Abstract
Acute kidney injury is defined as any of the following (not graded): increase in serum creatinine by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours; or increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or urine volume <0.5 ml/kg/ hour for 6 hours. The goal of a staging system is to classify the course of a disease in a reproducible manner that supports accurate identification and prognostication and informs diagnostic or therapeutic interventions. Risk factors for acute kidney injury include environmental, socioeconomic and/ or cultural factors, as well as factors related to the process of care, acute exposures and patients themselves. Loss of kidney excretory function implies disturbances in the main function of the kidneys (maintaining homeostasis), for example, through excretion of metabolic waste products. Patients were categorized according to the treatment for acute kidney injury as follows: conservative treatment; surgical treatment; and renal replacement therapy. The use of isotonic saline as the standard of care for intravascular volume expansion to prevent or treat AKI is thus based upon the lack of clear evidence that colloids are superior for this purpose, along with some evidence that specific colloids may cause AKI, in addition to their higher costs.
Keywords
Acute kidney injury, Definition, Pathophysiology, Risk factors, Stages, Treatment
Introduction
Acute kidney injury (AKI), previously called acute renal failure, is characterized by an abrupt decline in renal function, resulting in an inability to secrete wastes and maintain electrolyte and water balance. AKI has clinical manifestations ranging from a small elevation in serum creatinine (SCr) levels to anuric renal failure. The severity of AKI is defined by Risk Injury Failure Loss End Stage (RIFLE) and Acute Kidney Injury Network (AKIN) criteria, which are based on the presence of increased SCr levels and/or a decreased urine output. The AKIN definition also emphasizes the change in SCr levels over a short period of time (within 48 h). This serious disorder may aggravate pre- existing kidney disease, thus leading to a rapid loss of renal function [1]. AKI is a broad clinical syndrome encompassing various etiologies, including pre-renal azotemia, acute tubular necrosis, acute interstitial nephritis, acute glomerular and vasculitic renal diseases, and acute post renal obstructive nephropathy. More than one of these conditions may coexist in the same patient and epidemiological evidence supports the notion that even mild, reversible AKI has important clinical consequences, including increased risk of death. AKI can thus be considered more like acute lung injury or acute coronary syndrome. Furthermore, because the manifestations and clinical consequences of AKI can be quite similar (even indistinguishable) regardless of whether the etiology is predominantly within the kidney or predominantly from outside stresses on the kidney, the syndrome of AKI encompasses both direct injury to the kidney as well as acute impairment of function [2-4]. AKI is defined as any of the following (not graded): increase in SCr by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours; or increase in SCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or urine volume <0.5 ml/kg/hour (oliguria) for 6 hours. The cause of AKI should be determined whenever possible (not graded) [2]. AKI was defined as an absolute increase in SCr of at least 26.5 mmol/l (0.3 mg/dl) or a 50% or above increase in SCr from baseline according to the AKIN criteria [3]. The severity of AKI was defined by the AKIN staging criteria as follows: Stage I, SCr increase to 1.5-2 fold of baseline; Stage II, SCr increase to 2-3 fold of baseline; and Stage III, SCr increase to 3 fold of baseline or an absolute increase of 356.6 mmol/l (4.0 mg/dl) with an acute increase of at least 44.2 mmol/l (0.5 mg/dl). All patients who needed dialysis were categorized into Stage III [3]. The global burden of AKI-related mortality exceeds by far that of breast cancer, heart failure or diabetes, with mortality remaining high during the past 50 years. In general, the incidence of AKI is reported as either community-acquired or hospital-acquired AKI. In high-income countries (HIC), AKI is predominantly hospital- acquired, whereas community-acquired AKI is more common in lower-income settings. These patterns apply to both adults and children globally. In HIC overall, patients with AKI tend to be older, have multiple comorbidities, and have access to dialysis and intensive care if needed. Post-surgical or diagnostic interventions or iatrogenic factors are the main causes of AKI in HIC. However, in low-income settings, numerous community-acquired causes exist, such as sepsis, volume depletion, toxins (bites, remedies) and pregnancy [5].
Staging/Classification
The goal of a staging system is to classify the course of a disease in a reproducible manner that supports accurate identification and prognostication and informs diagnostic or therapeutic interventions. The group recognized that a number of systems for staging and classifying AKI are currently in use or have been proposed. The RIFLE (Risk, Injury, Failure, Loss, and End-stage kidney disease) criteria proposed by the ADQI group were developed by an interdisciplinary, international consensus process and are now being validated by different groups worldwide [6,7] (Table 1).
Table 1: Classification/staging system for acute kidney injury [4]

(a) Modified from RIFLE (Risk, Injury, Failure, Loss, and End- stage kidney disease) criteria [6]. The staging system proposed is a highly sensitive interim staging system and is based on recent data indicating that a small change in serum creatinine influences outcome. Only one criterion (creatinine or urine output) has to be fulfilled to qualify for a stage. (b) 200% to 300% increase=2- to 3-fold increase.
(c) Given wide variation in indications and timing of initiation of renal replacement therapy (RRT), individuals who receive RRT are considered to have met the criteria for stage 3 irrespective of the stage they are in at the time of RRT.
Risk Factors
Risk factors for AKI include environmental, socioeconomic and/ or cultural factors, as well as factors related to the process of care, acute exposures and patients themselves. Environmental factors include inadequate drinking and waste water systems, insufficient control of infectious diseases and insufficient health care systems. Patient-related factors can be modifiable, for example, volume depletion, hypotension, anaemia, hypoxia and use of nephrotoxic drugs, or non-modifiable, for example, chronic kidney, heart, and liver or gastrointestinal disease, diabetes and severe infections and sepsis. Rarer causes include genetic predispositions to myoglobinuria, haemoglobinuria and urolithiasis Further important risk factors for AKI are severe diseases, acute infections, sepsis, malaria, severe trauma, hypovolaemia, old age, pre- existing CKD, acute organ failures, major surgeries (including cardiac surgery), being in the ICU with exposure to nephrotoxic drugs and opportunistic infections, chemotherapy for leukaemia or cancer, delayed graft function upon kidney transplantation, autoimmune disorders with rapid progressive kidney injury, cholesterol crystal embolism and urinary tract obstruction. In HIC, despite severe AKI occurring more frequently in the context of hospital-related risk factors, such as major surgery, bleeding, septic shock or drug toxicity in older patients with multiple diseases, milder forms of AKI can also be community-acquired. The three main causes of AKI are summarized below as: Prerenal AKI is secondary to under perfusion of otherwise normal, functioning kidneys. The hallmark of prerenal AKI is rapid reversibility. Prerenal kidney injury can result from volume depletion that is the result of hypovolaemia (haemorrhage, volume depletion, renal or extrarenal losses, fluid sequestration, renal fluid loss (over-diuresis), third space (burns, peritonitis, muscle trauma)); Impaired cardiac function (congestive heart failure, inadequate perfusion pressures secondary to heart failure, acute myocardial infarction, massive pulmonary embolism; Systemic vasodilatation (anti-hypertensive medications, gram negative bacteraemia, cirrhosis, anaphylaxis, sepsis); Increased vascular resistance (anaesthesia, surgery, hepatorenal syndrome, NSAID medications, drugs that cause renal vasoconstriction (i.e. cyclosporine)). For patients with prerenal AKI, urinalysis is typically bland or with hyaline casts, urine sodium is low (ie, 1%), and urine osmolality is high. Intrinsic AKI can be challenging to evaluate because of the wide variety of injuries that can occur to the kidney. Generally, four structures of the kidney are involved including tubules, glomeruli, the interstitium, and intra- renal blood vessels. Acute tubular necrosis (ATN) is the term used to designate AKI resulting from damage to the tubules. It is the most common type of intrinsic kidney injury. AKI from glomerular damage occurs in severe cases of acute glomerulonephritis (GN). AKI from vascular damage occurs because injury to intra-renal vessels decreases renal perfusion and diminishes GFR and finally acute interstitial nephritis occurs due to an allergic reaction to a variety medications or an infection. Tubular (renal ischaemia (shock, complications of surgery, haemorrhage, trauma, bacteraemia, pancreatitis, pregnancy), nephrotoxic drugs (antibiotics, antineoplastic drugs, contrast media, organic solvents, anaesthetic drugs, heavy metals), endogenous toxins (myoglobin, haemoglobin, uric acid); Glomerular (acute post-infectious glomerulonephritis, lupus nephritis, IgA glomerulonephritis, infective endocarditis, good pasture syndrome, wegener disease); Interstitium infections ((bacterial, viral), medications (antibiotics, diuretics, NSAIDs, and many more drugs)), Vascular (large vessels (bilateral renal artery stenosis, bilateral renal vein thrombosis), small vessels (vasculitis, malignant hypertension, atherosclerotic or thrombotic emboli, haemolytic uraemic syndrome, thrombotic thrombocytopenic purpura)). Postrenal causes of AKI are characterized by acute obstruction to urinary flow. Urinary tract obstruction increases intratubular pressure and thus decreases GFR. In addition, acute urinary tract obstruction can lead to impaired renal blood flow and inflammatory processes that also contribute to diminished GFR. Obstruction of the urinary tract at any level may produce AKI. In general, obstruction must involve both kidneys and a solitary kidney to produce significant renal failure. However, a patient with pre-existing renal insufficiency may develop AKI with obstruction of only one kidney. Urinary obstruction may present as anuria or intermittent urine flow (such as polyuria alternating with oliguria) but may also present as nocturia or nonoliguric AKI. Causes of postrenal AKI include benign prostatic hyperplasia and prostate cancer in men, gynecologic cancers especially cervical cancer in women, retroperitoneal fibrosis, ureteral stones, papillary necrosis, neurogenic bladder, and intratubular obstruction due to precipitation of various substances such as acyclovir or indinavir. Extrarenal obstruction (prostate hypertrophy, improperly placed catheter, bladder, prostate or cervical cancer, retroperitoneal fibrosis), Intrarenal obstruction (nephrolithiasis, blood clots, papillary necrosis) [5,8-13] (Figure 1).
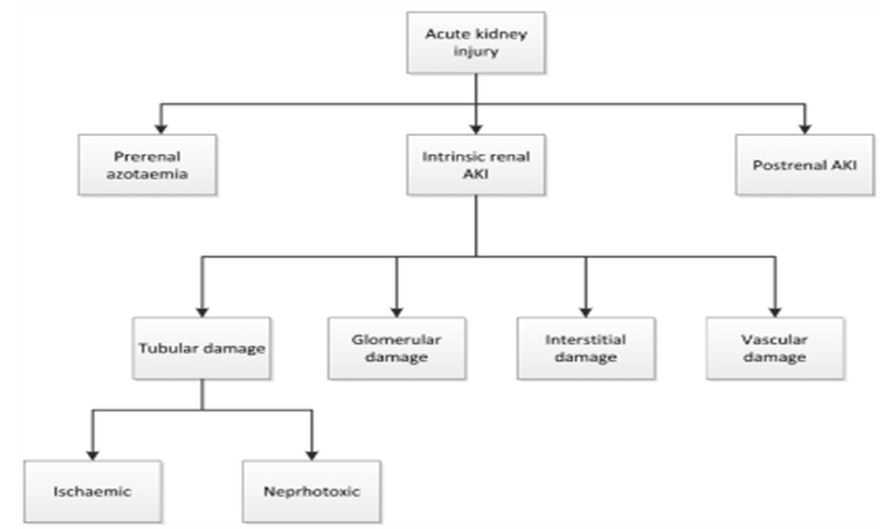
Figure 1: Causes of AKI
Pathophysiology of Kidney Failure
Loss of kidney excretory function implies disturbances in the main function of the kidneys (maintaining homeostasis), for example, through excretion of metabolic waste products. Serum creatinine and urea nitrogen levels are often used as biomarkers of reduced kidney function, but their use skews awareness towards the kidneys’ excretory function. Fluid homeostasis is affected, as declining glomerular filtration rate (GFR) and activation of the renin angiotensin system promote fluid retention, which presents as peripheral oedema, third- space effusions, and pulmonary congestion, especially in those with heart failure. In addition, as urinary output determines potassium excretion, hyperkalaemia is a common complication of severe AKI. When hyperkalaemia leads to electrocardiogram changes, AKI constitutes a medical emergency and warrants immediate intervention. Both hyponatraemia and hypernatraemia may occur when the kidney loses the capacity for urine concentration or dilution as needed. Impaired phosphate clearance leads to hyperphosphataemia. AKI also affects acid-base homeostasis. A declining capacity for the excretion of fixed acids in patients with AKI causes tubular metabolic acidosis and respiratory compensation via an increased ventilatory drive. Although a hyperchloraemic metabolic acidosis develops initially, widening of the anion gap is often seen as the result of accumulation of phosphate, sulfate and small organic anions in the bloodstream. A decline in the capacity to excrete metabolic waste products is indicated by azotaemia but implies disturbance of homeostasis of hundreds, if not thousands, of other metabolites that are not waste products, which all together account for symptoms of uraemia, such as fatigue, tremor or confusion. Importantly, kidney failure affects most organ systems of the body. Many of the AKI-related uraemic toxins originate from the intestinal microbiota, such as indoxyl sulfate or p-cresyl sulfate. The microbiota itself undergoes shifts in its composition, owing to AKI and the accompanying acidosis, azotaemia, intestinal ischaemia, and other alterations of the intestinal microenvironments, which affects the microbiota’s secretome and metabolites needed for normal human physiology. The lungs are affected by hyperpnoea to compensate for metabolic acidosis, hypervolaemia, cytokines, oxidative stress and cytotoxic elements of necrotic cell debris (released by parenchymal necrosis in the kidneys, causing microvascular injury, and eventually acute respiratory distress syndrome). AKI affects cardiac function via acidosis, hyperkalaemia, uraemic toxins, hypervolaemia, hypertension, and systemic inflammation46. Uraemic encephalopathy also involves systemic oxidative stress responses [5,14-20] (Figure 2).
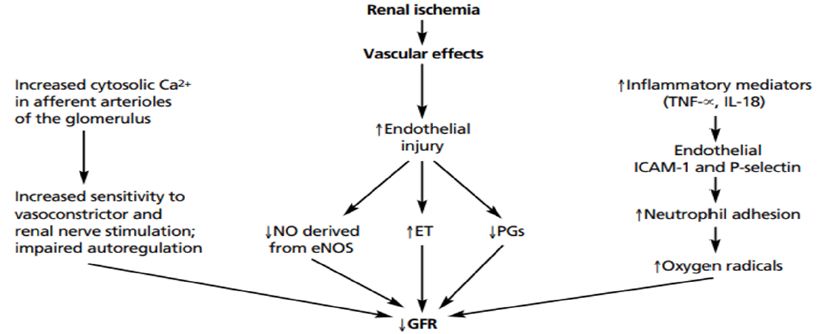
Figure 2: Mechanisms of acute kidney injury: a molecular viewpoint. Cascade of events involved in the pathophysiology of acute kidney injury.
Treatment of AKI
As previously noted, treatment plans for patients with AKI are varied and depend on etiologic factors. Prerenal azotemia from volume depletion is usually responsive to isotonic saline repletion. Treatment of ATN requires the discontinuation of nephrotoxic agents, maintenance of optimum hemodynamics, and close surveillance for complications of renal dysfunction (eg, acidosis, electrolyte abnormalities). Postrenal etiologies dictate obstruction removal. Patients were categorized according to the treatment for AKI as follows: conservative treatment; surgical treatment; and renal replacement therapy (RRT). RRT included intermittent hemodialysis (IHD), continuous renal replacement therapy (CRRT) and peritoneal dialysis (PD). Surgical treatment included surgical removal of obstructive tumors, stones, or prostate hypertrophy. Patients without RRT and surgical treatment were included in the conservative treatment group. Treatment included correction of the primary cause of AKI, fluid replacement therapy, and nutritional support. If AKI was due to serious trauma, heart failure and acute hemorrhage, patients were administered normal saline to correct intravascular volume depletion. In AKI patients with severe hyperkalemia, 5-10 units of insulin and 50% dextrose were used to promote uptake of potassium into cells. Supportive therapies such as antibiotics and adequate nutrition were given according to standard management practice. All medications that could potentially affect renal function were discontinued [5,22- 25]. Delayed antibiotic administration in septic shock was associated with early AKI development. However, certain nephrotoxic agents involving in the treatment such as aminoglycosides, vancomycin particularly in combination with piperacillin-tazobactam, and amphotericin B, as well as diagnostic agents such as intravenous radiocontrast media should be used with caution to prevent kidney injury according to the KDIGO AKI guidelines. Strict therapeutic drug monitoring should be considered when applicable [26,27].
Fluids Resuscitation
Fluid resuscitation followed by vasopressor medications is cornerstones in the treatment of shock. Hydroxyethylstarch (HES) is a widely used, relatively inexpensive alternative to human albumin for correcting hypovolemia. The use of isotonic saline as the standard of care for intravascular volume expansion to prevent or treat AKI is thus based upon the lack of clear evidence that colloids are superior for this purpose, along with some evidence that specific colloids may cause AKI, in addition to their higher costs. It is acknowledged that colloids may be chosen in some patients to aid in reaching resuscitation goals, or to avoid excessive fluid administration in patients requiring large volume resuscitation, or in specific patient subsets (for example, a cirrhotic patient with spontaneous peritonitis, or in burns). Similarly, although hypotonic or hypertonic crystalloids may be used in specific clinical scenarios, the choice of crystalloid with altered tonicity is generally dictated by goals other than intravascular volume expansion (for example, hypernatremia or hyponatremia). In addition, isotonic saline solution contains 154 mmol/l chloride and when administration in large volumes will result in relative or absolute hyperchloremia. All intravenous fluids can contribute to adverse renal and patient outcomes by fluid overload and renal edema. Fluids containing non- physiologic ratios of sodium and chloride may worsen AKI. Balanced electrolyte solutions, such as lactated Ringer’s solution, are preferable in most patients [2]. Albumin solutions have generally been found to be safe in sepsis resuscitation; however, evidence that hyperoncotic albumin might deteriorate AKI and ICU outcomes is growing [28,29].
Vasopressors
Norepinephrine is recommended as an agent of choice for septic shock treatment. Dopamine is not recommended for renal protection and is associated with more adverse events than norepinephrine is. Vasopressin does not appear to increase AKI risk [30-32].
Human recombinant alkaline phosphatase (AP) is an endogenous enzyme that confers renal protection during sepsis via the dephosphorylation of various compounds, including bacterial endotoxins and proinflammatory mediators such as extracellular adenosine triphosphate, which is released by mitochondria in response to inflammation and hypoxia. Intravenous infusion of AP (bolus injection followed by continuous infusion for 48 hours or placebo) starting within 48 hours of AKI onset and showed improvement of endogenous creatinine clearance, requirement for and duration of dialysis, decreased urinary biomarkers of renal injury (KIM-1 and interleukin-18), and inflammatory biomarkers from baseline to day 28 in patients receiving AP. Human recombinant AP is a highly stable, biologically active enzyme [32,33].
Angiotensin II (ATII) is potent vasoconstrictor acting via angiotensin II type 1 receptors and appears to cause vasoconstriction of efferent more than afferent arterioles, resulting in increasing glomerular perfusion pressure and filtration rate. Sepsis leads to relative scarcity of ATII. In addition, ATII is a potent vasopressor without inotropic or chronotropic properties. Unlike norepinephrine, ATII may preserve medullary perfusion and oxygenation [34].
Diuretics
Loop diuretics (especially furosemide) have long been prescribed in the acute-care setting, and a number of RCTs have tested whether furosemide is beneficial for prevention or treatment of AKI. Specifically, prophylactic furosemide was found to be ineffective or harmful when used to prevent AKI after cardiac surgery, and to increase the risk of AKI when given to prevent contrast-induced AKI. Furosemide may be useful in achieving fluid balance to facilitate mechanical ventilation according to the lung-protective ventilation strategy in hemodynamically stable patients with acute lung injury. Mannitol is often added to the priming fluid of the cardiopulmonary bypass system to reduce the incidence of renal dysfunction, but the results of these studies are not very convincing. Mannitol is beneficial in rhabdomyolysis by stimulating osmotic diuresis and by lowering the intracompartmental pressure in the affected crushed limbs [35,36].
Deferoxamine
A key early feature of AKI is the generation of reactive oxygen species. The iron chelator deferoxamine is a widely known free radical scavenger. In several models of AKI, deferoxamine was proved effective. The protective effect of deferoxamine in various models suggests the central role of free radicals in AKI. Studies in AKI are planned to test the efficacy of iron chelation [21].
Vasodilator Therapy: Dopamine, Fenoldopam, and Natriuretic Peptides
There is also limited evidence that the use of dopamine to prevent or treat AKI causes harm. Dopamine can trigger tachyarrhythmias and myocardial ischemia, decrease intestinal blood flow, cause hypopituitarism, and suppress T-cell function. Fenoldopam mesylate is a pure dopamine type-1 receptor agonist that has similar hemodynamic renal effects as low-dose dopamine, without systemic αadrenergic or β-adrenergic stimulation. The guideline recommendation against using fenoldopam places a high value on avoiding potential hypotension and harm associated with the use of this vasodilator in high-risk perioperative and ICU patients, and a low value on potential benefit, which is currently only suggested by relatively low-quality single-center trials. Nesiritide (b-type natriuretic peptide) is the latest natriuretic peptide introduced for clinical use, and is approved by the US Food and Drug Administration only for the therapy of acute, decompensated congestive heart failure [37-39].
Caspase Inhibitors
Caspases are a family of proteases that are involved in the initiation and execution phase of apoptosis. Nonselective and selective caspase inhibitors are effective in attenuating renal injury in ischemia- or endotoxemia-induced AKI when administered before or at the time of injury [40].
Minocycline
Minocycline are second-generation tetracycline antibiotics with proven human safety data. Minocycline is known to have antiapoptotic and anti-inflammatory effects. When administered 36 h before renal ischemia, minocycline reduced tubular cell apoptosis and mitochondrial release of cytochrome c, p53, and bax [41].
Ethyl Pyruvate
Pyruvate has been known as a potent endogenous antioxidant and free radical scavenger.In addition to an effect on mortality, ethyl pyruvate reduced kidney injury using the technique cecal ligation puncture as a model of sepsis. Ethyl pyruvate is a widely used food additive and has been shown to be safe in phase I clinical trials. It now is being tested in a phase II trial in patients who undergo cardiopulmonary bypass surgery [42].
Activated Protein C
Activated protein C (APC) is a physiologic anticoagulant that is generated by thrombin-thrombomodulin complex in endothelial cells. In addition to its effect on coagulation, APC has been shown to have anti-inflammatory, antiapoptotic effects. APC also attenuated renal IRI by inhibiting leukocyte activation. APC is approved by the Food and Drug Administration for treating patients who have severe sepsis and an Acute Physiology, Age, Chronic Health Evaluation (APACHE) score of 25 or higher [43].
Insulin
Insulin resistance and hyperglycemia are common in critically ill patients, and intensive insulin therapy that targeted blood glucose level between 80 and 110 mg/dl reduced the incidence of AKI that required dialysis or hemofiltration [21,44].
Conclusion
Acute kidney injury (AKI), previously called acute renal failure, is characterized by an abrupt decline in renal function, resulting in an inability to secrete wastes and maintain electrolyte and water balance. The severity of AKI was defined by the AKIN staging criteria as follows: Stage I, SCr increase to 1.5-2 fold of baseline; Stage II, SCr increase to 2-3 fold of baseline; and Stage III, SCr increase to 3 fold of baseline or an absolute increase of 356.6 mmol/l (4.0 mg/dl) with an acute increase of at least 44.2 mmol/l (0.5 mg/dl). Patient-related factors can be modifiable, for example, volume depletion, hypotension, anaemia, hypoxia and use of nephrotoxic drugs, or non-modifiable, for example, chronic kidney, heart, and liver or gastrointestinal disease, diabetes and severe infections and sepsis. Fluid homeostasis is affected, as declining glomerular filtration rate (GFR) and activation of the renin angiotensin system promote fluid retention, which presents as peripheral oedema, third-space effusions, and pulmonary congestion, especially in those with heart failure. In addition, as urinary output determines potassium excretion, hyperkalaemia is a common complication of severe AKI. Hypotonic or hypertonic crystalloids may be used in specific clinical scenarios; the choice of crystalloid with altered tonicity is generally dictated by goals other than intravascular volume expansion (for example, hypernatremia or hyponatremia). In addition, isotonic saline solution contains 154 mmol/l chloride and when administration in large volumes will result in relative or absolute hyperchloremia.
Abbreviations
AKI: Acute Kidney Injury; AKIN: Acute Kidney Injury Network; GFR: Glomerular Filtration Rate; HIC: High-Income Countries; ICU: Intensive Care Unit; RRT: Renal Replacement Therapy; RIFLE: Risk Injury Failure Loss End Stage; Scr: Serum Creatinine
Acknowledgments
The author would be grateful to anonymous reviewers by the comments that increase the quality of this manuscript.
Data Sources
Sources searched include Google Scholar, Research Gate, PubMed, NCBI, NDSS, PMID, PMCID, Scopus database, Science direct, Scielo and Cochrane database. Search terms included: definition, stages, risk factors, pathophysiology and treatment of acute kidney injury.
Funding
None
Availability of Data and Materials
The datasets generated during the current study are available with correspondent author.
Competing Interests
The author has no financial or proprietary interest in any of material discussed in this article.
References
- Yang F, Zhang L, Wu H, Zou H, Du Y (2014) Clinical Analysis of Cause, Treatment and Prognosis in Acute Kidney Injury PLoS ONE 9. [crossref]
- Kellum, Norbert L, KDIGO AKI Guideline Work Group (2013) Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical Care 17. [crossref]
- Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, et (2006) RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 10. [crossref]
- Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C (2006) An assessment of the RIFLE criteria for acute renal failure in hospitalized Crit Care Med 34: 1913- 1917. [crossref]
- Kellum JA, Paola R, Gloria A, Claudio R, Alexander Z, et al. (2021) Acute kidney injury. Disease primers 7.
- Mehta, John AK, Sudhir VS, Bruce AM, Claudio R, et (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care 11. [crossref]
- Mehta RL, Chertow GM (2003) Acute renal failure definitions and classification: time for change?. J Am Soc Nephrol 14: 2178-2187. [crossref]
- Mehta RL, Emmanuel AB, Jorge C, John F, Fredric F, et al. (2016) Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional Lancet 387: 2017-2025. [crossref]
- Bairey Merz CN, Laura MD, Julie RI, Amanda V, Joel N, et al. (2019) Sex and the kidneys: current understanding and research opportunities. Rev. Nephrol. 15: 776-783. [crossref]
- Basile, et (2012) Pathophysiology of Acute Kidney Injury. Compr Physiol 2: 1303- 1353. [crossref]
- Beers K, Huei HW, Aparna S, Kinsuk C, Mihir D, et al. (2020) Racial and ethnic disparities in pregnancy-related acute kidney Kidney 360 1: 169-178.
- Makris K, Loukia S (2016) Acute Kidney Injury: Definition, Pathophysiology and Clinical Clin Biochem Rev 37: 85-98. [crossref]
- Cerdá J, Norbert L, Paul E, Neesh P, Sigehiko U, et (2008) Epidemiology of acute kidney injury. Clin. J. Am. Soc. Nephrol. 3: 881-886. [crossref]
- Prowle JR, Kirwan CJ, Bellomo R (2014) Fluid management for the prevention and attenuation of acute kidney injury. Overview of fluid status assessment and analysis of cardiovascular and renal targets for the prevention and attenuation of AKI. Rev. Nephrol 10: 37-47. [crossref]
- Weyker PD, Pérez XL, Liu KD (2016) Management of acute kidney injury and acid- base balance in the septic Clin. Chest Med. 37: 277-288. [crossref]
- Lee SA, Cozzi M, Bush EL, Rabb H (2018) Distant organ dysfunction in acute kidney injury: a Am. J. Kidney Dis. 72, 846-856. [crossref]
- Meijers B, Evenepoel P, Anders HJ (2019) Intestinal microbiome and fitness in kidney disease. Rev. Nephrol. 15: 531-545. [crossref]
- Li X, Hassoun HT, Santora R, Rabb H (2009) Organ crosstalk: the role of the Curr. Opin. Crit. Care 15: 481-487. [crossref]
- Faubel S, Edelstein CL (2016) Mechanisms and mediators of lung injury after acute kidney Nat. Rev. Nephrol. 12: 48-60. [crossref]
- Nakazawa, D, Santhosh VK, Julian M, Jyaysi D, Alexander H, et (2017) Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in Ischemic AKI. J. Am. Soc. Nephrol. 28: 1753-1768. [crossref]
- Jo SK, Mitchell HR, Mark DO, et al. (2007) Pharmacologic Treatment of Acute Kidney Injury: Why Drugs Haven’t Worked and What Is on the Horizon. Clin J Am Soc Nephrol 2: 356-365. [crossref]
- Lattanzio MR, Nelson PK (2009) Acute Kidney Injury: New Concepts in Definition, Diagnosis, Pathophysiology, and J Am Osteopath Assoc. 109: 13-19. [crossref]
- Miller SB, Martin DR, Kissane J, Hammerman MR (1992) Insulin-like growth factor I accelerates recovery from ischemic acute tubular necrosis in the Proc Natl Acad Sci USA 89: 11876-11880. [crossref]
- Acker CG, Singh AR, Flick RP, Bernardini J, Greenberg A, et al. (2000) A trial of thyroxine in acute renal Kidney Int. 57: 293-298. [crossref]
- Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, et (2008) Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis 197: 1548-1557. [crossref]
- Bagshaw SM, Lapinsky S, Dial S, Yaseen A, Peter D, et (2009) Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 35: 871-881. [crossref]
- Luther MK, Timbrook TT, Caffrey AR, David D, Thomas PL, et (2018) Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med 46:12-20. [crossref]
- Caironi P, Tognoni G, Masson S, et al. (2014) Albumin replacement in patients with severe sepsis or septic N Engl J Med 370: 1412-1421. [crossref]
- Finfer S, McEvoy S, Bellomo R, Colin McA, John M, et (2011) Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 37: 86-96. [crossref]
- Udeh CI, You J, Wanek MR, Jarrod D, Belinda LU, et al. (2018) Acute kidney injury in postoperative shock: is hyperoncotic albumin administration an unrecognized resuscitation risk factor?. Perioper Med (Lond) [crossref]
- Rhodes A, Evans LE, Alhazzani W, et al. (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med 43: 304-377.
- De Backer D, Biston P, Devriendt J, et al. (2010) Comparison of dopamine and norepinephrine in the treatment of N Engl J Med 362: 779-789.
- Peters E, Masereeuw R, Pickkers P (2014) The potential of alkaline phosphatase as a treatment for sepsis-associated acute kidney Nephron Clin Pract 127: 144-148. [crossref]
- Heemskerk S, Masereeuw R, Moesker O, Martijn PWJMB, Johannes GH, et (2009) Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med 37: 417-423. [crossref]
- Lombardi R, Ferreiro A, Servetto C (2003) Rena l function after cardiac surgery: adverse eff ect of Ren Fail 25: 775-786. [crossref]
- Karajala V, Mansour W, Kellum JA (2009) Diuret ics in acute kidney Minerva Anestesiol 75: 251-257. [crossref]
- Murray PT (2003) Use of dopaminergic agents fo r renoprotection in the In Yearbook of Intensive Care and Emergency Medicine. Berlin: Springer-Verlag 637- 648.
- Murray PT (2006) Fenoldopam: renal-dose dopami ne redux? Crit Care Med 34: 910- 911. [crossref]
- Jonathan D, Sackner-B, Marcin K, Marshal F, Keith A (2005) Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled JAMA 293: 1900-1905. [crossref]
- Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR (2005) Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: Role of nitric oxide and Am J Physiol Renal Physiol 289: F1324-F1332. [crossref]
- Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, et al. (2004) Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 288: F91-F97. [crossref]
- Miyaji T, Hu X, Yuen PS, Muramatsu Y, Iyer S, et (2003) Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int 64: 1620-1631. [crossref]
- Mizutani A, Okajima K, Uchiba M, Noguchi T (2000) Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood 95: 3781-3787.
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, et al. (2001) Intensive insulin therapy in the critically ill patients. N Engl J Med 345: 1359-1367. [crossref]