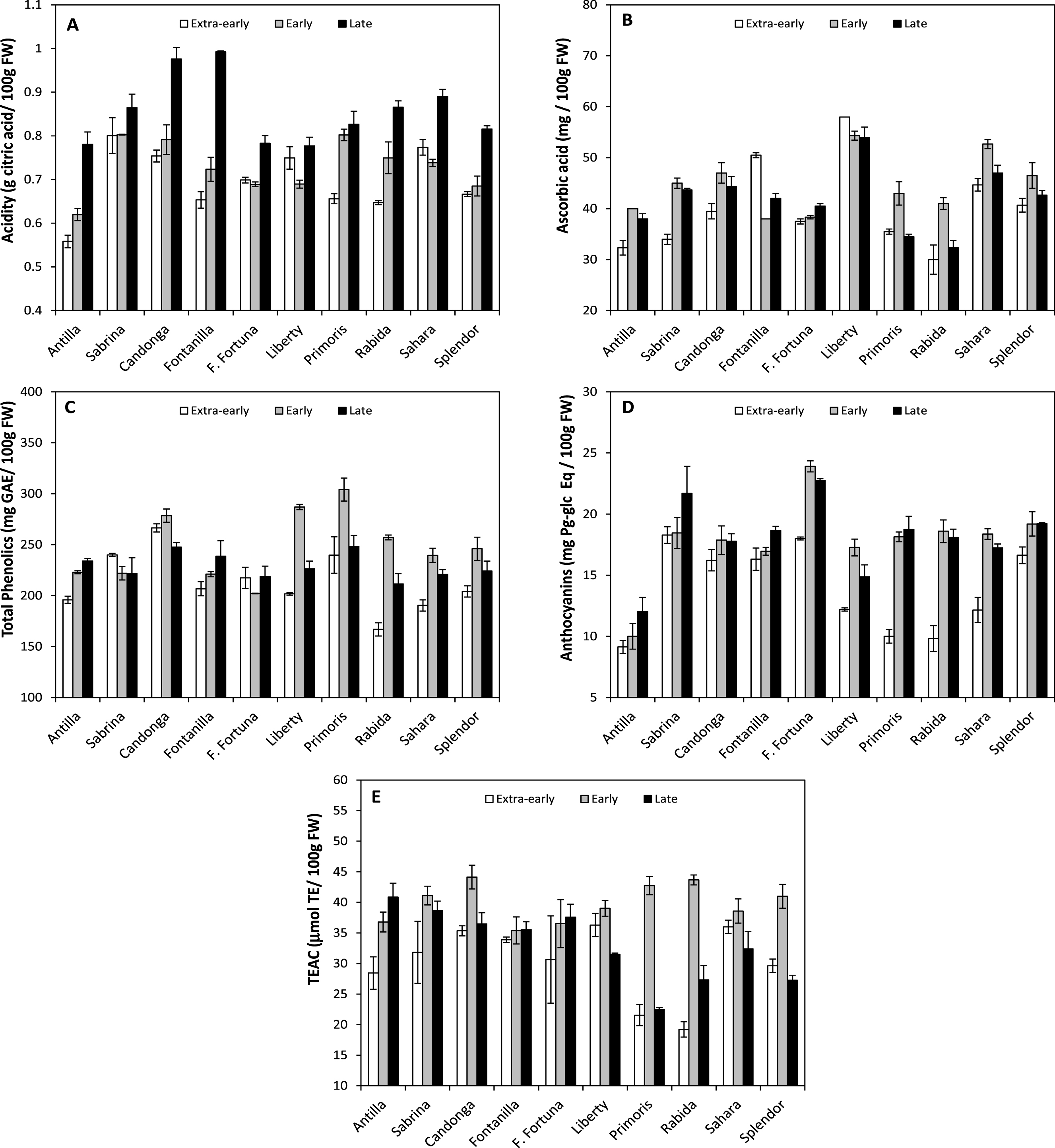
Table 1
Content of citric acid, ascorbic acid, total phenolics, anthocyanins, and antioxidant capacity (TEAC) in fruits of ten strawberry cultivars. Data are the mean ± SE (n = 9). Means within the same column followed by different letters were significantly different at p < 0.001
| Cultivar | Citric acid (g CA/100 g FW) | Ascorbic acid (mg AA / 100 g FW) | Phenolics (mg GAE / 100 g FW) | Anthocyanins (mg Pg-glc Eq / 100 g FW) | TEAC (μmol TEq / g FW) |
| ‘Antilla’ | 0.65 ± 0.04 b | 36.4 ± 1.3 de | 217.6 ± 6.3 b | 10.4 ± 0.7 d | 35.4 ± 2.3 ab |
| ‘Sabrina’ | 0.82 ± 0.02 a | 41.3 ± 1.8 bcde | 227.0 ± 6.4 ab | 19.5 ± 1.0 ab | 37.0 ± 2.4 ab |
| Candonga ® | 0.84 ± 0.04 a | 43.7 ± 1.4 bc | 263.9 ± 5.7 a | 17.3 ± 0.6 abc | 39.1 ± 1.7 a |
| ‘Fontanilla’ | 0.76 ± 0.05 ab | 43.5 ± 2.0 bcd | 222.2 ± 7.1 b | 17.3 ± 0.5 abc | 35.0 ± 0.9 ab |
| ‘Fortuna’ | 0.73 ± 0.02 ab | 38.7 ± 0.5 cde | 212.7 ± 5.3 b | 21.4 ± 1.0 a | 34.3 ± 2.9 ab |
| ‘Liberty’ | 0.74 ± 0.02 ab | 55.3 ± 0.8 a | 232.2 ± 12.8 ab | 14.4 ± 0.8 cd | 36.1 ± 1.3 ab |
| ‘Primoris’ | 0.76 ± 0.03 ab | 38.4 ± 1.7 cde | 264.0 ± 12.9 a | 15.6 ± 1.5 bc | 25.5 ± 4.0 b |
| ‘Rabida’ | 0.75 ± 0.04 ab | 34.4 ± 2.1 e | 217.4 ± 13.8 b | 15.5 ± 1.6 bc | 30.1 ± 3.0 ab |
| ‘Sahara’ | 0.81 ± 0.03 a | 48.1 ± 1.4 b | 216.8 ± 8.2 b | 15.8 ± 1.1 bc | 35.2 ± 1.5 ab |
| ‘Splendor’ | 0.72 ± 0.03 ab | 42.9 ± 1.1 bcd | 224.8 ± 8.1 ab | 18.3 ± 0.6 abc | 33.8 ± 2.5 ab |
Table 2
Content of citric and ascorbic acid, total phenolics, total anthocyanins and antioxidant capacity (TEAC) in fruits across the ten strawberry cultivars in three harvesting times (19th February, 21st March and 9th April). Data are the mean ± SE (n = 30). Means within the same line followed by different letters were significantly different at p < 0.05
| 19th February | 21st March | 9th April | |
| Citric acid (g CA / 100 g FW) | 0.70 ± 0.01 c | 0.73 ± 0.01 b | 0.85 ± 0.01 a |
| Ascorbic acid (mg AA / 100 g FW) | 39.7 ± 1.6 c | 44.8 ± 1.1 a | 41.8 ± 1.1 b |
| Phenolics (mg GAE / 100 g FW) | 212.2 ± 5.2 c | 248.4 ± 6.2 a | 229.0 ± 3.5 b |
| Anthocyanins (mg Pg-glc Eq / 100 g FW) | 13.9 ± 0.7 b | 17.9 ± 0.7 a | 18.1 ± 0.6 a |
| TEAC (μmol TEq / g FW) | 30.1 ± 1.4 b | 39.9 ± 0.7 a | 32.8 ± 1.2 b |
Table 3
Pearson correlation coefficient (R2) of the different parameters analyzed (citric acid, antioxidant capacity (TEAC), Anthocyanins, and phenolics compounds) in strawberry
| TEAC | Anthocyanins | Phenolics | Ascorbic acid | |
| Citric acid | 0.1875 ns | 0.4668 ** | 0.2749 ns | 0.1284 ns |
| TEAC | 0.3613 * | 0.5176 ** | 0.5044 ** | |
| Anthocyanins | 0.3002 ns | 0.1335 ns | ||
| Phenolics | 0.2459 ns |
n = 90. ns: not significant, *and **: significant differences at p < 0.05 and at p < 0.01, respectively.



