J.ophthalmol.(Ukraine).2019;1:17-22.
|
http://doi.org/10.31288/oftalmolzh201911722 Received: 27 November 2018; Published on-line: 28 February 2019 Comprehensive treatment of patients with refractory glaucoma complicated by bullous keratopathy O.V. Guzun, Cand. Med. Sc.; G.I. Drozhzhyna, Prof., Dr. Med. Sc. SI “Filatov Institute of Eye Diseases and Tissue Therapy of NAMS of Ukraine” Odessa (Ukraine) E-mail: olga.v.guzun@gmail.com TO CITE THIS ARTICLE: GuzunOV, DrozhzhynaGI. Comprehensive treatment of patients with refractory glaucoma complicated by bullous keratopathy. J.ophthalmol.(Ukraine).2019;1:17-22.http://doi.org/10.31288/oftalmolzh201911722 Introduction. Patients with refractory glaucoma complicated by bullous keratopathy often suffer from insupportable pain and uncomfortable sensations in the eye, and visual acuity loss, which worsens the patients’ quality of life. Purpose. To study the efficacy of a complex treatment of patients with refractory glaucoma complicated by bullous keratopathy through transscleral cyclophotocoagulation (TS CPC ) (λ=1.06 µm) followed by laser stimulation for corneal regeneration (λ=0.63 µm) and in combination with a preservative-free tear substitute bio-protector, containing 3% trehalose and 0.15% sodium hyaluronate. Material and Methods. 23 patients (23 eyes) with refractory glaucoma complicated by bullous keratopathy were treated. TS CPC was performed using neodymium laser (λ=1.06 µm; 3 sessions). On completion of a TS CPC course, each patient underwent a course of helium-neon laser stimulation of the cornea (λ=0.63 µm, t=300 sec, 10 sessions) and a 3-month course of a bio-protective tear substitute, containing 3% trehalose and 0.15% sodium hyaluronate (1 drop thrice a day). Each patient underwent visual acuity assessment, IOP measurement, Ocular Surface Disease Index (OSDI) evaluation before treatment, after a course of TCCSC, and after a three-month follow-up. Results. The TS CPC course resulted in pain management in all patients. The post-treatment IOP level decreased to 26.0 mmHg, by 30%, as compared to pre-treatment IOP level and remained stable at 3 months. Visual acuity improved in 10 of 23 patients (43%). Dry eye severity decreased by 34% (OSDI=59.4 scores). Conclusions. The patients with refractory glaucoma complicated by bullous keratopathy had a significantly decreased IOP level (by 30% in 65% of the patients) after a TS CPC course. The following laser stimulation of the cornea in combination with a course of preservative-free tear substitute, containing 3% trehalose and 0.15% sodium hyaluronate, decreased the severity of dry eye disease and corneal syndrome by 34% and significantly improved the patients’ quality of life. Keywords: refractory glaucoma, bullous keratopathy, transscleral laser cyclophotocoagulation, laser stimulation of the cornea, a substitute-bioprotector, 3% trehalose, 0.15% sodium hyaluronate.
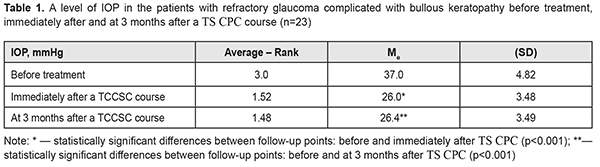
Introduction Drainage implant surgery is common for refractory glaucoma. However, any kind of surgery is traumatizing and can be accompanied by various complications, pain, and inflammation. The efficacy of drainage implant surgery does not exceed 70-80%, according to different authors. In the post-operative period recurs a question on a search for methods to normalize increased intraocular pressure. A long-lasting increase in intraocular pressure (IOP) is accompanied by significant visual acuity loss and persistent pain condition and followed by failure in a barrier function of the endothelial cell layer and development of bullous keratopathy [1, 17, 19]. Gradually, the anterior epithelium is involved in the pathologic process with the formation of folds and bullae, which is accompanied by a pronounced corneal syndrome. It is known that the pain sensation of different intensity is noted in 50-75% of patients with bullous keratopathy (BK) [7]. BK develops as a result of endothelial decompensation and is one of the major indications for keratoplasty [5]. However; according to Kaleem M., patients with pseudophakic bullous keratopathy after Descemet stripping automated endothelial keratoplasty had a 54% higher incidence of IOP elevation [14]. Besides, keratoplasty is often impossible to perform due to a lack of donor corneas and a high cost of the procedure. That is why, in bullous keratopathy and poor visual acuity, most of the focus is on symptomatic relief to improve the quality of patient’s life. I. B. Pedersen and colleagues have reported that glaucoma in BK increases the risk of graft rejection after Descemet’s stripping endothelial keratoplasty [20]. The health of the ocular surface is crucial for a patient’s quality of life and can be compromised through diseases or dystrophies [22]. A. Galor and colleagues have compared dry eye syndrome (DES) with neuropathic pain which requires treatment [9]. A prospective randomized clinical trial, performed by Kheirkhah A. and colleagues, has shown no improvement in DES patients with significant damage to corneal nerve fibres after treatment using topical artificial tears and low-dose steroids [15]. Another reason why surgery for BK-complicated refractory glaucoma is not always available is a somatic condition and, at times, insupportable pain of a patient. Thus, considering major biological effects, including anti-edematous, anti-inflammatory, desensitizing, immunomodulating, and anti-oxidant ones, which occur in low level laser therapy, as well as stimulating neurogenesis and synaptogenesis [10, 24], we decided, when performing transscleral cyclophotocoagulation with (TS CPC) scleral compression and decreasing IOP, to subsequently use of helium-neon laser stimulation of the cornea in combination with a preservative-free tear substitute bio-protector, containing 3% trehalose and 0.15% sodium hyaluronate. The purpose of the present paper was to study the efficacy of a complex treatment of patients with refractory glaucoma complicated by bullous keratopathy through transscleral cyclophotocoagulation (TS CPC) of the ciliary body (CB) (λ=1.06 µm) followed by laser stimulation for corneal regeneration (λ=0.63 µm) and in combination with a preservative-free tear substitute bio-protector, containing 3% trehalose and 0.15% sodium hyaluronate. Material and Methods It was an open non-controlled study of case series. Twenty-three patients (23 eyes) with refractory glaucoma complicated by bullous keratopathy were followed up; the age averaged 64.4 (SD, 7.7) years; there were 13 men (57%) and 10 women (43%). Each patient had undergone either extracapsular cataract extraction (ECCE) (16 patients) or cataract phacoemulsification (Phaco) (7 patients). Baseline (before treatment) visual acuity averaged 0.02 (SD 0.017) with 0 (null) in 6 eyes (26%), count fingers in 1 eye (4%), 0.01 in 8 eyes (35%), 0.02 to 0.03 in 5 eyes (22%), and 0.04 to 0.06 in 3 eyes 913%). The baseline level of IOP by the Maklakov method averaged 37 (SD 4.8) mmHg, ranging from 29.0 to 45.0 mmHg. TS CPC of the ciliary body was performed using neodymium laser (Nd laser) with λ=1.06 µm and followed the technique having been described previously [2]. A treatment course comprised three sessions every other day. A number of laser applications varied from 15 to 40, averaging 27.4 (SD 4.93). Preoperatively, subconjunctival anaesthesia was performed using 2% lidocaine solution. In each patient, pars plicata was visualized in all quadrants using infrared diaphanoscopy in order to position a laser probe accurately within the course of TS CPC [12, 13]. On completion of the treatment course, anti-hypertensive instillation regimen was not changed and an additional course of non-steroidal anti-inflammatory therapy was prescribed. After a TS CPC course, each patient underwent a course of helium-neon laser stimulation of the cornea (λ=0.63 µm, t=300 sec, 10 sessions). A long duration of the disease and intensive anti-hypertensive therapy in the patients resulted in the occurrence of DES of different intensity, which aggravated the patients’ objective status. Each patient was performed the Schirmer test. The test findings were differentiated according to tear production levels as follows: normal, which is ≥15 mm wetting of the paper; mild, which is 14-10 mm wetting of the paper; moderate, which is 9-5 mm wetting of the paper; and severe, which is <4 mm wetting of the paper. The test showed 12 patients (52%) with a mild tear production level and 11 patients (48%) with a moderate tear production level; the mean tear production level equalled 9.4 (SD 2.73). Thus, to treat DES and control the symptoms of discomfort, burning, and irritation, a bio-protective tear substitute, not containing preservatives and phosphates, which are toxic for glaucoma patients, was recommended with a dosage of 1 drop thrice a day for three months. Inclusion criteria were refractory glaucoma with severe pain syndrome (IOP ≥ 29.0 mmHg), BK with photophobia, tearing, foreign body sensation, and blepharospasm. Biomicroscopy showed pronounced hyperemia, no signs of infectious inflammation in anterior eye tissues, loss of corneal lustre, uneven surface, pronounced bullous changes in the corneal epithelium, corneal deepithelialization, opaque and swollen corneal stroma, and folded Descemet's membrane. The IOL position was correct and an ophthalmoscopic picture of deeper media was shaded. Each patient underwent visual acuity assessment and IOP measurement before treatment, after a course of TS CPC , and after a three-month follow-up. To assess the ocular surface, the patients completed an Ocular Surface Disease Index (OSDI) questionnaire [11]. OSDI was assessed according to answers to 12 questions on ocular discomfort. Each answer was scored from 0 to 4. Afterwards, dry eye severity was determined using the formula: (sum of scores x 25)/# of questions answered. OSDI was assessed on a scale with a maximum of 100 scores, representing severe disability; and the lower the score, the milder the subjective dry eye symptoms, representing normal, mild to moderate dry eye disease. STATISTICA 10.0 (StatSoftInc.) was used to perform statistical analyses. Changes in IOP levels were assessed using the Friedman rank analysis of variance and the Wilcoxon signed-rank test. To demonstrate differences in the studied indices over time, medians (Ме) and standard deviations (SD) were calculated. Spearman correlation coefficient (rs) was calculated for correlation analysis. Results and Discussion After the first TS CPC session, most patients noted an abatement of pain in the eye; after 3 sessions, the pain was managed in all patients. The IOP level after the treatment course significantly decreased, by 30%, compared to the baseline IOP level, with a median of 26.0 (SD 3.48) mmHg (Table 1). Table 1 demonstrates that the mean level decreased immediately after treatment (χF2=39.7; p<0.0001) and stabilized to 26.4 (SD 3.49) mmHg after 3 months.
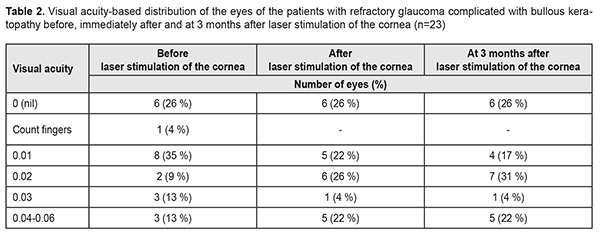
After the TS CPC course, the IOP level was within normal ranges (lower than 26.0 mmHg) in 65% of the eyes and this outcome was stable within a 3-month follow-up. Our findings are in agreement with several studies which have shown the efficacy and relative safety of transscleral laser cyclophotocoagulation, particularly the 30% reduction in IOP, the 73.5% efficacy, and relief of pain in patients with refractory glaucoma [8]. Glaucoma tube implants, noncontact YAG laser cyclophotocoagulation, and contact transscleral diode laser cyclophotocoagulation for refractory glaucoma were successful in 78%, 69%, and 71%, respectively. Complications were more frequently noted in the patients in the tube group [3]. J.K. Ndulue and colleagues have reviewed the international literature and have reported that laser cyclophotocoagulation has been recognized as a treatment option for end-stage glaucoma due to its simplicity, noninvasiveness, efficacy, and possibility to repeat the procedure. Herewith, due to incomplete compensation of IOP in 5 eyes (22%), a TS CPC course was repeated 3 months later [18]. On completion of a TS CPC course, each patient underwent a course of helium-neon laser stimulation of the cornea. Immediately after the course of corneal LS, visual acuity improved in 8 eyes (35%) due to better corneal transparency and, at three months, visual acuity improved in 10 eyes (43%). Table 2 demonstrates the visual acuity-based distribution of the eyes in the patients with refractory glaucoma complicated by bullous keratopathy at different follow-up points.
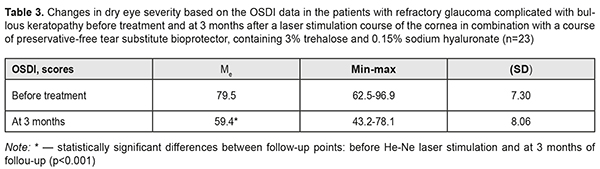
Table 3 demonstrates changes in dry eye severity in the patients with refractory glaucoma complicated by bullous keratopathy.
V. E. Raivio has reported that cyclophotocoagulation with the 670-nm diode laser does not impair corneal innervation [21]. Epithelial cells in the cornea have a trophic dependence on sensory nerves innervating the cornea. So, the neuropeptidergic nerve fibres have an effect on neurotrophic activities of the corneal epithelial cells and the corneal sensory nerves play an important role in homeostasis of the corneal epithelium [6]. Our finding also demonstrated that helium-neon laser irradiation to the cornea has an apparent neurotrophic action, thus, improving the cornea. Analysis of the OSDI questionnaire data showed such scores as follows: eyes, sensitive to light before treatment, were all the time, most of the time, and half of the time in 1 (4%), 8 (35%), and 14 (61%) patients, respectively, while those at 3 months were most the time, half of the time, and some of the time in 6 (26%), 12 (52%), and 5 (22%) patients, respectively; eyes, feeling gritty, were all the time, most of the time, and half of the time in 1 (4%), 12 (52%), and 10 (44%) patients, respectively, at baseline vs. most the time, half of the time, and some of the time in 7 (30%), 13 (57%), and 3 (13%) patients, respectively, at 3 months; painful or sore eyes were all the time in all 23 (100%) patients before treatment while those were some of the time in 4 (17%) patients and none of the time in 19 (83%) patients at 3 months; blurred vision was experienced by 18 (78%) patients all the time and 5 (22%) patients most of the time with no improvements at 3 months; poor vision was experienced by 20 (87%) patients all the time and 3 (13%) patients most of the time with no improvements at 3 months. Only 8 (35%) patients had problems with eyes limiting them in reading, watching TV, and working with the computer with no changes at 3 months. The eyes felt uncomfortable in windy conditions in 3 (13%), 14 (61%), and 6 (26%) patients, respectively, all the time, most of the time, and half of the time at baseline vs. 8 (35%), 14 (61%), and 1 (4%) patients who felt discomfort in their eyes, respectively, most of the time, half of the time, and some of the time at three months. Before treatment, when being in places and areas with low humidity, 8 (35%) and 15 (65%) patients felt discomfort in the eyes all the time and most of the time, respectively. At 3 months, when being in places and areas with low humidity, 8 (35%) patients felt uncomfortable most of the time and 14 (65%) patients half of the time, and 1 (4%) patient some of the time. In areas that are air- conditioned, the eyes felt uncomfortable all the time and most of the time, respectively, in 16 (70%) and 7 (30%) patients before treatment while at 3 months 12 (52%) and 1 (4%) patients felt discomfort half of the time and some of the time. Schiffman R. M. and colleagues have noted that the OSDI system is a valid and reliable instrument for measuring dry eye severity and it possesses the necessary psychometric properties [25]. Considering the patients’ complaints, specified in the OSDI questionnaire, we noted a reduction in the dry eye severity by 34%, which scored 59.4 (SD 8.06) at 3 months. The reduction was associated, basically, with a decrease in sensitivity to light (17%), feeling gritty (15%), and painful or sore eyes (95%) as well as less discomfort in windy conditions (95%), being in places or areas with low humidity (27%) and areas that are air-conditioned (35%). However, complaints of poor vision, problems when working with a computer and watching TV were characterized by a high score due to the specificity of this cohort of patients and initial severity of the cornea. Given that, there was a strong negative correlation relationship between the Schirmer test and OSDI findings (rs= - 0.66). Various authors have reported a relationship between elevated IOP and damage to the corneal endothelial cells as well as a negative correlation relationship between the density of the corneal endothelial cells and an acute IOP elevation [4, 16, 26]. Confocal laser scanning microscopy revealed a decrease in the number and diameter of nerve fibres as well as a reduction in corneal sensitivity in glaucoma patients [23, 27], which, in our opinion, aggravates the course of bullous keratopathy, lowers the patient’s quality of life, and requires necessary treatment of the cornea. According to the latest recommendations of Tear Film and Ocular Surface Society Dry Eye Workshop (TFOS DEWS II, 2017), glaucoma patients, due to the need for long-term instillations, are recommended to use preservative-free drugs, which are maximally safe for the ocular surface. The 3-month follow-up after a course of TS CPC with the IOP stabilization, followed by LS of the cornea with bio-protection using preservative-free tear substitute, containing 3% trehalose and 0.15% sodium hyaluronate, showed a quiet eye and visual stabilization. No blepharospasm, photophobia, and tearing were noted in 19 (83%) patients. The surface corneal layers were epithelized and had less swelling; no bullae were noted in 15 (65%) patients. Thus, the state of the cornea was better; the patients made fewer complaints and their life quality improved. To conclude, the patients with refractory glaucoma complicated by bullous keratopathy had a significantly decreased IOP level (by 30% in 65% of the patients) after a TS CPC course and remained stable within 3 months. The following LS of the corneal regeneration in combination with a course of a preservative-free tear substitute, containing 3% trehalose and 0.15% sodium hyaluronate, decreased the severity of dry eye disease and corneal syndrome by 34%, and significantly improved the patients’ quality of life. References 1.Boiko EV, Shishkin MM, Gudakovskii YuP, Yan AV. [On the treatment of endothelial-epithelial dystrophy of the cornea by the method of pancorneal photocoagulation using a ytterbium-erbium laser]. Oftalmokhirurgiia. 2002;2:3-7. (In Russian). 2.Chechin PP, Guzun OV, Khramenko NI, Peretyagin OA. Efficacy of transscleral Nd:YAG laser cyclophotocoagulation and changes in blood circulation in the eye of patients with absolute glaucoma. J.ophthalmol.(Ukraine).2018;2:34-39. 3.Bloom PA, Clement CI, King A, Noureddin B, Sharma K, Hitchings RA, Khaw PT. A comparison between tube surgery, ND:YAG laser and diode laser cyclophotocoagulation in the management of refractory glaucoma. Biomed Res Int. 2013. – 371951. Published online 2013 Oct 7. 4.Chen MJ, Liu CJ, Cheng CY, Lee SM. Corneal status in primary angle-closure glaucoma with a history of acute attack. J Glaucoma. 2012; Jan 21(1):12-6. 5.Cursiefen C, Kuchle M, Naumann GO. Changing indications for penetrating keratoplasty: histopathology of 1,250 corneal buttons. Cornea.1998;17:468–70. 6.Eguchi H, Hiura A, Nakagawa H, Kusaka S, Shimomura Y. Corneal Nerve Fiber Structure, Its Role in Corneal Function, and Its Changes in Corneal Diseases. Biomed Res Int. 2017. 3242649. 7.Filippova ЕО, Sokhoreva VV, Pichugin VF. Study the possibility of using nuclear track membranes for ophthalmology. Membranes and membrane technology. 2014;4(4): 1. 8.Frezzott P, Mittica V, Martone G, Motolese I, Lomurno L, Peruzzi S, Motolese E. Longterm follow-up of diode laser transscleral cyclophotocoagulation in the treatment of refractory glaucoma. Acta ophthalmologica. 2010;88:150-155. 9.Galor A, Moein HR, Lee C, Rodriguez A, Felix ER, Sarantopoulos KD, Levitt RC. Neuropathic pain and dry eye. Ocul Surf. 2018;16(1):31-44. 10.Hamblin MR. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol. 2017. Nov 22. 11.Jones LT. The lacrimal secretory system and its treatment. Ophthalmol. 1966. 62(1):47-60. 12.Zadorozhnyy O, Korol A, Nevska A, Kustryn T, Pasyechnikova N. Сiliary body imaging with transpalpebral near-infrared transillumination (Pilot study). Klinika oczna. 2016;3:184-6. 13.Zadorozhnyy OS, Guzun OV, Bratishko AIu et al. Infrared thermography of external ocular surface in patients with absolute glaucoma in transscleral cyclophotocoagulation: a pilot study. J. ophthalmol. (Ukraine). 2018;2:23-8. 14.Kaleem M, Ridha F, Shwani Z, Swenor B, Goshe J, Singh A. Rates of Intraocular Pressure Elevation and Use of Topical Antihypertensive Medication After Descemet Stripping Automated Endothelial Keratoplasty. Cornea. 2017;36(6):669-74. 15.Kheirkhah A, Dohlman TH, Amparo F, Arnoldner MA, Jamali A, Hamrah P, Dana R. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology. 2015;122:662–8. 16.Li X, Zhang Z, Ye L, Meng J, Zhao Z, Liu Z, Hu J. Acute ocular hypertension disrupts barrier integrity and pump function in rat corneal endothelial cells. Sci Rep. 2017; Jul 31; 7(1):6951. 17.Melamed S, Ben-Sira I, Ben-Shaul Y. Ultrastructure of fenestrations in endothelial choriocapillaries of the rabbit – a freeze-fracturing study. The British journal of ophthalmology. 1980;64:537-43. 18.Ndulue JK, Rahmatnejad К, Sanvicente С, Wizov SS, Moster MR. Evolution of Cyclophotocoagulation. J Ophthalmic Vis Res. 2018;13 (1):55-61. 19.Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Kyne BM, Saban DR, Steppet MA al. Partial denervation of sub-basal axons persists following debridement wounds to the mouse cornea. Lab Invest. 2015;95:1305–18. 20.Pedersen IB, Ivarsen А, Hjortdal J. Graft rejection and failure following endothelial keratoplasty (DSAEK) and penetrating keratoplasty for secondary endothelial failure. Acta Ophthalmol. 2015;93(2):172-7. 21.Raivio VE, Vesaluoma MH, Tervo TM, Immonen IJ, Puska PM. Corneal innervation, corneal mechanical sensitivity, and tear fluid secretion after transscleral contact 670-nm diode laser cyclophotocoagulation. J Glaucoma. 2002;11(5):46-453. 22.Rowsey TG, Karamichos D. A role of lipids is in the diseases of cornea and dystrophy : systematic review. Clin Transl Med. 2017;6:30. 23.Saini M, Vanathi M, Dada T, Agarwal T, Dhiman R, Khokhar S. Ocular surface evaluation in eyes with chronic glaucoma on long term topical antiglaucoma therapy. Int J Ophthalmol. 2017;10(6):931–8. 24.Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR. Brain Photobiomodulation Therapy: a Narrative Review /F. Salehpour, // Mol Neurobiol. 2018; Jan 11. doi: 10.1007/s12035-017-0852-4. [Epub ahead of print] 25.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118: 615-21. 26.Tham CC, Kwong YY, Lai JS, Lam DS. Effect of a previous acute angle closure attack on the corneal endothelial cell density in chronic angle closure glaucoma patients. J Glaucoma. 2006; Dec 15(6):482-5. 27.Yüksel N, Emre E, Pirhan D. Evaluation of Corneal Microstructure in Pseudoexfoliation Syndrome and Glaucoma: In Vivo Scanning Laser Confocal Microscopic Study. Curr Eye Res. 2016;41(1):34-40.
|