Demographics and Medication Use of Patients with Late-Onset Alzheimer’s Disease in Hong Kong
Abstract
Background:
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly population. However, epidemiological studies on the demographics of AD in Hong Kong population are lacking.
Objective:
We investigated the demographics, comorbidities, mortality rates, and medication use of patients with AD in Hong Kong to understand how the disease has been managed locally.
Methods:
This was a collaborative study of The Hong Kong University of Science and Technology and the Hospital Authority Data Collaboration Lab. We analyzed the demographic data, clinical records, diagnoses, and medication records of patients with AD under the care of the Hospital Authority between January 1, 2007 and December 31, 2017.
Results:
We identified 23,467 patients diagnosed with AD. The median age at diagnosis was 84 years old, and 71% of patients were female. The most common comorbidity was hypertension (52.6%). 39.9% of patients received medications for dementia; of those, 68.4% had taken those medications for > 1 year. Compared to nonusers, long-term AD medication users had a significantly younger age of AD onset and were taking more lipid-regulating medication, diabetes medication, or antidepressants. Surprisingly, the use of antipsychotics in patients with AD was quite common; 50.7% of patients had received any type of antipsychotic during disease progression.
Conclusion:
This study provides detailed information on the demographics and medication use of patients with AD in Hong Kong. The data from this AD cohort will aid our future research aiming to identify potential AD risk factors and associations between AD and other diseases.
INTRODUCTION
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly population. By 2040, an estimated 80 million people worldwide will have dementia [1]. In 2018, more than 7 million elderly people in China had AD. In 2020, the estimated total costs of managing AD in China exceeded 200 billion USD, highlighting the disease’s immense burden on society [2]. In Hong Kong, the elderly population (≥65 years old) has grown 4.3% annually over the past decade, reaching 1 million people in 2016 [3]. A 2018 meta-analysis based on two studies published in 1998 and 2008 estimated the prevalence of dementia in Hong Kong to be 7.2% [4–6]. However, studies on the prevalence of AD specifically in Hong Kong are lacking. Moreover, few studies have investigated the demographics or medication use of patients with AD in Hong Kong. There is an urgent need to study patients with AD in Hong Kong to improve patient care, guide government planning, estimate the social and economic costs of AD, and provide valuable information for research on AD pathogenesis and novel treatment strategies.
To understand the demographics and medication use of local patients with AD, The Hong Kong University of Science and Technology and Data Collaboration Lab of the Hospital Authority (HA) in Hong Kong conducted a collaborative study. The Data Collaboration Lab, which provides the ‘Big Data Analytics Platform’ to researchers for clinical data analysis, granted our team access to the demographic data, clinical records, diagnoses, investigations, and medication records of patients under the care of the HA. Data provided by the Big Data Analytics Platform is generated based on both written medical records and the electronic medical system, which hosts and manages the medical records in the public healthcare system under the Hospital Authority in Hong Kong. To identify patients with AD, we made the clinical diagnosis based on the ICD-10 codes. Using the Big Data Analytics Platform, we identified patients documented as having AD over a 10-year period and investigated their demographics, comorbidities, medication use, and mortality rates. As the public healthcare services under the HA cover more than 90% of the secondary and tertiary services in Hong Kong [7], this cohort is representative of patients with AD in the Hong Kong elderly population.
MATERIALS AND METHODS
Subject selection
Using the Big Data Analytics Platform, we identified subjects who were≥65 years old and had at least one inpatient or outpatient record of an AD-related ICD-10 code between January 1, 2007 and December 31, 2017 (n = 28,838). Meanwhile, we excluded subjects with documented neurodegenerative disorders other than AD (n = 2,572) since the underlying pathological changes in non-AD dementia differ from those in AD (Supplementaryv Tables 1 and 2). We subsequently applied a case filter according to the patients’ drug dispensing histories and clinical notes (Supplementary Figure 1).
Data analysis
We then analyzed the demographic characteristics, comorbidities, clinical notes, and medication use of the AD cohort. We determined the presence of comorbidities by identifying diagnoses based on ICD-10 codes (Supplementary Table 3). We used the keyword ‘death’ to search the clinical notes for records relating to the episode of death. Two neurologists determined each subject’s principal cause of death based on the clinical notes. We subsequently calculated annual mortality rates based on the cumulative number of mortalities and the cumulative number of AD cases per year.
Medication use
We defined medication use as a single medication-dispensing episode for at least 21 consecutive days after a diagnosis of AD was made. British National Formulary codes were used to identify the different types of medication dispensed (Supplementary Table 4). We excluded prescription records from when a patient was < 65 years old and/or before an AD diagnosis was made. From the prescription records, we identified patients who had at least one dispensing episode of a medication for dementia. Next, we determined the duration of each dispensing record. If the record of one dispensing episode overlapped with the subsequent dispensing record, the interval between the earliest start date and latest end date was used as the drug-dispensing duration of those consecutive prescriptions. Finally, we calculated the total duration of medication use by summing the duration of all dispensing episodes for each patient (Supplementary Figure 2). We also identified those patients who had at least one dispensing record of antipsychotics, antidepressants, or hypnotics.
Protection of personal health information
All personal health information (i.e., names, Hong Kong Identity Card numbers, hospital record numbers, etc.) was removed from the study dataset. All information from the Big Data Analytics Platform was de-identified and encrypted to ensure confidentiality. Access to the platform was restricted to researchers involved in this study. This project was approved by the Committee on Research Practices of The Hong Kong University of Science and Technology.
Statistical analysis
Continuous variables were compared using 2-sided Student’s t-tests, whereas categorical variables were compared using Yates’ χ2-test. p-values < 0.05 were considered statistically significant. We performed all analyses using RStudio Desktop version 1.4.1106 (Boston, MA, USA).
RESULTS
Demographics and comorbidities
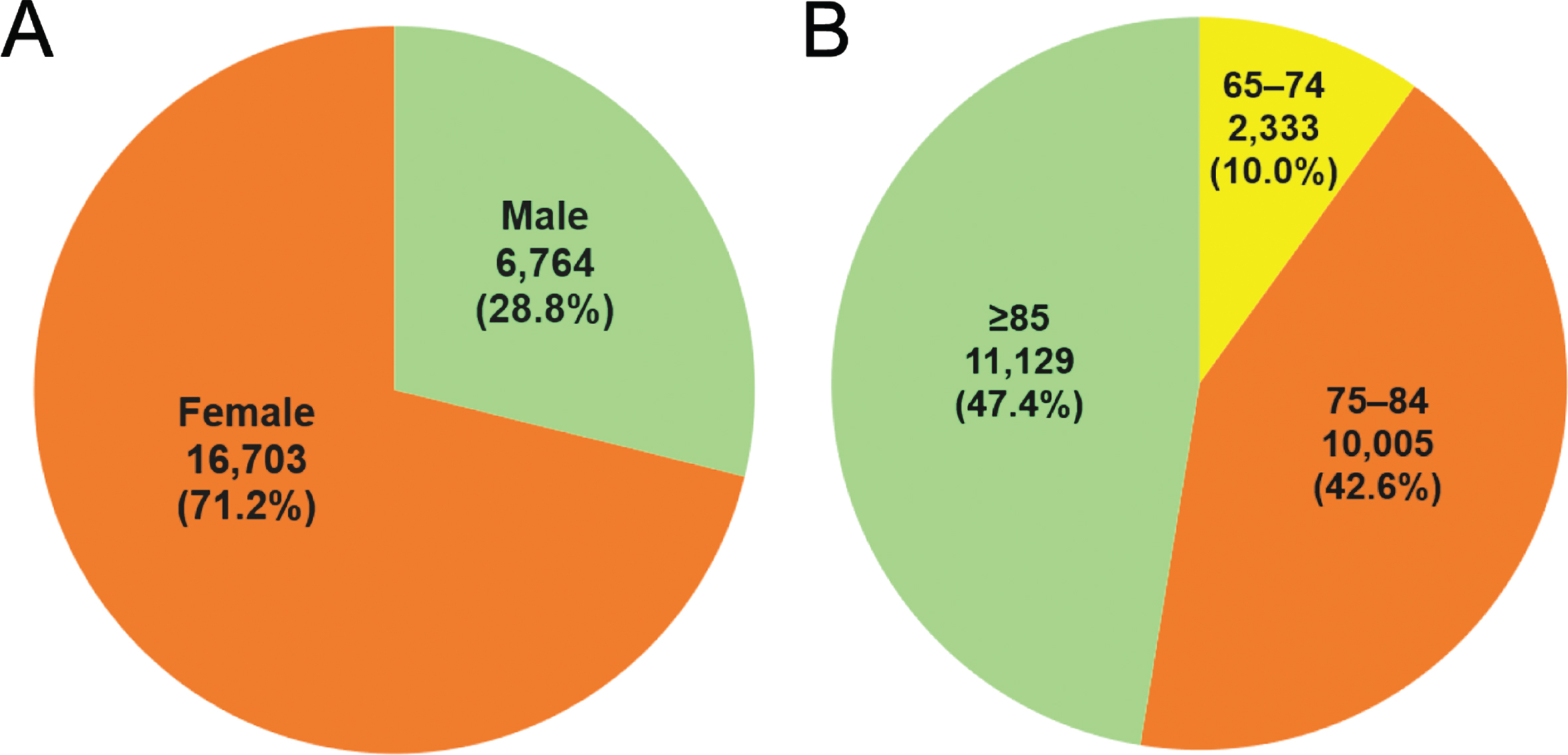
The AD cohort comprised 23,467 patients diagnosed with AD between January 1, 2007 and December 31, 2017; of these patients, 71% (n = 16,703) were female and 29% (n = 6,764) were male (Fig. 1A). The median age at AD diagnosis was 84 years old (range: 77–91). Regarding age at diagnosis, 90% of patients (n = 21,134) were≥75 years old, 47.4% (n = 11,129) were≥85 years old, and 10% (n = 2,333) were < 75 years old (Fig. 1B).
Fig. 1
Sex and age at Alzheimer’s disease (AD) diagnosis in the Hong Kong population from 2007–2017. A total of 23,467 patients with documented AD were selected from the Big Data Analytics Platform of the Hospital Authority Data Collaboration Lab from 2007–2017. (A) Sex and (B) age at the time of AD diagnosis.
The most common comorbidity among patients with AD was hypertension (52.6%, n = 12,343) followed by diabetes mellitus (46.7%, n = 10,969) and chronic renal diseases (18.8%, n = 4,409) (Table 1). Approximately 17% of patients had a history of cerebrovascular accidents (n = 4,148), the majority of which were ischemic strokes (n = 3,463), and 12% had a history of coronary heart disease (n = 2,833). In addition, 11.4% of patients (n = 2,682) had a history of cancer, with colorectal cancers being the most common type (2.5%, n = 576) followed by lung cancers (1.6%, n = 369).
Table 1
Comorbidities of patients with Alzheimer’s disease
| Comorbidities | Number (%) |
| Hypertension | 12,343 (52.6) |
| Diabetes Mellitus | 10,969 (46.7) |
| Hyperlipidemia | 2,501 (10.7) |
| Cerebrovascular accident | 4,148 (17.7) |
| Ischemic stroke | 3,463 (14.8) |
| Hemorrhagic stroke | 685 (2.9) |
| Coronary heart diseases | 2,833 (12.1) |
| Cancers | 2,682 (11.4) |
| Colorectal | 576 (2.5) |
| Lung | 369 (1.6) |
| Breast | 320 (1.4) |
| Chronic renal diseases | 4,409 (18.8) |
| Chronic respiratory diseases | 2,650 (11.3) |
| Asthma | 573 (2.4) |
| COAD | 2,077 (8.9) |
| Chronic liver diseases | 1,039 (4.4) |
COAD, chronic obstructive airway diseases
Mortality rates
Between 2007 and 2017, 15.3% of patients with AD (n = 3,599) died. Annual mortality rates gradually increased from 80 deaths (3.2%) in 2007 to 427 deaths (15.4%) in 2017 (Fig. 2). The most common principal cause of death was pneumonia (54.3%, n = 1,954) followed by acute myocardial infarction (5.6%, n = 203) and cancers (5.3%, n = 192). Over 70% of patients passed away at an age of≥85 (73.4%, n = 2,643). Lung cancers accounted for most cancer-related deaths (n = 55) followed by colorectal cancers (n = 31) and hepatocellular carcinoma (n = 16). Septicemia (3.5%, n = 125), urinary tract infections (2.9%, n = 104), and bedsores (1.8%, n = 65) were causes of death in patients with AD (Table 2).
Fig. 2
Alzheimer’s disease (AD) mortality in Hong Kong from 2007–2017. A) Cumulative AD cases (blue bars) and cumulative mortality of patients with AD (black line with triangles) between January 1, 2007 and December 31, 2017. B) Cumulative AD cases (blue bars) and annual mortality rates (yellow line with circles).
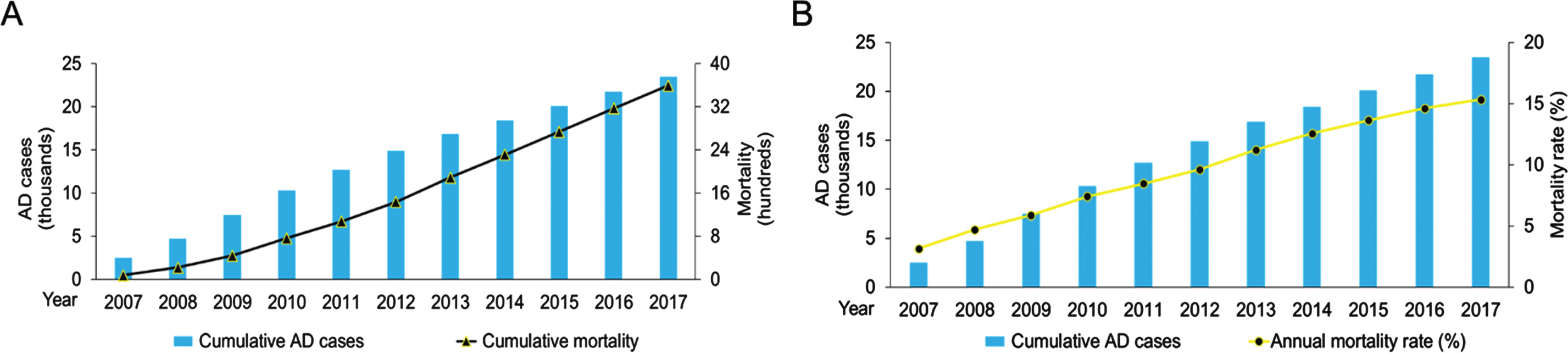
Table 2
Top ten causes of death in Alzheimer’s disease
| Causes of death (Top 10) | Number (%) |
| Pneumonia | 1,954 (54.3) |
| Acute myocardial infarction | 203 (5.6) |
| Cancers | 192 (5.3) |
| Sepsis | 125 (3.5) |
| Congestive heart failure | 116 (3.2) |
| Cerebrovascular accident | 112 (3.1) |
| Urinary tract infections | 104 (2.9) |
| Renal failure | 72 (2.0) |
| Infected bedsores | 65 (1.8) |
| Gastrointestinal bleeding | 55 (1.5) |
Medication use
The most commonly prescribed medications for patients with AD are listed in Table 3. The most dispensed medications were those for hypertension (67.4%, n = 15,813) including calcium-channel blockers (52.3%, n = 12,275), angiotensin-converting enzyme inhibitors (27.3%, n = 6,395), and beta-adrenoceptor blocking drugs (23.7%, n = 5,570). Antiplatelet medications, which serve as both treatment and prophylaxis for atherosclerotic arterial diseases, were also commonly prescribed (42%, n = 9,856). Meanwhile, 26.5% of patients (n = 6,216) took lipid-regulating medications. Surprisingly, only 22.2% of patients (n = 5,215) took medications for diabetes.
Table 3
Medication use in patients with Alzheimer’s disease
| Medication | Number (%) |
| Drugs for dementia | |
| Total | 9,351 (39.9) |
| Donepezil | 4,879 (20.8) |
| Galantamine | 4,013 (17.1) |
| Memantine | 4,024 (17.2) |
| Rivastigmine | 2,529 (10.8) |
| Drugs for hypertension | |
| Total | 15,813 (67.4) |
| Calcium-channel blockers | 12,275 (52.3) |
| ACEI | 6,395 (27.3) |
| Beta-adrenoceptor blocking drugs | 5,570 (23.7) |
| Drugs for diabetes | |
| Total | 5,215 (22.2) |
| Sulphonyureas | 3,184 (13.6) |
| Metformin | 3,526 (15.0) |
| Short acting insulins | 1,709 (7.3) |
| Intermediate and long acting insulins | 1,238 (5.3) |
| Lipid-regulating drugs | |
| Total | 6,216 (26.5) |
| Simvastatin | 5,682 (24.2) |
| Atorvastatin | 500 (2.1) |
| Gemfibrosil | 194 (0.8) |
| Antiplatelets | |
| Total | 9.856 (42.0) |
| Antipsychotics | |
| Total | 11,895 (50.7) |
| Haloperidol | 5,908 (25.2) |
| Quetiapine | 5,128 (21.9) |
| Risperidone | 2,333 (9.9) |
| Antidepressants | |
| Total | 9,457 (40.3) |
| TCA and related anti-depressants | 5,697 (24.3) |
| Selective serotonin reuptake inhibitors | 5,231 (22.3) |
| Hypnotics | |
| Total | 6,819 (29.1) |
ACEI, angiotensin-converting enzyme inhibitors; TCA, tricyclic anti-depressants
In addition, 39.9% of patients with AD (n = 9,351) received medications for dementia. Antipsychotic drug use was common: 50.7% of patients (n = 11,895) had taken antipsychotics at least once, with haloperidol being the most prescribed type (25.2%, n = 5,908). Antidepressant use was also common (40.3%, n = 9,457), and 29.1% of patients (n = 6,819) used hypnotics.
Among patients who received medications for dementia (‘AD medication users’), 68.4% (n = 6,395) did so for > 1 year— half of whom (n = 3,241) did so for > 3 years (Fig. 3). Accordingly, we found several significant differences between patients who did not take any medications for dementia (‘AD medication nonusers’) (n = 13,077) with those who took such medications for > 1 year (‘long-term AD medication users’) (n = 6,395) (Table 4). First, long-term AD medication users had a younger median age at AD diagnosis than nonusers (82 versus 86 years, respectively; p < 0.001). In addition, more long-term AD medication users than nonusers used lipid-regulating medications (37.6% versus 21.7%, respectively; p < 0.001), diabetes medications (25.5% versus 21.9%, respectively; p < 0.001), and antidepressants (45.1% versus 41.1%, respectively; p < 0.001). However, antipsychotic use was less common in long-term AD medication users than nonusers (49.0% versus 56.3%, respectively; p < 0.001).
Fig. 3
Duration of medication use for dementia in patients with Alzheimer’s disease in Hong Kong from 2007–2017. A total of 9,351 patients received medication for dementia during the study period.
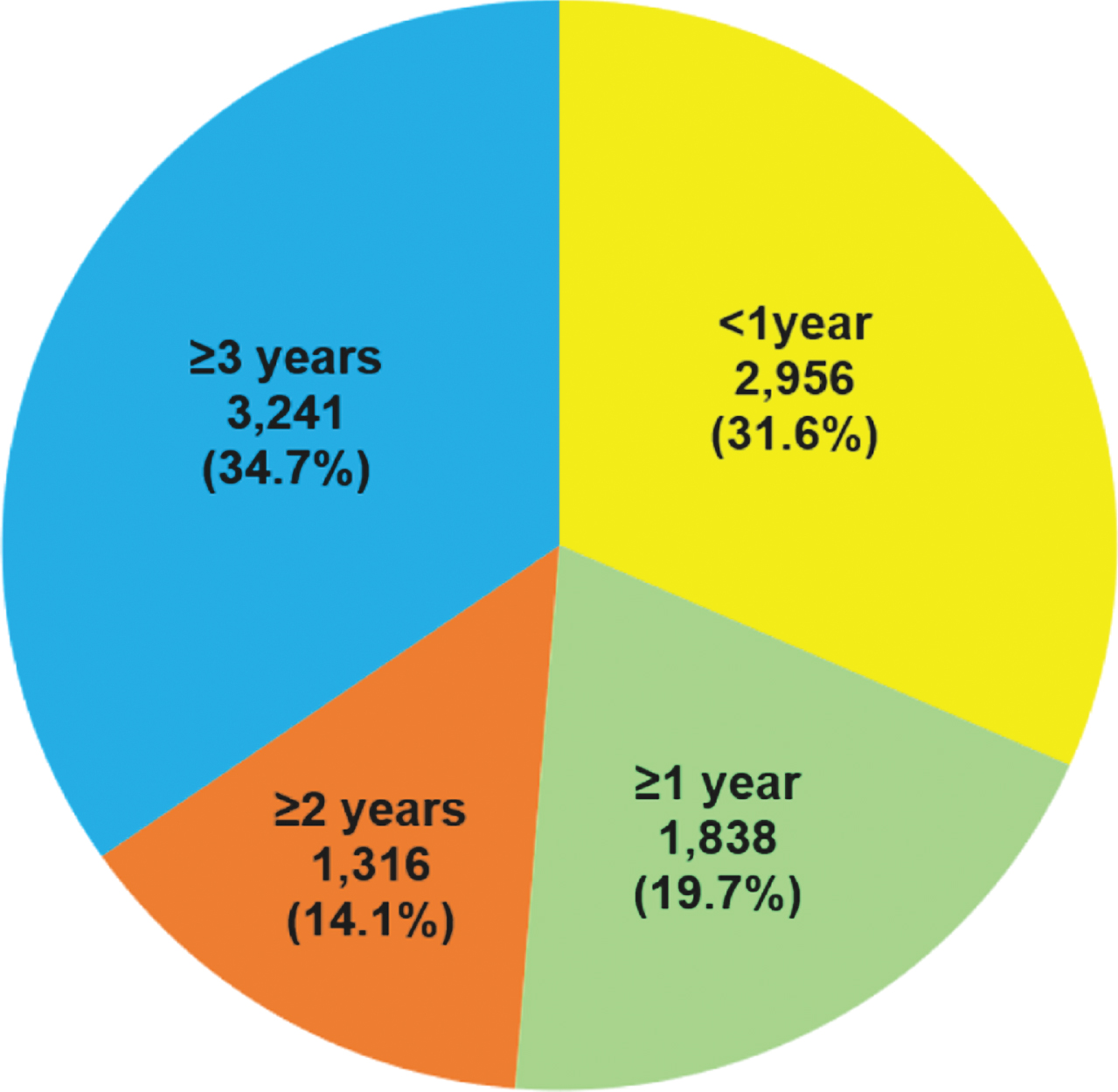
Table 4
Comparison between patients who had not taking medication for Alzheimer’s disease and those who took medication for longer than one year
| AD medication nonusers Number (%) | AD medication long-term users Number (%) | p | |
| Total | 13,077 | 6,395 | |
| Male | 3,692 (28.2) | 1,815 (28.4) | 0.84 |
| Female | 9,385 (71.8) | 4,580 (71.6) | |
| Median age at diagnosis | 86 | 82 | < 0.001 |
| Medication | |||
| Drugs for hypertension | 9,273 (70.9) | 4,570 (71.5) | 0.44 |
| Calcium-channel blockers | 7,098 (54.3) | 3,678 (57.5) | < 0.001 |
| ACEI | 3,748 (28.7) | 1,873 (29.3) | 0.37 |
| Beta-adrenoceptor blocking drugs | 3,307 (25.3) | 1,566 (24.5) | 0.23 |
| Drugs for diabetes | 2,863 (21.9) | 1,633 (25.5) | < 0.001 |
| Oral hypoglycemic drugs | 2,416 (18.5) | 1,548 (24.2) | < 0.001 |
| Insulins | 1,416 (10.8) | 580 (9.1) | < 0.001 |
| Lipid regulating drugs | 2,832 (21.7) | 2,405 (37.6) | < 0.001 |
| Antiplatelets | 5,763 (44.1) | 2,865 (44.8) | 0.34 |
| Antipsychotics | 7,366 (56.3) | 3,133 (49.0) | < 0.001 |
| Antidepressants | 5,375 (41.1) | 2,886 (45.1) | < 0.001 |
| Hypnotics | 4,113 (31.5) | 1,938 (30.3) | 0.11 |
ACEI, angiotensin-converting enzyme inhibitors
As patients with moderate-to-severe AD can present with neuropsychiatric symptoms, we examined patients’ simultaneous use of medications for dementia, antipsychotics, antidepressants, and hypnotics (Table 5). Combining antipsychotics and hypnotics was significantly lower among long-term AD medication users than nonusers (16.3% versus 19%, respectively; p < 0.001). Approximately 14–20% of patients with AD required more than one type of medication to control neuropsychiatric symptoms, while 6–7% had a history of simultaneously using antipsychotics, antidepressants, and hypnotics.
Table 5
Combination of medication use in patients with Alzheimer’s disease
| AD medication nonusers Number (%) | AD medication long-term users Number (%) | p | |
| Total | 13,077 | 6,395 | |
| Medication combination | |||
| Antipsychotics + Antidepressants | 2,528 (19.3) | 1,275 (19.9) | 0.33 |
| Antipsychotics + Hypnotics | 2,481 (19.0) | 1,040 (16.3) | < 0.001 |
| Antidepressants + Hypnotics | 1,801 (13.8) | 902 (14.1) | 0.25 |
| All three drugs | 908 (6.9) | 417 (6.5) | 0.28 |
DISCUSSION
Our study is the first to provide detailed information about the demographics, comorbidities, mortality rates, and medication use of patients with AD in the Hong Kong elderly population over a 10-year period (2007–2017). While several local studies have focused on patients with dementia, AD-focused studies are limited and mostly single-center studies; therefore, those studies may not be representative of the whole Hong Kong population. Accordingly, in this study, we used the Big Data Analytics Platform of the HA Data Collaboration Lab to examine medical records from public healthcare services in Hong Kong. Specifically, we investigated the demographics, comorbidities, mortality rates, and medication use of patients with AD in Hong Kong. These data may help our local authorities, clinicians, scientists, and the public become more aware of how AD has been managed locally.
Our AD cohort was predominantly female (71%), which may be related to the longer life expectancy of the female population in Hong Kong. In 2016, the female-to-male ratio in the Hong Kong population aged > 85 years was 1.9:1 [3]. In 2019, the life expectancy of females and males was 88.1 and 82.2 years, respectively (the Hong Kong Census and Statistics Department). Since the risk of developing AD increases with age, a high female-to-male sex ratio is expected among patients with AD. A comparison of our cohort with 2 previous local studies indicates that the sex ratio of AD has remained stable over the past decade [8, 9].
Most patients with AD were diagnosed after age 85, with a median age at diagnosis of 84 years. Our cohort was older than those of studies conducted in mainland China, Taiwan, the United Kingdom, and the United States, in which most patients were 75–84 years old [10–13]. It is difficult to postulate the reason for the more advanced age for AD diagnosis in our study, as our study is retrospective and lacked a control population. One possible reason is that we only included subjects with an AD diagnosis made at age≥65. We applied an age filter to ensure a fair comparison with the general elderly population in Hong Kong, which is defined as people aged≥65 [3]. However, a previous cross-sectional survey revealed that there is insufficient public education about dementia in Hong Kong; as dementia is the second-most feared disease among the elderly, people tend to seek medical advice only when cognitive decline is advanced [14]. Another possible cause is related to APOE ɛ4 genotype in Chinese population. Our group has published a study on the genetics of an AD cohort in the Hong Kong Chinese population. Compared to the European-descent population, the Hong Kong population exhibits a lower allele frequency of APOE ɛ4 (frequency = 0.089 and 0.149, in the Hong Kong Chinese and European populations, respectively) [15]. The lower APOE ɛ4 frequency may explain, at least in part, the onset of AD in the Hong Kong Chinese population.
The number of patients taking hypertension and lipid-regulating medications exceeded the number of patients documented with comorbidities (Table 1). There are two possibilities for this finding. First, some hypertension medications are also used for treatment of heart failure and coronary heart diseases. Lipid-regulating medications can also be used as a primary prevention for atherosclerotic artery diseases. Second, comorbidities were defined based on the clinical records with ICD-10 codes; the numbers of patients with comorbidities may be underestimated if attending physicians had not used ICD-10 codes in their documentation.
In our study, the mortality rate of AD patients increased for more than three-fold over the past ten years. Similar observation is seen in other demographic studies [13, 16, 17]. The improvement of medical care quality in the past decade may improve the survival duration of AD patients, other than the overall mortalities. Pneumonia was the leading cause of death for more than half of the patients with AD in our study. Meanwhile, malignancy is the leading cause of death in the general Hong Kong population. Pneumonia is the most commonly identified immediate cause of death among older adults with AD or other dementias [13]. Our findings are concordant with those of a recent meta-analysis in which autopsy-confirmed pneumonia accounted for approximately 50% of deaths in patients with dementia. The risk of pneumonia-associated death in patients with dementia is double that of patients without dementia [18]. Loss of muscle mass and skeletal muscle strength, reduced physical activity, and immune dysregulation are common during aging, and the impaired cognitive function in AD patients further increases the risk of infection. These findings collectively highlight the need for clinicians to pay careful attention to pneumonia-related symptoms in patients with AD, especially those with difficulty in swallowing and prolonged immobilization in advanced-stage AD. A recent review on the aging population in China summarizes a number of challenges and strategies for ensuring the wellbeing of our elderly population [19]. It will be important to educate our AD patients on maintaining a healthy lifestyle, including a balanced diet and regular exercise; encouraging social engagement especially for patients who live alone; and implementing multidimensional geriatric care including palliative care programs for end-stage AD patients.
This study focused on medication use by patients with AD in Hong Kong. Medication use is closely related to disease control, specifically the control of dementia and comorbidities. Elderly patients with AD have more comorbidities than elderly people without dementia [20]. In this study, drugs for hypertension were the most commonly prescribed medication among patients with AD, which is concordant with our observation that hypertension was the most common comorbidity among these patients. Antiplatelet drug use was also common, as 12.1–18.8% of patients with AD had chronic kidney diseases, cerebrovascular accidents, or coronary artery diseases.
In our study, approximately 40% of patients with AD received medications for dementia. We compared our cohort with other large-scale studies on medication use in patients with AD. A Swedish study revealed that 73% of patients with AD took cholinesterase inhibitors and 9.8% of patients took NMDA (N-methyl-d-aspartate) antagonists [21], while a study in Taiwan found that 7.6% of patients with AD took drugs for dementia [22]. The large variation in medication use for dementia across studies is multifactorial. In Hong Kong, medications for dementia were introduced to the HA drug formulary as ‘special drugs’ between 1999 and 2011; special drugs could only be prescribed by a certain group of specialists. However, donepezil and rivastigmine were changed from ‘special drugs’ to ‘general drugs’ in 2015, while memantine became a ‘general drug’ in 2017. Therefore, these drugs would have had limited availability before 2015, which may explain why only 40% of the patients in our cohort received medications for dementia.
Meanwhile, the use of antipsychotics in our local AD cohort was high; half of the patients had taken antipsychotics at least once during the disease progression. Antipsychotics are prescribed to patients with AD to manage symptoms related to psychosis, behavioral problems, and euphoria. One local study examining the patterns of hospitalization and emergency room use of long-term care facility residents with AD revealed that psychotropic medication use was negatively associated with acute medical care, especially emergency services [8]. Another local study of Hong Kong Chinese patients with AD also identified that psychosis, behavioral problems, and mood disturbance were strongly associated with caregiver stress [23]. Thus, prescribing antipsychotics is a common local practice for treating neuropsychiatric symptoms in AD.
Moreover, we compared medication use between patients who had taken medication for dementia for > 1 year (i.e., long-term AD medication users) and those who had never been prescribed medication for dementia (i.e., AD medication nonusers). Interestingly, long-term AD medication users used lipid-regulating drugs, diabetes drugs, and antidepressants significantly more than nonusers; in particular, the difference between these 2 subgroups in the use of lipid-lowering drugs exceeded 15%. On the other hand, long-term AD medication users had much lower antipsychotic use. The variable medication use among the AD patient subgroups reflects the diversity of disease management practices. Furthermore, long-term AD medication users had a significantly lower rate of simultaneous use of antipsychotics and hypnotics than AD medication nonusers. Hypnotics are prescribed to patients with AD with sleep disturbances. The effects of medications for controlling behavioral and psychological symptoms of dementia in AD remains controversial. One meta-analysis on the pharmacological management of agitation in dementia supports the use of cholinesterase inhibitors for behavioral and psychological symptoms [24], but such benefits have not been observed in the Chinese population [25].
Nevertheless, our study has a few limitations. First, we included subjects who were documented as having AD. In previous years when advanced imaging modalities (e.g., positron emission tomography scanning) and plasma assays for biomarkers were unavailable, AD diagnoses were mainly made clinically and by exclusion. Second, in conducting a retrospective study, we were unable to extract subgroups among patients with AD for further comparison. Moreover, although all patients suffering from dementia underwent the Mini–Mental State Examination (MMSE), some of the MMSE records were not in electronic forms; The data quality of some of the hand-written forms was not clear to the Data Lab so the data were not included in Data Catalogue. Therefore, we were unable to retrieve full MMSE records for all patients with AD through the Big Data Analytics Platform. Although we tried to find the related clinical notes by searching the keyword ‘MMSE,’ we could only retrieve MMSE records for approximately half of the patients. As a result, we did not present any data on MMSE scores here. In collaboration with Data Collaboration Lab, this is the first systematic analysis of AD in the elderly population in Hong Kong. To optimize the data collection and better utilize the Platform database, it has been suggested that data, including case and medication record filtering, and details of death statistics, should be collected and included in the Platform. We believe this will benefit future studies of diseases and medications associated with the elderly in Hong Kong.
Conclusion
Our study provides previously unreported, detailed information about the demographics, comorbidities, mortality rates, and medication use of patients with AD in the Hong Kong elderly population who were under the care of the HA between January 1, 2007 and December 31, 2017. The advanced age at AD diagnosis and increasing mortality rates we observed among patients with AD deserve further attention. Moreover, many patients with AD required antipsychotics and antidepressants to control neuropsychiatric symptoms, while only 40% of patients with AD used medications for dementia. Accordingly, the following should be enhanced to improve the management of AD: 1) the availability of medications for dementia; 2) healthcare support for prompt diagnosis; 3) AD awareness among the general public; and 4) support to patients and family members. Moreover, greater resources should be allocated to AD research, as studies on AD-related plasma biomarkers and genomics in our local population will contribute to the development of new diagnostic tools and therapies.
ACKNOWLEDGMENTS
This study was supported in part by the National Key R&D Program of China (2018YFE0203600), the Hong Kong Research Grants Council Theme-based Research Scheme (T13-605/18-W), the Area of Excellence Scheme of the University Grants Committee (AoE/M-604/16), the Innovation and Technology Commission (ITCPD/17-9 and INNOHK18SC01), and the Chow Tai Fook Charity Foundation (CTFCF18SC01). We also thank other members of the Ip Laboratory for their many helpful discussions.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosure13-Apr-22s/21-5312r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-215312.
REFERENCES
[1] | Ballard C , Gauthier S , Corbett A , Brayne C , Aarsland D , Jones E ((2011) ) Alzheimer’s disease. Lancet 377: , 1019–1031. |
[2] | Jia J , Wei C , Chen S , Li F , Tang Y , Qin W , Zhao L , Jin H , Xu H , Wang F , Zhou A , Zuo X , Wu L , Han Y , Han Y , Huang L , Wang Q , Li D , Chu C , Shi L , Gong M , Du Y , Zhang J , Zhang J , Zhou C , Lv J , Lv Y , Xie H , Ji Y , Li F , Yu E , Luo B , Wang Y , Yang S , Qu Q , Guo Q , Liang F , Zhang J , Tan L , Shen L , Zhang K , Zhang J , Peng D , Tang M , Lv P , Fang B , Chu L , Jia L , Gauthier S ((2018) ) The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement 14: , 483–491. |
[3] | Population By-census Thematic Report: Older Persons. Census and Statistics Department, Hong Kong Special Administrative Region. |
[4] | Wu YT , Ali GC , Guerchet M , Prina AM , Chan KY , Prince M , Brayne C ((2018) ) Prevalence of dementia in mainland China, Hong Kong and Taiwan: An updated systematic review and meta-analysis. Int J Epidemiol 47: , 709–719. |
[5] | Chiu HF , Lam LC , Chi I , Leung T , Li SW , Law WT , Chung DW , Fung HH , Kan PS , Lum CM , Ng J , Lau J ((1998) ) Prevalence of dementia in Chinese elderly in Hong Kong. Neurology 50: , 1002–1009. |
[6] | Lam LC , Tam CW , Lui VW , Chan WC , Chan SS , Wong S , Wong A , Tham MK , Ho KS , Chan WM , Chiu HF ((2008) ) Prevalence of very mild and mild dementia in community-dwelling older Chinese people in Hong Kong. Int Psychogeriatr 20: , 135–148. |
[7] | Hospital Authority Statistical Report 2016-2017. Hospital Authority, Hong Kong Special Administrative Region. |
[8] | Leung AY , Kwan CW , Chi I ((2013) ) Residents with Alzheimer’s disease in long-term care facilities in Hong Kong: Patterns of hospitalization and emergency room use. Aging Ment Health 17: , 959–965. |
[9] | Yang YH , Wang H , Lam L , Chan WC , Yu X , Li T , Wang WF , Chiu PY , Lin YT , Hu CJ , Fuh JL , Morris JC ((2014) ) Characteristics of Alzheimer’s disease among patients in Taiwan, Hong Kong, and Beijing. J Alzheimers Dis 42: , 193–200. |
[10] | Jia J , Wang F , Wei C , Zhou A , Jia X , Li F , Tang M , Chu L , Zhou Y , Zhou C , Cui Y , Wang Q , Wang W , Yin P , Hu N , Zuo X , Song H , Qin W , Wu L , Li D , Jia L , Song J , Han Y , Xing Y , Yang P , Li Y , Qiao Y , Tang Y , Lv J , Dong X ((2014) ) The prevalence of dementia in urban and rural areas of China. Alzheimers Dement 10: , 1–9. |
[11] | Hung YN , Kadziola Z , Brnabic AJ , Yeh JF , Fuh JL , Hwang JP , Montgomery W ((2016) ) The epidemiology and burden of Alzheimer’s disease in Taiwan utilizing data from the National Health Insurance Research Database. Clinicoecon Outcomes Res 8: , 387–395. |
[12] | Imfeld P , Brauchli Pernus YB , Jick SS , Meier CR ((2013) ) Epidemiology, co-morbidities, and medication use of patients with Alzheimer’s disease or vascular dementia in the UK. J Alzheimers Dis 35: , 565–573. |
[13] | ((2020) ) 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 16: , 391–460. |
[14] | Lam TP , Sun KS , Chan HY , Lau CS , Lam KF , Sanson-Fisher R ((2019) ) Perceptions of Chinese towards dementia in Hong Kong-diagnosis, symptoms and impacts. Int J Environ Res Public Health 16: , 128. |
[15] | Zhou X , Chen Y , Ip FCF , Lai NCH , Li YYT , Jiang Y , Zhong H , Chen Y , Zhang Y , Ma S , Lo RMN , Cheung K , Tong EPS , Ko H , Shoai M , Mok KY , Hardy J , Mok VCT , Kwok TCY , Fu AKY , Ip NY ((2020) ) Genetic and polygenic risk score analysis for Alzheimer’s disease in the Chinese population. Alzheimers Dement (Amst) 12: , e12074. |
[16] | Niu H , Alvarez-Alvarez I , Guillen-Grima F , Al-Rahamneh MJ , Aguinaga-Ontoso I ((2017) ) Trends of mortality from Alzheimer’s disease in the European Union, 1994-2013. Eur J Neurol 24: , 858–866. |
[17] | Darbà J , A M ((2021) ) Hospital incidence, mortality and costs of Alzheimer’s disease in Spain:Aretrospective multicenter study. Expert Rev Pharmacoecon Outcomes Res 21: , 1101–1106. |
[18] | Manabe T , Fujikura Y , Mizukami K , Akatsu H , Kudo K ((2019) ) Pneumonia-associated death in patients with dementia: A systematic review and meta-analysis. PLoS One 14: , e0213825. |
[19] | Fang EF , Xie C , Schenkel JA , Wu C , Long Q , Cui H , Aman Y , Frank J , Liao J , Zou H , Wang NY , Wu J , Liu X , Li T , Fang Y , Niu Z , Yang G , Hong J , Wang Q , Chen G , Li J , Chen HZ , Kang L , Su H , Gilmour BC , Zhu X , Jiang H , He N , Tao J , Leng SX , Tong T , Woo J ((2020) ) A research agenda for ageing in China in the 21st century (2nd edition): Focusing on basic and translational research, long-term care, policy and social networks. Ageing Res Rev 64: , 101174. |
[20] | Wang JH , Wu YJ , Tee BL , Lo RY ((2018) ) Medical comorbidity in Alzheimer’s disease: A nested case-control study. J Alzheimers Dis 63: , 773–781. |
[21] | Cermakova P , Fereshtehnejad SM , Johnell K , Winblad B , Eriksdotter M , Religa D ((2014) ) Cardiovascular medication burden in dementia disorders: A nationwide study of 19,743 dementia patients in the Swedish Dementia Registry. Alzheimers Res Ther 6: , 34. |
[22] | Lin SK , Tsai YT , Lai JN , Wu CT ((2015) ) Demographic and medication characteristics of traditional Chinese medicine users among dementia patients in Taiwan: A nationwide database study. J Ethnopharmacol 161: , 108–115. |
[23] | Cheng ST , Lam LC , Kwok T ((2013) ) Neuropsychiatric symptom clusters of Alzheimer disease in Hong Kong Chinese: Correlates with caregiver burden and depression. Am J Geriatr Psychiatry 21: , 1029–1037. |
[24] | McDermott CL , Gruenewald DA ((2019) ) Pharmacologic management of agitation in patients with dementia. Curr Geriatr Rep 8: , 1–11. |
[25] | Leung KC , Li V , Ng YZ , Chan TT , Chang RS , Wong RY ((2017) ) Systematic review of cholinesterase inhibitors on cognition and behavioral symptoms in patients of Chinese descent with Alzheimer’s disease, vascular dementia, or mixed dementia. Geriatrics (Basel) 2: , 29. |



