J.ophthalmol.(Ukraine).2022;3:18-23.
|
http://doi.org/10.31288/oftalmolzh202231823 Received: 27.04.2022; Accepted: 14.06.2022; Published on-line 15.06.2022 Changes in clinical and biochemical characteristics in combination treatment for diabetic macular edema V. O. Drozdov, V. M. Sakovych Dnipro State Medical University; Dnipro (Ukraine) TO CITE THIS ARTICLE:Drozdov VO, Sakovych VM. Changes in clinical and biochemical characteristics in combination treatment for diabetic macular edema. J.ophthalmol.(Ukraine).2022;3:18-23. http://doi.org/10.31288/oftalmolzh202231823
Background: Diabetic macular edema (DME) can be seen in both types of diabetic retinopathy (DR), and is a major cause of vision loss. Purpose: To evaluate the efficacy of the combination treatment for DME in type 2 diabetic patients with non-proliferative diabetic retinopathy (NPDR). Material and Methods: An open-label, controlled study included 45 type 2 diabetic patients with both NPDR and DME. Patients of the aflibercept-plus-hyperbaric oxygen (HBO) group and those of the aflibercept-only (control) group received a monthly intravitreous aflibercept at a dose of 2 mg (50 µL) for 3 months. In addition, the former patients received an adjunctive treatment with ten sessions of HBO in the period between the first and the third injections. Changes in clinical and biochemical characteristics were assessed at 3 months after initiation of treatment. Results: At 3 months, patients of the aflibercept-plus-HBO group showed a statistically significant improvement in visual acuity, improvement in retinal light sensitivity, reduction in manifestations of DME, and statistically significant reductions in blood glucose and glycated hemoglobin levels. At 3 months, the changes in the activities of antioxidant enzymes were more substantial in this group, and these improvements were statistically significant compared to baseline and compared to controls. Particularly, superoxide dismutase (SOD) activity, catalase (CAT) activity, and glutathione reductase (GSR) activity decreased by 33.9%, 22.4% and 17.7%, respectively, compared to baseline, and 34.78%, 28.9%, and 25.3%, respectively, compared to controls. Conclusion: Treatment for DME with the use of aflibercept and adjunctive HBO caused a substantial reduction in the frequency of DME and improvements in visual acuity and retinal light sensitivity in type 2 diabetic patients NPDR. Under these conditions, a decrease in the activity of CAT was larger than that of GSR but smaller than that of SOD, likely indicating an improvement in antioxidant protection. Key words: diabetic macular edema, aflibercept, hyperbaric oxygen therapy, enzymes.
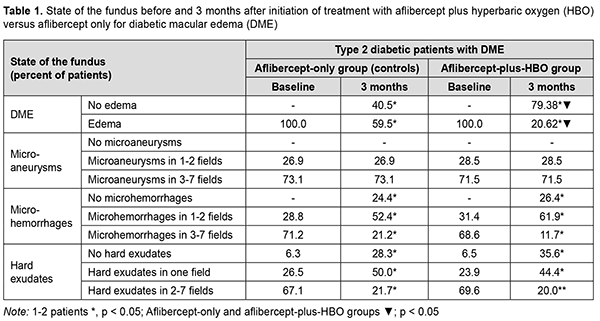
Introduction Diabetic macular edema (DME) can be seen in both types of diabetic retinopathy (DR) [1], in type 1 and type 2 diabetes mellitus (DM), and is a major cause of vision loss [2-4]. The value of DME as a factor of deterioration of quality of life increases with increases in diabetes duration, blood pressure and glycated hemoglobin (HbA1c). A reduced retinal oxygen level is important in the pathogenesis of DME [5], is maintained by a reduced blood flow in the choroid plexus tissue [6], and impairs retinal pigment epithelium (RPE) function and antioxidant enzyme (AOE) activation [7-9]. Of the above enzymes, superoxide dismutase (SOD; E.C. 1.15.1.1), catalase (CAT; E.C. 1.11.1.6) and glutathione reductase (GSR, E.C. 1.8.1.7) are worthy of the most attention [10]. Activation of oxidative processes causes the formation of a branched system protecting tissues from damage by aggressive oxygen radicals [superoxide anion radical (O2-), hydroxyl radical (HO-) and hydrogen peroxide Н2О2]. SOD family enzymes catalyze the dismutation of superoxide radicals into oxygen and hydrogen peroxide peroxide, and CAT catalyzes the conversion of hydrogen peroxide to water and molecular oxygen [11-16]. GSR provides restoration of the reduced form of glutathione [17], which significantly decreases the risk of oxidative stress. A combined anti-VEGF and hyperbaric oxygen (HBO) therapy is a possible approach for improved efficacy of treating DME in non-proliferative DR (NPDR) in the presence of type 2 DM. It has been reported that the use of HBO in type 2 DM reduced hypoxia in tissues, reduced blood glucose, increased insulin secretion, increased target cell insulin sensitivity, and normalized an imbalance of the antioxidant system [15, 16]. Material and Methods This open-label study was conducted at the Department of Ophthalmology of Dnipro State Medical University. The study is a portion of Optimizing the Methods of Diagnosis and Treatment of Retinal and Optic Nerve Diseases in the Presence of Diabetes Mellitus, a research program (Ukrainian State Registration No. 0118U001275; with the research to be conducted from January 2018 through December, 2020) and Improving the Diagnosis and Pathogenetically Grounded Treatment of Ocular Degenerative, Vascular and Inflammatory Disorders, a research program (Ukrainian State Registration No. 0121U111440; with the research to be conducted from January 2021 through December, 2024). Seventy-one type 2 diabetic patients with both NPDR and DME were included in the study after obtaining informed consent. Diabetes mellitus was diagnosed according to the guidelines of the Association of Endocrinologists of Ukraine, European Society of Endocrinology [18] and other normative documents outlined by the Ministry of Health (MOH) of Ukraine. Diabetic retinopathy was diagnosed and patients were treated in accordance with MOH Order No. 117 dated March 15, 2007, On Approval of Protocols for Provision of Eye Care. Diabetic macular edema was graded on clinical examination according to recognized severity scales based on Early Treatment Diabetic Retinopathy Study (ETDRS) criteria [19]. The inclusion criteria were as follows: moderate type 2 diabetic patients with moderate glycemic control, NPDR and DME, and no other retinal and/or optic nerve disorders. The exclusion criteria were as follows: progression to severe diabetes or poor glycemic control, occurrence of uncontrolled arterial hypertension, myocardial infarction or stroke, proliferative DR, media opacity affecting best-corrected visual acuity measurements; refraction errors associated with amblyopia; other retinal and/or optic disc disorders. Mean patient age was 62.41 ± 0.74 years; mean diabetes duration, 11.61 ± 0..573 years; mean body mass index, 27.48 ± 0.31; mean systolic blood pressure, 135.08±1.13 mmHg, and mean diastolic blood pressure, 83.42 ± 0.77 mmHg. Patients were divided into two groups. Patients of the aflibercept-plus-HBO group (34 patients; 34 eyes) and those of the aflibercept-only group (37 patients; 37 eyes) received a monthly intravitreous aflibercept (Eylea; Bayer, Basel, Switzerland) at a dose of 2 mg (50 µL) for 3 months. In addition, the former patients received ten 45-minute sessions of hyperbaric 95-percent medical oxygen at 1.4 atmospheres in a BLKS 301M monoplace chamber in the period between the first and the third injections. Biochemical and clinical characteristics were assessed before treatment and at 3 months after initiation of treatment. Patients received a comprehensive eye examination including visual acuity, refractokeratometry, intraocular pressure, biomicroscopy, ophthalmoscopy, Humphrey automated perimetry (Humphrey Field Analyzer II Carl Zeiss Meditec; Humphrey 10-2 threshold visual field testing), optical coherence tomography (OCT), and fundus photography. In addition, clinical features of the fundus (microaneurysms, microhemorrhages, and hard exudates) were described in the seven standard fields of the modified ETDRS Airlie House Classification of diabetic retinopathy. Serum glucose levels were determined with an enzymatic, colorimetric PZ Cormay test (Liquick Cor-GLUCOSE mini, PZ Cormay S.A., Poland; Cat No. 2-218). Serum HbA1c levels were determined by ion exchanged chromatography using the Glycohemoglobin HbA1-Test kit (China), and the results were read on a photometer (Stat Fax 303). Serum insulin levels were determined by enzyme-linked immunosorbent assay (ELІSA) (Insulin AccuBind Elisa kit, Monobind Inc., Lake Forest, CA, USA; Cat No. 2425-300). Serum activities of superoxide dismutase, catalase and glutathione reductase were measured by sandwich ELISA assays using an ELISA reader (Sirio-S; Italy) and SOD, CAT and GSR ELISA kits (FineTest, China; Cat Nos. EH0643, EH4706-1, EH3170) according to the manufacturer’s instructions. Mathematical statistics methods were used for data analysis. Methods of primary statistical analysis were employed for statistical description of the parameters considered in the study. Mean values and standard error of mean values were calculated. Data was assessed for normality using the Kolmogorov-Smirnov test. Distributions of most characteristics were not normal. The Student t test was used when the distribution of the data was normal, whereas the Mann-Whitney when the distribution of the data was not normal. Median and interquartile range values were calculated when the distribution of quantitative variables was not normal. Spearman’s correlation was employed to assess associations between variables. The correlation coefficient was considered significant if P ≤ 0.05. Results At baseline, all subjects exhibited DME (Table 1) which was accompanied by symptoms of NPDR. At 3 months the frequency of DME substantially decreased in both groups, and the percentage of eyes with DME was lower in the aflibercept-plus-HBO group than in the aflibercept-only group. In addition, there was a significant reduction in manifestations of NPDR (number of hemorrhages and hard exudates) compared to baseline values in both groups, with no significant difference between the groups.
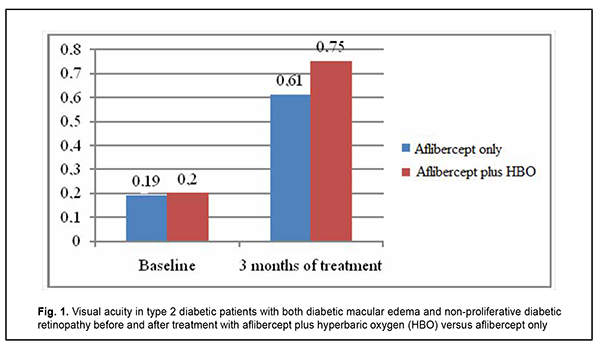
As a result of the above changes, visual acuity substantially improved, from 0.19 [0.17; 0.21] to 0.61 [0.36; 0.74] in the aflibercept-only group, and from 0.2 [0.18; 0.22] to 0.75 [0.48; 0.79] in the aflibercept-plus-HBO group, at 3 months after initiation of treatment (Fig. 1).
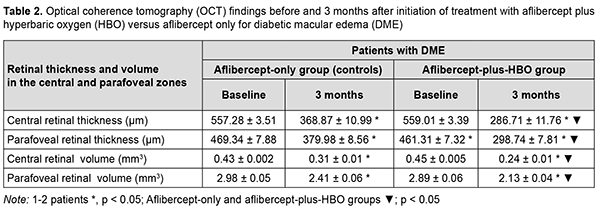
Under these conditions, retinal sensitivity to light increased, from 12.44 dB [11.78 dB; 13.07 dB] to 4.79 Db [3.89 dB; 8.67 dB] in the former group, and from 12.39 Db [11.85 dB; 12.84 dB] to 3.74 dB [3.15 dB; 6.75 dB] in the latter group. Treatment with aflibercept only or with aflibercept plus HBO resulted in a significant improvement in OCT-measured characteristics (Table 2). Of note, at 3 months, reductions OCT-measured retinal thicknesses and volumes were 21% to 23% more substantial in the latter group than in the former group.
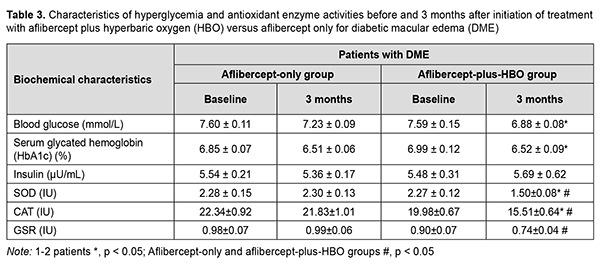
Because the treatment effect of aflibercept-plus-HBO therapy was most pronounced at 3 months, it was decided to determine changes in the registered biochemical characteristics at this time point (Table 3).
It was found that the treatment effect of aflibercept-plus-HBO therapy was more substantial than that of aflibercept-only therapy. At baseline, blood glucose and HbA1c levels were significantly lower in the aflibercept-plus-HBO group than in the aflibercept-only group. There was no significant change in insulin level with time in both groups. At 3 months, the changes in the activities of antioxidant enzymes were more substantial in the aflibercept-plus-HBO group, and these improvements were statistically significant compared to baseline and compared to controls. Particularly, superoxide dismutase (SOD) activity, catalase (CAT) activity, and glutathione reductase (GSR) activity decreased by 33.9%, 22.4% and 17.7%, respectively, compared to baseline, and 34.78%, 28.9%, and 25.3%, respectively, compared to controls. Discussion Studies have demonstrated that a reduced retinal oxygen level is important in the pathogenesis of DME [5], is maintained by a reduced blood flow in the choroid plexus tissue [6], and impairs RPE function and AOE activation [7-9]. When cells become hypoxic, HIF-1α starts to accumulate, triggering the activation of the genes that produce VEGF [20]. VEGF increases endothelial vascular permeability and endothelial cell proliferation. Long-term hypoxia and hyperglycemia cause loss of pericytes, endothelial cell destruction, and loss and atrophy of capillaries [21]. It has been reported that the use of HBO in type 2 DM reduced hypoxia in tissues, reduced blood glucose, increased insulin secretion, increased target cell insulin sensitivity, and normalized an imbalance of the antioxidant system [15, 16]. Until recently, there have been limited reports on the use of HBO in DR. However, a study by Maalej and colleagues [22] demonstrated an improvement in visual acuity and a decrease in retinal thickness in patients with both NPDR and DME treated with HBO. We hypothesized that the treatment effect of aflibercept for DME in NPDR may be improved by two adjunctive courses of HBO before the first and third injections, in an attempt to reduce glycemia, hypoxia and manifestations of oxidative stress, and to improve visual acuity and reduce manifestations of DME. Both in the aflibercept-only and aflibercept-plus-HBO groups, visual acuity improved; NPDR manifestations (number of microhemorrhages and hard exudates) and retinal thickness and volume in the central and paracentral zones reduced; with no change in insulin level, at month 3 compared to baseline. Blood glucose and HbA1c levels in the aflibercept-plus-HBO group significantly decreased at month 3 compared to baseline. We compared the effects of three-month treatment in the aflibercept-plus-HBO group with those of the aflibercept-only group, and the improvements in the former group were found to be statistically significant: visual acuity improved by 18.67% (р < 0.05); retinal sensitivity to light increased by 21.92% (р < 0.05); central and paracentral retinal thickness and volume decreased approximately by 21– 23% (р < 0.05); and the levels of antioxidant enzymes, SOD, CAT and GSR, decreased by 34.78% (р < 0.05), 28.9% (р < 0.05), and 25.3% (р < 0.05), respectively. Our findings suppose that the use of aflibercept plus HBO therapy reduces manifestations of hypoxia and oxidative stress, leading to improvements in treatment outcomes in patients with DME in NPDR. Therefore, further studies are warranted to determine changes in other clinical and biochemical characteristics for advanced understanding of the role of HBO in the treatment of DME in NPDR. Conclusion The treatment for diabetic macular edema including monthly intravitreous aflibercept at a dose of 2 mg (50 µL) for 3 months with adjunctive two courses of hyperbaric oxygen (ten 45-minute sessions at 1.4 atmospheres in a monoplace chamber) resulted in a substantial decrease in the number of patients with DME, reduction in DME manifestations, improvement in visual acuity, and reduction in retinal thickness and volume in type 2 diabetic patients with NPDR. Under these conditions, a decrease in the activity of CAT was larger than that of GSR but smaller than that of SOD, likely indicating an improvement in antioxidant protection.
References 1.Warboys C, Fraser P. Hyperglycemia attenuates acute permeability response to advanced glycation end products in retinal microvasculature. Microvasc Res. 2010 Jul;80(1):174-6. 2.Browning D, Stewart M, Lee Ch. Diabetic macular edema. Evidence-based management. Indian J Ophthalmol. 2018 Dec;66(12):1736-50. 3.Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology. 2008 Mar;115(3):533-539.e2. 4.Yu DY, Cringle SJ, Su E, Yu PK., Humayun MS, Dorin G, et al. Laser induced changes in intraretinal oxygen distribution in pigmented rabbits. Invest Ophthalmol Vis Sci. 2005 Mar;46(3):988-99. 5.Stefánsson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. Jul-Aug 2006;51(4):364-80. 6.de Andrade GC, de Oliveira Dias JR, Maia A, Farah ME, Meyer CH, et al. Intravitreal ziv aflibercept for diabetic macular edema: 48 week outcomes. Ophthalmic Surg Lasers Imaging Retina. 2018 Apr 1;49(4):245-250. 7.Ivankiv IaI, Oleshchuk OM, Datsko TV, Fedoniuk LIa. [Characteristics of prooxidative-antioxidative homeostasis, carbohydrate metabolism and morphological changes in the liver under conditions of melatonin injection in experimental type 2 diabetes]. Visnyk morphologii. 2016;22(2):253-8. Ukrainian. 8.Rykov SO, Vydyborets SV. [Glycated proteins, oxidative stress and interleukins: risk factors for the onset and progression of diabetic retinopathy]. Arkhiv oftalmologii Ukrainy. 2019;7(1):81-5. 9.Noma H, Yasuda K, Shimura M. Involvement of Cytokines in the Pathogenesis of Diabetic Macular Edema. Int J Mol Sci. 2021. 2021 Mar 26;22(7):3427. 10.Bokhary K, Aljaser F, Abudawood M. Role of Oxidative Stress and Severity of Diabetic Retinopathy in Type 1 and Type 2 Diabetes. Ophthalmic Res. 2021;64(4):613-621. 11.Kowluru RA., Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabet Res. 2007;2007:43603. 12.Case AJ. On the Origin of Superoxide Dismutase: An Evolutionary Perspective of Superoxide-Mediated Redox Signaling. Antioxidants (Basel). 2017 Oct 30;6(4):82. 13.Giordano CR, Roberts R, Krentz KA, et al. Catalase therapy corrects oxidative stress-induced pathophysiology in incipient diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015 May;56(5):3095-102. 14.Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol. Vis Sci. 2006. 2006 Apr;47(4):1594–99. 15.Resanović I, Zarić B, Radovanović J, et al. Hyperbaric oxygen therapy and vascular complications in diabetes mellitus. Angiology. 2020 Nov;71(10):876-85. 16.Baitule S, Patel AH, Murthy N. A systematic review to assess the impact of hyperbaric oxygen therapy on glycaemia in people with diabetes mellitus. Medicina (Kaunas). 2021 Oct 19;57(10):1134. 17.Satoh N, Watanabe N, Kanda A, Sugaya-Fukazawa M, Hisatomi H. Expression of glutathione reductase splice variants in human tissues. Biochem Genet. 2010 Oct;48(9-10):816-21. 18.Petersmann A, Müller-Wieland D, Müller UA, et al. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp Clin Endocrinol Diabetes. 2019 Dec;127(S 01):S1-S7. 19.Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003 Sep;110(9):1677-82. 20.Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp Eye Res. 2006. 2006 Sep;83(3):473-83. 21.Gupta A., Bhatnagar S. Vasoregression: a shared vascular pathology underlying macrovascular and microvascular pathologies? OMICS. 2015 Dec;19(12):733-53. 22.Maalej A, Khallouli A, Choura R, et al. The effects of hyperbaric oxygen therapy on diabetic retinopathy: A preliminary study. J Fr Ophtalmol. 2020. 2020 Feb;43(2):133-8.
Disclosures Corresponding Author: Drozdov Volodymyr, vladimirandco@gmail.com Disclaimer: The opinions stated and presented in the article are solely the author's and not the official position of the institution. Sources of support: none. Conflict of Interest: Not all authors have a conflict of interest
|