Abstract
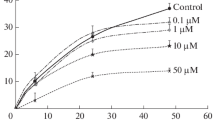
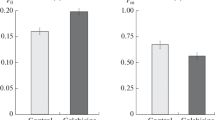
Tyrosine phosphorylation plays a vital role in the variety of signal transduction pathways in eukaryotic cells, however its role and relevance in plants are still largely unknown. To investigate the functional role of tubulin tyrosine phosphorylation in plant cells the interplay between the effects of tyrosine kinases (herbimycin A) as well as tyrosine phosphatases (sodium orthova nadate) inhibitors on microtubules sensitivity to cold in A. thaliana root cells were studied. Since it was found that inhibition of tyrosine kinases significantly increased the microtubules sensitivity to cold, while inhibition of tyrosine phophatases enhanced their cold-resistance, we suggest an existence of certain functional interaction between the phosphorylation on tyrosine residues and sensitivity of cortical microtubules to low temperatures.
Similar content being viewed by others
References
Mayer, U. and Jürgens, G., Microtubule Cytoskeleton: a Track Record, Curr. Opin. Plant Biol., 2002, vol. 5, no. 6, pp. 494–501.
Hasezawa, S. and Kumagai, F., Dynamic Changes and the Role of the Cytoskeleton During the Cell Cycle in Higher Plant Cells, Int. Rev. Cytol., 2002, vol. 214, pp. 161–191.
Ehrhardt, D.W. and Shaw, S.L., Microtubule Dynamics and Organization in the Plant Cortical Array, Ann. Rev. Plant Biol., 2006, vol. 57, pp. 859–875.
Guo, L., Ho, C.-M.K., Kong, Z., Lee, Y.-R.J., Qian, Q., and Liu, B., Evaluating the Microtubule Cytoskeleton and Its Interacting Proteins in Monocots by Mining the Rice Genome, Ann. Bot., 2009, vol. 103, no. 3, pp. 387–402.
Goddard, R.H., Wick, S.M., Silflow, C.D., and Snustad, D.P., Microtubule Components of the Plant Cell Cytoskeleton, Plant Physiol., 1994, vol. 104, no. 1, pp. 1–6.
Cheng, Z., Snustad, D.P., and Carter, J.V., Temporal and Spatial Expression Patterns of TUB9, β-Tubulin Gene of Arabidopsis thaliana, Plant. Mol. Biol., 2001, vol. 47, no. 3, pp. 389–398.
Abdrakhamanova, A., Wang, Q., Khokhlova, L., and Nick, P., Is Microtubule Disassembly a Trigger for Cold Acclimation?, Plant Cell Physiol., 2003, vol. 44, no. 7, pp. 676–686.
Parrotta, L., Cai, G., and Cresti, M., Changes in the Accumulation of α- and β-Tubulin during Bud Development in Vitis vinifera L, Planta, 2010, vol. 231, no. 2, pp. 277–291.
Yu, Y., Li, Y., Li, L., Lin, J., Zheng, C., and Zhang, L., Overexpression of PwTUA1, a Pollen-Specific Tubulin Gene, Increases Pollen Tube Elongation by Altering the Distribution of α-Tubulin and Promoting Vesicle Transport, J. Exp. Bot., 2009, vol. 60, no. 9, pp. 2737–2749.
Cai, G., Assembly and Disassembly of Plant Microtubules: Tubulin Modifications and Binding to MAPs, J. Exp. Bot., 2010, vol. 61, no. 3, pp. 623–626.
Duckett, C.M. and Lloyd, C.W., Gibberellic Acid-Induced Microtubule Reorientation in Dwarf Peas Is Accompanied by Rapid Modification of an α-Tubulin Isotypes, Plant J., 1994, vol. 5, pp. 363–372.
Blume, Ya.B., Smertenko, A., Ostapets, N.N., Viklick, V., and Draber, P., Post-Translational Modifications of Plant Tubulin, Cell Biol. Int., 1997, vol. 21, pp. 918–920.
Gilmer, S., Clay, P., MacRae, T.H., and Fowke, L.C. Acetylated Tubulin Is Found in All Microtubule Arrays of Two Species of Pine, Protoplasma, 1999, vol. 207, pp. 174–185.
Gilmer, S. and Clay, P., MacRae T.H., Fowke L.C. Tyrosinated, but not Detyrosinated, α-Tubulin Is Present in Root Tip Cells, Protoplasma, 1999, vol. 210, pp. 92–98.
Huang, Y., Li, H., Gupta, R., Morris, P.C., Luan, S., and Kieber, J.J., AtMPK4, an Arabidopsis Homolog of Mitogen-Activated Protein Kinases, Is Activated in vitro by At-MEK1 through Threonine Phosphorylation, Plant Physiol., 2000, vol. 122, no. 4, pp. 1301–1310.
Blume, Ya., Yemets, A., Sulimenko, V., Sulimenko, T., Chan, J., Lloyd, C., and Dráber, P., Tyrosine Phosphorylation of Plant Tubulin, Planta, 2008, vol. 229, pp. 143–150.
Yemets, A.I., Krasylenko, Yu.A., Lytvyn, D.I., Sheremet, Ya.A., and Blume, Ya.B., Nitric Oxide Signaling via Cytoskeleton in Plants, Plant Sci., 2011, vol. 181, no. 5, pp. 545–554.
Blume, Ya., Yemets, A., Sheremet, Ya., Nyporko, A., Sulimenko, V., Sulimenko, T., and Dárber, P., Exposure of Beta-Tubulin Regions Defined by Antibodies on an Arabidopsis thaliana Microtubule Protofilament Model and in the Cells, BMC Plant Biol, 2010, vol. 10, no. 29, pp. 1–10.
Sheremet, I., Yemets, A., Dráber, P., and Blume, Ya.B., Plant Microtubules Are Modified by the Tyrosine Phosphorylation in Arabidopsis Root Cells, in Annual Main Meeting of Society for Experimental Biology: Abstract Book, Prague, 2010, p. 246.
Yemets, A., Sheremet, Y., Vissenberg, K., Van Orden, J., Verbelen, J.P., and Blume, Ya.B., Effects of Tyrosine Kinase and Phosphatase Inhibitors on Microtubules in Arabidopsis Root Cells, Cell Biol. Int., 2008, vol. 32, no. 6, pp. 630–637.
Wallin, M. and Strmberg, E., Cold-Stable and Cold-Adapted Microtubules, Int. Rev. Cytol., 1995, vol. 157, pp. 1–31.
Mizuno, K., Induction of Cold Stability of Microtubules in Cultured Tobacco Cells, Plant Physiol., 1992, vol. 100, no. 2, pp. 740–748.
Baluka, F., Mancuso, S., Volkmann, D., and Barlow, P.W., Root Apex Transition Zone: a Signaling-Response Nexus in the Root, Trends Plant Sci., 2010, vol. 15, no. 7, pp. 402–408.
Zhao, J.L., Li, X.J., Zhang, H., and Li, Y., Chilling Stability of Microtubules in Root-Tip Cells of Cucumber, Plant Cell Rep., 2003, vol. 22, no. 1, pp. 32–37.
Farajalla, M.R. and Gulick, P.J., The α-Tubulin Gene Family in Wheat (Triticum aestivum L.) and Differential Gene Expression during Cold Acclimation, Genome, 2007, vol. 50, pp. 502–510.
Cyr, R.J. and Palevitz, B.A., Organization of Cortical Microtubules in Plant Cells, Curr. Opin. Cell Biol., 1995, vol. 7, no. 1, pp. 65–71.
Blume, Ya.B., Lloyd, C.W., and Yemets, A.I., Plant Tubulin Phosphorylation and Its Role in Cell Cycle Progression, in The Plant Cytoskeleton: A Key Tool for Agro-Biotechnology, Blume, Ya.B., Baird, W.V., Yemets, A.I., and Breviario, D., Eds., Berlin: Springer-Verlag, 2008, pp. 145–159.
Sheremet, Ya.A., Yemets, A.I., Verbelen, J.-P., and Blume, Ya.B., The Effect of Okadaic Acid on Arabidopsis thaliana Root Morphology and Microtubule Organization in Its Cells, Cytol. Genet., 2009, vol. 43, no. 1, pp. 3–10.
Sheremet, Ya.A., Yemets, A.I., Vissenberg, K., Verbelen, J.-P., and Blume, Ya.B., Effects of Inhibitors of Serine/Threonine Protein Kinases on Arabidopsis thaliana Root Morphology and Microtubule Organization in Its Cells, Cell Tiss. Biol., 2010, vol. 4, no. 4, pp. 399–409.
Mathur, J. and Chua, N.H., Microtubule Stabilization Leads To Growth Reorientation in Arabidopsis Trichomes, Plant Cell, 2000, vol. 12, no. 4, pp. 465–477.
Yemets, A.I., Krasylenko, Yu.A., Sheremet, Ya.A., and Blume, Ya.B., Microtubule Reorganization as a Response to Implementation of NO Signals in Plant Cells, Cytol. Genet., 2009, vol. 43, no. 2, pp. 73–79.
Baluka, F., Parker, J.S., and Barlow, P.W., The Microtubular Cytoskeleton in Cells of Cold-Treated Roots of Maize (Zea mays L.) Shows Tissue-Specific Responses, Protoplasma, 1993, vol. 172, pp. 84–96.
Kalinina, I., Shevchenko, G., and Kordyum, E., Tubulin Cytoskeleton in Arabidopsis thaliana Root Cells under Clinorotation, Microgravity Sci. Technol., 2009, vol. 21, nos. 1/2, pp. 187–190.
Krasylenko, Yu.A., Yemets, A.I., Sheremet, Ya.O., and Blume, Ya.B., Nitric Oxide as a Critical Factor for Arabidopsis Microtubules Perception of UV-B Irradiation, Physiol Plant, 2011. doi:10.1111/j.1399-3054.2011.01520x
Monroy, A.F., Sangwan, V., and Dhindsa, R.S., Low Temperature Signal Transduction during Cold Acclimation: Protein Phosphatase 2A as an Early Target for Cold Inactivation, Plant J., 1998, vol. 13, pp. 653–660.
Sangwan, V., Foulds, I., Singh, J., and Dhindsa, R.S., Cold-Activation of Brassica napus BN115 Promoter Is Mediated by Structural Changes in Membranes and Cytoskeleton, and Requires Ca2+ Influx, Plant J., 2001, vol. 27, no. 1, pp. 1–12.
Kameyama, K., Kishi, Y., Yoshimura, M., Kanzawa, N., Sameshima, M., and Tsuchiya, T., Tyrosine Phosphorylation in Plant Bending, Nature, 2000, vol. 407, no. 6800, p. 37.
MacRobbie, E.A., Evidence for a Role for Protein Tyrosine Phosphatase in the Control of Ion Release from the Guard Cell Vacuole in Stomatal Closure, Proc. Nat. Acad. Sci. USA., 2002, vol. 99, no. 18, pp. 11963–11968.
Zi, H., Xiang, Y., Li, M., Wang, T., and Ren, H., Reversible Protein Tyrosine Phosphorylation Affects Pollen Germination and Pollen Tube Growth via the Actin Cytoskeleton, Protoplasma, 2007, vol. 230, nos. 3/4, pp. 183–191.
Bretz, J.R., Mock, N.M., Charity, J.C., Zeyad, S., Baker, C.J., and Hutcheson, S.W., A Translocated Protein Tyrosine Phosphatase of Pseudomonas syringae pv. Tomato DC3000 Modulates Plant Defense Response to Infection, Mol. Microbiol., 2003, vol. 49, pp. 389–400.
Espinosa, A., Guo, M., Tam, V.C., Fu, Z.Q., and Alfano, J.R., The Pseudomonas syringae Type III-Secreted Protein HopPtoD2 Possesses Protein Tyrosine Phosphatase Activity and Suppresses Programmed Cell Death in Plants, Mol. Microbiol., 2003, vol. 49, no. 2, pp. 377–387.
Huang, S., Blanchoin, L., Kovar, D.R., and Staiger, C.J., Arabidopsis Capping Protein (AtCP) Is a Heterodimer That Regulates Assembly at the Barbed Ends of Actin Filaments, J. Biol. Chem., 2003, vol. 278, no. 45, pp. 44832–44842.
Luan, S., Tyrosine Phosphorylation in Plant Cell Signaling, Proc. Nat. Acad. Sci. U.S.A., 2002, vol. 99, no. 18, pp. 11567–11569.
Ghelis, T., Bolbach, G., Clodic, G., Habricot, Y., Miginiac, E., Sotta, B., and Jeannette, E., Protein Tyrosine Kinases and Protein Tyrosine Phosphatases Are Involved in Abscisic Acid-Dependent Processes in Arabidopsis Seeds and Suspension Cells, Plant Physiol., 2008, vol. 148, no. 3, pp. 1668–1680.
Bentem van, S. and Hirt, H., Protein Tyrosine Phosphorylation in Plants: More Abundant Than Expected? Trends Plant Sci., 2009, vol. 14, no. 2, pp. 71–76.
Jaillais, Y., Hothorn, M., Belkhadir, Y., Dabi, T., Nimchuk, Z.L., Meyerowitz, E.M., and Chory, J., Tyrosine Phosphorylation Controls Brassinosteroid Receptor Activation by Triggering Membrane Release of Its Kinase Inhibitor, Genes Dev., 2011, vol. 25, no. 3, pp. 232–237.
Mithoe, C.S. and Menke, F.L.H., Phosphoproteomics Perspective on Plant Signal Transduction and Tyrosine Phosphorylation, Phytochemistry, 2011, vol. 72, no. 10, pp. 997–1006.
Blume, Ya.B., Karpov, P.A., Nyporko, A.Yu., Samofalova, D.A., Sheremet, Ya.O., and Yemets, A.I., Bioinformatic Analysis of Arabidopsis Kinome and Phosphatome for Investigation of Microtubule Functions and Applied Aspects of Their Regulation, in Biotechnology Conference: Science and Advance in the Black Sea Region, Albena, 2008, pp. 1–3.
Dash, A., Evidence for the Presence of Non-Receptor Protein Tyrosine Kinases in Algal Cells, Acta Physiol. Plant., 2010, vol. 32, no. 1, pp. 177–182.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Sheremet, Y.A., Yemets, A.I. & Blume, Y.B. Inhibitors of tyrosine kinases and phosphatases as a tool for the investigation of microtubule role in plant cold response. Cytol. Genet. 46, 1–8 (2012). https://doi.org/10.3103/S0095452712010112
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452712010112