More Information
Submitted: 04 July 2019 | Approved: 30 July 2019 | Published: 31 July 2019
How to cite this article: Fauvel C, Bubenheim M, Raitière O, Vallet C, Bauer F, et al. Preclinical stiff heart is a marker of cardiovascular morbimortality in apparently healthy population. J Cardiol Cardiovasc Med. 2019; 4: 083-089.
DOI: 10.29328/journal.jccm.1001045
Copyright License: © 2019 Fauvel C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Heart failure; Preserved ejection fraction; Prognosis; Stiff heart; Preclinical; Relaxation
Preclinical stiff heart is a marker of cardiovascular morbimortality in apparently healthy population
Charles Fauvel1, Michael Bubenheim2, Olivier Raitière1, Charlotte Vallet1, Nassima Si Belkacem1 and Fabrice Bauer1*
1Rouen University Hospital, Heart Failure Clinic and Pulmonary Hypertension Referral Center, Pole Thorax, Vaisseaux, Rouen, F-76031, France
2Rouen University Hospital, Department of Statistics, Rouen, F-76031, France
*Address for Correspondence: Fabrice Bauer, Heart Failure Clinic and Pulmonary Hypertension Referral Center, Pole Thorax Vaisseaux, Rouen University Hospital, Rouen, France, Tel: +33 2 32 88 82 32; Fax: + 33 2 32 88 87 14; Email: fabrice.bauer@chu-rouen.fr
Background: The prognostic significance of impaired left ventricular (LV) relaxation and increased LV stiffness as precursor of heart failure with preserved ejection fraction and death is still largely unknown in apparently healthy subjects.
Methods: We constituted a cohort of 353 patients with normal ejection fraction (>45%) and no significant heart disease, based on a total of 3,575 consecutive left-sided heart catheterizations performed. We measured peak negative first derivative of LV pressure (-dP/dt) and operating chamber stiffness (Κ) using a validated equation. Patients were categorized as having: 1) normal diastolic function, 2) isolated relaxation abnormalities (-dP/dt > 1860mm Hg/sec and K <0.025mm Hg/ml), or 3) predominant stiff heart (K ≥0.025mm Hg/ml).
Results: During a follow-up of at least 5 years, the incidence of the primary composite endpoint (death, major arterial event, heart failure, and arrhythmia) was 23.2% (82 patients). Compared to isolated relaxation abnormalities, predominant stiff heart showed stronger prognostic significance for all events (p=0.002), namely heart failure (HR, 2.9; p=0.0499), cardiac death (HR, 5.8; p=0.03), and heart failure and cardiac death combined (HR, 3.7; p=0.003).
Conclusion: In this apparently healthy population referred to our center for cardiac catheterization, the prevalence of diastolic dysfunction was very high. Moreover, predominant stiff heart was a better predictor of cardiovascular outcomes than isolated relaxation abnormalities.
Left ventricular (LV) stiffness is defined by the relationship between pressure and volume [1]. Increased LV stiffness is found in ventricular hypertrophy, ischemic heart disease, end-stage LV dysfunction and senescent heart leading to heart failure and preserved ejection fraction. Stiff heart puts the patient at increased risk for pulmonary capillary hypertension, impairs exercise capacity and plays a role to the transition from compensated to decompensated heart failure [2-4]. Changing the ventricular PV relationship by therapy could be an exciting prospect if the question to detect non-invasively increased LV stiffness and to link stiff heart to prognosis is solved.
Non-invasive direct measurement of LV stiffness is in the process of validation. A dedicated new ultrasound platform is today capable to create a myocardial shear wave in the low kHz frequency and to image it at an ultrahigh frame rate of 12 000 images/s using a single probe [5]. Left ventricular stiffness is automatically calculated from the Young’s modulus, also known as the tensile modulus or elastic modulus. After validation, this tool may replace the invasive and expansive conductance catheter and supplant the use of complex algorithm utilized as surrogate of left ventricular diastolic function assessment [3,6]. Prognostic value of diastolic wall strain in patients with chronic heart failure has to be validated and clearly linked to LV stiffness hence prognosis [7-9]. Diastolic wall strain is apparently correlated to myocardial shear wave elastography [10]. Further, drugs management of increased LV stiffness could be relevant if the link between PV relationship and cardiovascular endpoint is explored [11]. Therefore, the purpose of our study was to examine the prognostic significance of left ventricular stiffness as a potential new marker of cardiovascular event and a possible target to drugs.
Population: From January 1, 2001 to December 31, 2003, a total of 3,575 consecutive patients were referred to our center for coronary angiography due to suspected coronary artery disease based on symptoms, positive stress test, or incidental ECG abnormalities (bundle branch block or repolarization abnormality), or for preoperative assessment in high-risk patients. No patient had heart failure (HF) at the entry of the study.
Upon completion of the coronary angiography, we selected patients if they met the following inclusion criteria: no prior history of heart disease, no significant valvular heart disease, EF>45%, no pericardial disease, no significant coronary artery disease (stenosis<50%), and no significant dyspnea associated with HF (Framingham’s criteria). Therefore, we constituted a cohort of 353 patients considered to be free of established heart disease in spite of being exposed to traditional risk factors.
This cohort was prospectively followed up at our institution for cardiac events and death. Information on post diagnostic events was obtained for all patients except 2 loss of follow-up until March 2009 and confirmed by requesting a short medical report from the patients’ physicians and reviewing hospital medical reports. All patients gave their informed consent to participate in the study.
Left-sided heart catheterization
Cardiac catheterization was performed in a conventional manner. In short, a dedicated catheter was inserted from the right or left femoral artery under local anesthesia, advanced through the aorta into the left ventricle (LV) where pressures were measured. To calculate LV volumes, contrast medium was injected into the cavity at constant output from the right oblique anterior incidence after calibrating the image. Thereafter, dedicated catheters were placed at the origin of the coronary arteries, with a radiopaque contrast medium being selectively injected to rule out stenosis.
Invasive measurements
LV silhouettes at end-diastole and end-systole were manually traced onto a digital screen, with volumes calculated using the area-length method. EF was determined using the following formula: (end-diastolic volume, end-systolic volume)/end-diastolic volume. With the use of fluid filled catheter, computer-assisted analysis provided (Philips, Inc.) peak positive and negative first derivative of LV pressure (+dP/dt and -dP/dt, respectively) for LV contraction and relaxation. LV peak systolic pressure and end-diastolic pressure were measured, with LV stiffness being calculated from a simplified validated equation [12]. The diastolic pressure–volume relation can be described by an exponential equation, P=AeβV, where P is the left ventricular diastolic pressure, V is the left ventricular diastolic volume, and A and β are curve-fitting constants used to quantify passive stiffness. To model this exponential equation, we chose 2 and ideally 3 pressure-volume (PV) points: end-diastolic pressure and volume, pre-A diastolic pressure and volume, and early pressure and volume at the time of minimal diastolic pressure. We did not correct for the effects of incomplete LV relaxation as it was previously suggested [12].
Subjects were classified into three groups based on the values published for normal or abnormal diastolic parameters [13]: normal diastolic function was defined as -dP/dt<-1860 mmHg/s and LV stiffness <0.025mm Hg/ml, and isolated relaxation abnormality as -dP/dt≥-1860mm Hg/s and LV stiffness as <0.025mm Hg/ml. As both parameters overlap, the third group was designated as predominant stiff heart characterized by LV stiffness ≥0.025mm Hg/ml. No patients had isolated stiff heart. These 2 cut-off values were verified and validated posteriorly with the use of receiver-operator characteristic curve analysis performed from our data. A ≥0.023 mm Hg/ml stiffness and a >-1700 mm Hg/s dP/dt were selected by receiver-operator characteristic curve analysis as the best cut-off value for distinguishing the patient’s issue, considered close to published data.
Statistical analysis
Continuous variables were expressed as mean±SD and nominal ones as number and percentage. Patient groups were compared at baseline using the Kruskal-Wallis or Fisher’s exact test, wherever appropriate. The purpose of our study was to gain insight into the probability of cardiovascular (CV) morbidity and mortality, i.e., death or the occurrence of selected non-fatal events following heart catheterization. Therefore, the combined primary endpoint included the following events: coronary syndrome, congestive HF, arrhythmia, vascular peripheral event, stroke, cardiac death, and non-cardiac death [14].
Given the small number of subjects in the normal group, our aim was to compare the probability of CV morbidity and mortality in subjects with a predominantly stiff heart versus that of those with an isolated relaxation abnormality using the Kaplan-Meier method. Differences between both groups were assessed using the log-rank test, which was also separately used for each patient group to identify predictors of CV morbimortality among baseline patient characteristics. For this purpose, values close to the observed first and second tertiles were chosen to categorize each baseline characteristic that was not already dichotomized. As our approach was exploratory, baseline characteristics were considered potential predictors when the log-rank test’s p-value was <0.05. As the occurrence of each event may result from different processes, a second aim of our study was to compare both patient groups in order to identify the different processes at work. Since the occurrence of a first non-fatal event implied especially survival up to this point, analyses were carried out using methods for competing risks. Hence, process-specific figures show the cumulated probability of a specific event occurring given the presence of other events. Given the assumption of independent process-specific hazards, differences between patient groups were assessed using Cox’s proportional hazard model. Due to the exploratory nature of the study, no corrections for multiple testing were carried out, and a p-value <0.05 was considered to be statistically significant.
Baseline characteristics and hemodynamics
Indications for left-sided heart catheterization were positive stress test for chest pain (88.4%), preoperative assessment for non-cardiovascular surgery and ECG abnormality (11.2%). Among the 353 subjects with satisfactory hemodynamic assessment at 60±11 years of age, 337 (95%) exhibited diastolic dysfunction, classified into isolated relaxation abnormality (56%) and predominant stiff heart (39%). Five percent of patients had no diastolic dysfunction. When examining patient characteristics at baseline, subjects with diastolic dysfunction presented similar risk factors, age, gender, and EF compared to those with normal function (Table 1). However, patients with predominant stiff heart were more likely to have higher LV end-diastolic pressure as well as smaller LV end-diastolic volume and stroke volume. In addition, subjects with stiff hearts were more likely to have deteriorated LV relaxation compared to normal subjects.
| Table 1: Clinical and hemodynamic characteristics | ||||
| Characteristics | Normal N=16 (5%) | Isolated relaxation abnormality N=198 (56%) |
Predominant stiff heart N=139 (39%) |
P |
| Categorical variables | ||||
| Male sex | 9 (56%) | 126 (64%) | 84 (60%) | 0.72 |
| Hypertension | 13 (81%) | 116 (59%) | 75 (54%) | 0.11 |
| Diabetes | 4 (25%) | 43 (22%) | 32 (23%) | 0.88 |
| Hypercholesterolemia | 7 (44%) | 99 (50%) | 63 (45%) | 0.66 |
| Obesity | 6 (38%) | 57 (29%) | 34 (24%) | 0.44 |
| Smoking | 5 (31%) | 82 (41%) | 54 (39%) | 0.72 |
| Continuous variables | ||||
| Age (y) | 63±11 | 59±11 | 60±11 | 0.56 |
| Heart rate (bpm) | 68±15 | 66±13 | 70±15 | 0.09 |
| Mean BP (mm Hg) | 105±13 | 99±15 | 98±14 | 0.12 |
| LVED pressure (mm Hg) | 13±6 | 14±6 | 15±5 | 0.03 |
| + dP/dt (mm Hg/s) | 1858±354 | 1474±280 | 1603±332 | <0.001 |
| - dP/dt (mm Hg/s) | 2015±153 | 1408±296 | 1591±304 | <0.001 |
| LVED volume (ml) | 75±19 | 75±22 | 58±14 | <0.001 |
| LVES volume (ml) | 22±12 | 23±10 | 19±8 | <0.001 |
| Stroke volume (ml) | 53±11 | 52±16 | 39±10 | <0.001 |
| Ejection fraction (%) | 73±11 | 70±9 | 68±10 | 0.08 |
| K (mm Hg/ml) | 0.016±0.006 | 0.016±0.006 | 0.036±0.012 | <0.001 |
| BP: Blood pressure; dP/dt: First derivative of pressure; ED: End-diastole; ES: End-systole; K: passive stiffness; LV: Left ventricle. | ||||
Outcomes in the population
Patients were followed up at least 5 years. During this period, 15 were hospitalized for congestive HF, 6 had strokes, 11 suffered from acute coronary syndrome, 15 developed arrhythmias (atrial fibrillation or flutter), and 5 underwent vascular surgery. Furthermore, among the 353 patients, 30 died during the follow-up period, the cause of death being cardiac (sudden death) in 10 patients and non-cardiac in 20. Outcomes at the end of the follow-up period in relation to the presence or absence of diastolic dysfunction are shown in table 2. During follow-up, patients with predominant stiff heart were more likely to experience adverse outcomes, including HF and death, compared to those with isolated relaxation abnormality or to normal patients.
| Table 2: Outcomes. | |||
| Outcomes | Normal n=16 (5%) | Isolated relaxation abnormality n=198 (56%) |
Predominant stiff heart n=139 (39%) |
| No events | 14 (88%) | 164 (83%) | 93 (67%) |
| Arterial outcomes | 1 (6%) | 10 (5%) | 11 (8%) |
| Peripheral artery disease | 1 | 2 | 2 |
| Coronary artery disease | 0 | 6 | 5 |
| Stroke | 0 | 2 | 4 |
| Arrhythmia | 1 (6%) | 9 (4%) | 5 (4%) |
| Heart failure | 0 (0%) | 5 (3%) | 10 (7%) |
| Death | 0 (0%) | 10 (5%) | 20 (14%) |
| CV death | 0 | 2 | 8 |
| Non-cardiac death | 0 | 8 | 12 |
| CV: cardiovascular | |||
Predictors of CV morbimortality were body mass (p=0.02) and EF (p=0.01) for patients with isolated relaxation abnormality, and age (p=0.04) and LV early diastolic pressure (p=0.05) for those with predominant stiff heart.
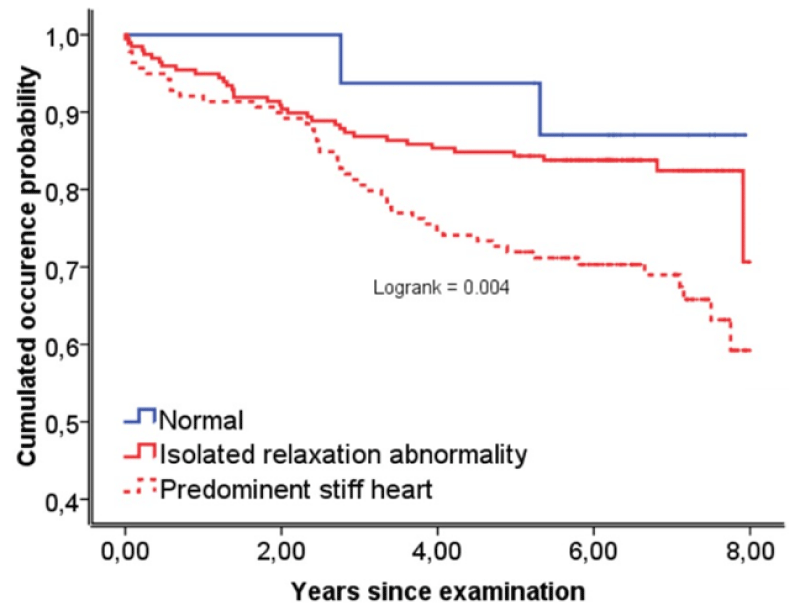
The risk of all-cause mortality and non-fatal events was significantly higher in patients with predominant stiff heart compared to those with impaired relaxation (p=0.002, Figure 1) and normal diastolic function. The difference between both patient groups with respect to CV morbidity and mortality only became noteworthy after a period of more than 2 years following catheterization (Figure 1).
Figure 1: Kaplan-Meier curves for event-free survival. Isolated relaxation abnormality indicates mild asymptomatic left ventricular diastolic ≥-1860mm Hg/s and K <0.025mm Hg/ml); predominant stiff heart indicates moderate-to-severe asymptomatic left ventricular diastolic dysfunction (K ≥0.025mm Hg/ml).
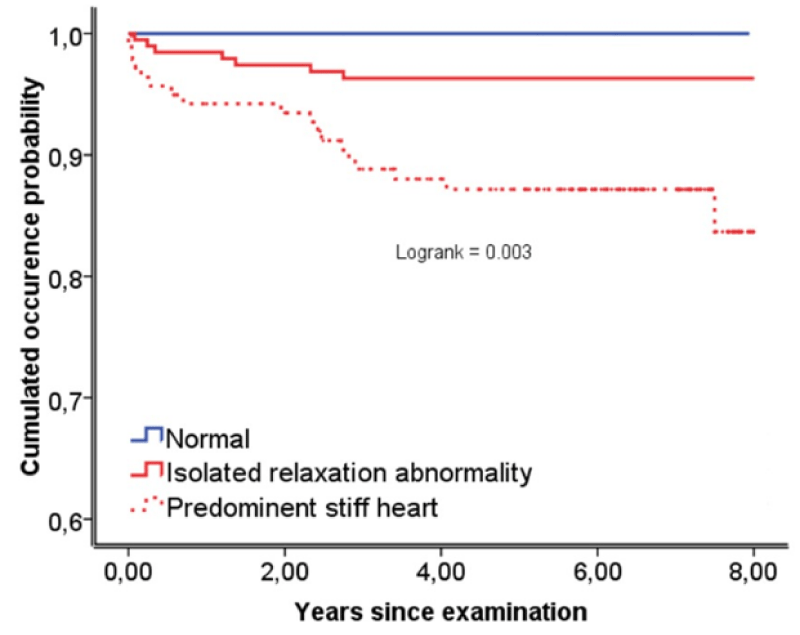
An exploratory sub-analysis of the different event-specific processes revealed that patients with predominant stiff heart exhibited a significantly higher risk of all-cause mortality (HR, 2.9; 95% CI, 1.3-6.2; p=0.006), HF (HR, 2.9; 95% CI; 1.0-8.6; p=0.0499), cardiac death (HR, 5.8; 95% CI, 1.2-27.3; p=0.03), and the combination of cardiac death and HF (HR, 3.7; 95% CI, 1.6-9.0; p=0.003; Figure 2) as compared with the others. However, patients with predominant stiff heart did not have a significantly higher risk for all non-fatal events (HR, 1.6; 95% CI, 0.9-2.8; p=0.10), other non-fatal events excepting HF (HR, 1.3; 95% CI, 0.6-2.4; p=0.51) and non-cardiac death (HR, 2.2; 95% CI, 0.9-5.3; p=0.09).
Figure 2: Patient’s probability of cumulated dysfunction (-dP/dt Heart failure and cardiac death before any other event.
Follow-up of patients with HF
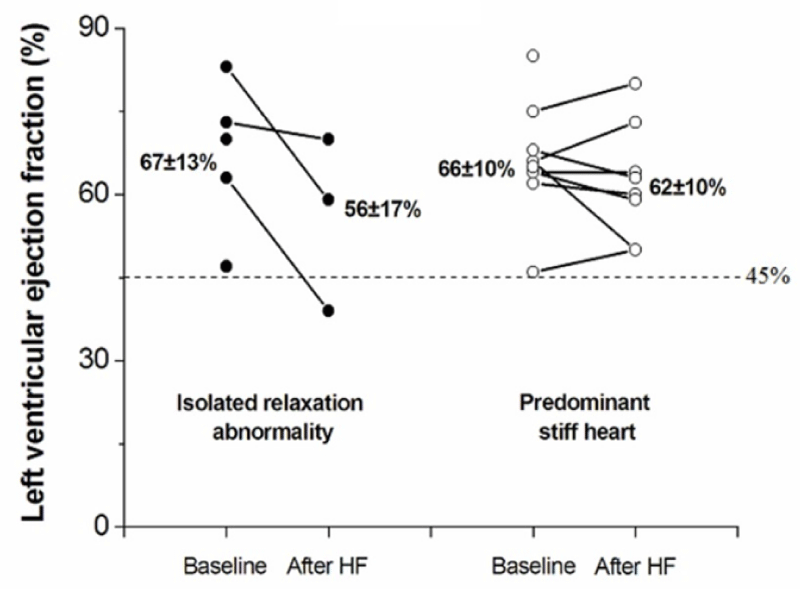
Of the 15 patients developing HF at follow-up, LVEF was available in 11 patients shortly after the episode of decompensation. Figure 3 illustrates the serial evaluation of EF at baseline and after incident HF in both groups. Overall, mean EF was 61±12% after HF versus 66±11% at baseline, indicating an episode of heart failure and preserved ejection fraction (HFpEF). Only one patient from the isolated relaxation abnormality group had EF <45%. Four patients had no post-éjection fraction assessment.
Figure 3: Plot of ejection fraction course before and after an episode of HF in both groups. 4 patients had no post-ejection fraction assessment.
In this cohort, the prevalence of diastolic dysfunction was common, unpredictable, and not necessarily correlated with age, gender, or other risk factors. Among the two markers of diastolic function, LV stiffness ≥0.025mm Hg/ml was associated with a higher risk of progression to CV events, including cardiac death and HF. Our findings may have implications on the evaluation of screening strategies and consequently, the management of unselected patients with isolated diastolic dysfunction, particularly those suffering from stiff heart.
Isolated diastolic dysfunction in the community
Isolated diastolic dysfunction is suspected on the basis of echocardiography findings, revealing a typical inversion of the transmitral flow velocity (E/A ratio) in healthy subjects aged 60 years and older [15,16]. This value is considered to be normal, with the phenomenon known as the aging heart. The prevalence and phenotype of isolated diastolic dysfunction is non-invasively reported but with a gross assessment by echocardiography [17-20]. Our cohort of 353 consecutive subjects who were invasively explored showed a high prevalence (95%) of isolated diastolic dysfunction. Extrapolating our results, it would appear that the magnitude of isolated diastolic dysfunction would be presumably similar in the wider community, although our population was notably selected with a certain degree of structural disorders. In addition, exposure to comparable risk factors needs to be recognized. Our study demonstrated that in patients aged 60 years, cardiac catheterization provided different data about the diastolic phenotype compared to echocardiography. The traditional inverted E/A ratio found using echocardiography is widely known as impaired relaxation [15,21]. In fact, at a mechanistic level, isolated abnormal relaxation was only found in 56% of patients, while 39% exhibited predominant stiff heart a structural and functional deterioration not easily detected by echocardiography in patients of this age group. In 186 (53%) patients, LV end-diastolic pressure was <15mm Hg, meaning the LV operated at a normal filling pressure despite abnormal diastolic function. Given the prognostic significance of stiff heart as demonstrated in our paper, the weakness of the Doppler modality to characterize diastolic dysfunction is only reinforced [22,23]. Some studies have emphasized the need to reappraise certain basic measurements, such as left atrial area and LV volumes, as both of these could prove more revealing than any other complex parameters or equations found in the literature. It should be noted that in our study, LV volumes were smaller in subjects with predominant stiff heart. Epidemiologically, the proportion of individuals aged over 60 years in developed countries is approximately 20%. Considering the prevalence of isolated diastolic dysfunction in this age range, subjects with stiff heart should be methodically evaluated and followed up given their risk for CV events.
The cardiovascular continuum in patients with diastolic dysfunction.
The CV disease continuum refers to the chain of events that may help us to understand, prevent, and treat heart diseases. While elaborated for coronary artery disease and HF with systolic dysfunction, the CV continuum still has many missing links in relation to diastolic dysfunction and HFpEF, which explains the current lack of validated treatments.
In short, the CV continuum may be divided into three fundamental steps: related and unrelated risk factors, early or preclinical tissue remodeling or dysfunction, and target-organ damage (stage B from the ACC/AHA guidelines) [24]. Each of these steps is correlated to outcome, such as end-organ failure or cardiac death (stage C from the ACC/AHA guidelines). In our study, we did not explore all three stages, but rather focused on the preclinical scenario, i.e. isolated diastolic dysfunction or stage B. Our findings revealed that predominant stiff heart rather than abnormal relaxation was correlated to outcome, including cardiac death and HFpEF. At an experimental level, Masuyama et al. demonstrated that LV relaxation abnormality was an early sign of isolated diastolic dysfunction, while LV stiffening played a crucial role in the transition from asymptomatic diastolic dysfunction to HFpEF [4]. Our study is the first to confirm these findings in humans. LV fibrosis and cardiomyocyte rigidity impose a viscoelastic burden, which compromises the diastole, thus affecting the rate of relaxation, diastolic suction, passive stiffness, and elevated end-diastolic pressure, ultimately leading to HF symptoms [3,25,26]. The link between stiff heart and cardiac death is less obvious, although it may be due to collagen accumulation in the myocardium, which acts as a trigger for fatal arrhythmia with sudden death the most common in the continuum of HFpEF [27,28]. The lack of statistical significance between traditional risk factors and predominant stiff heart is surprising given that this continuum is well documented in animals as well as in humans [3,12]. Considering our results, this lack of significance may be explained by several factors, which include the underpower of the study, adequate management of traditional or non-traditional risk factors as well as the lack of information on the duration of risk factor exposure. In addition, the role of certain pathways, especially cardiac senescence, oxidative stress, and endothelial dysfunction, still needs to be clarified [29].
Despite these differing results, we strongly support the theory that at a mechanistic level, there is a chain of events starting with various CV risk factors that lead to increased LV stiffness and then progress to established heart disease and poor outcomes.
Challenges to improve prognosis of diastolic dysfunction and heart failure with preserved EF
Approximately 15 to 20% of the world’s population is aged over 65 years. The annual rate of incident heart failure events approaches 10‰ for those aged 65 to 74 years, 25% for 75 to 84 years, and 50% for those aged 85 years and older [30]. Half of these subjects will develop HFpEF [31], which is a syndrome involving HF criteria, normal ejection fraction (EF) >45-50%, and echocardiographic or invasive evidence of diastolic dysfunction [32]. While outcome of patients with HF and reduced EF has dramatically improved over the last two decades, the prognosis of patients with HFpEF, particularly linked to mortality [33], has not been affected by the use of β-blockers, angiotensin-converting enzyme inhibitors, or angiotensin II receptor antagonists.
Established HF with preserved ejection has a poor prognosis given the current lack of treatment certainly because of irreversible organ damage in these patients belonging to stage C. A growing body of evidence has shown that elevated LV stiffness is one of the major determinants of established HFpEF [3]. Hence, the key to prevention and treatment of the disease is to focus on its early stage B when structural and functional disorders are potentially reversible. To our knowledge, we reported for the first time on the role of LV stiffness at the preclinical stage. Our findings indicated that predominant stiff heart with LV stiffness ≥ 0.025mm Hg/ml was prognostically important, with death and HF hazard being 3.7 times higher compared to individuals with isolated abnormal relaxation.
In order to improve the prognosis of patients with stiffness ≥ 0.025mm Hg/ml, developing the means to diagnose and treat this particular phenotype is a priority. Diagnosis may involve cardiac elastometry using echocardiography speckle tracking, delayed-enhancement magnetic resonance imaging (MRI), or T1-weighted MRI, unless another method becomes available [25,34]. Management of stiff heart remains complex. LV stiffness results from cell deregulation as well as the production and degradation of extracellular matrix. The presence of cardiac fibrosis is an important determinant of abnormal LV stiffness that contributes to diastolic dysfunction [26,35,36]. recently introduced the concept of cardiomyocyte resting tension, which also elevates stiffness, particularly in cases of HFpEF [35]. Future treatments should focus on the extracellular matrix, cardiomyocyte compliance regulation, as well as their original triggers. Several studies have looked at arterial stiffness and have shown that treatment with Angiotensin-convertin-enzyme inhibitor and thiazide diuretics can improve it. However, to our knowledge, no molecule has shown how to combat ventricular stiffness.
We acknowledge that methods to characterize diastolic function are questionable. Micro manometer catheters with adequate frequency response are preferable to fluid-filled catheter predisposed to ringing and catheter whip artifacts. For economic reasons, it is impossible to conduct an epidemiology, cohort-based invasive cardiac catheterization study with micro manometer catheters.
Deriving the diastolic pressure-volume relation from 3 coordinates is a gross oversimplification of true LV stiffness normally calculated from multiple successive points obtained after an inferior vena cava or aortic occlusion. This maneuver is almost impossible in human. Furthermore, LV volumes were measured by monoplane LV angiography that requires multiple assumptions. Again, conductance catheter or 3D echocardiography are preferable but we were consistent to the methodology used by Zile et coll [12]. Peak -dP/dt is not the optimal method to assess LV relaxation as it is quite load-sensitive. The generally accepted gold-standard is the time constant of iso-volumic pressure decay (τ), which could not be calculated in our work from a fluid-filled catheter.
Our study identified a preclinical phenotype of CV disease linked to prognosis. Our results suggest that in apparently healthy subjects, predominant stiff heart rather than isolated relaxation abnormality should be the new target for preventing CV outcomes characterized by HFpEF and cardiac death.
- Wisneski JA, Bristow JD. Left ventricular stiffness. Annu Rev Med. 1978; 29: 475-483. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/348043
- Sinning D, Kasner M, Westermann D, Schulze K, Schultheiss HP, et al. Increased left ventricular stiffness impairs exercise capacity in patients with heart failure symptoms despite normal left ventricular ejection fraction. Cardiol Res Pract. 2011; 2011: 692-862. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21403885
- Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008; 117: 2051-2060. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18413502
- Masuyama T, Yamamoto K, Sakata Y, Doi R, Nishikawa N, et al. Evolving changes in doppler mitral flow velocity pattern in rats with hypertensive hypertrophy. J Am Coll Cardiol. 2000; 36: 2333-2338. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11127481
- Pernot M, Couade M, Mateo P, Crozatier B, Fischmeister R, et al. Real-time assessment of myocardial contractility using shear wave imaging. J Am Coll Cardiol. 2011; 58: 65-72. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21700091
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009; 22: 107-133. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27037982
- Soyama Y, Mano T, Goda A, Sugahara M, Masai K, et al. Prognostic value of diastolic wall strain in patients with chronic heart failure with reduced ejection fraction. Heart Vessels. 2017; 32: 68-75. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27115147
- Minamisawa M, Miura T, Motoki H, Ueki Y, Shimizu K, et al. Prognostic impact of diastolic wall strain in patients at risk for heart failure. Int Heart J. 2017; 58: 250-256. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28320997
- Kang MK, Ju S, Mun HS, Choi S, Cho JR, et al. Decreased diastolic wall strain is associated with adverse left ventricular remodeling even in patients with normal left ventricular diastolic function. J Echocardiogr. 2015; 13: 35-42. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25750578
- Song P, Bi X, Mellema DC, Manduca A, Urban MW, et al. Quantitative assessment of left ventricular diastolic stiffness using cardiac shear wave elastography: A pilot study. J Ultrasound Med. 2016; 35: 1419-1427. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27208201
- Sakata Y, Ohtani T, Takeda Y, Yamamoto K, Mano T. Left ventricular stiffening as therapeutic target for heart failure with preserved ejection fraction. Circ J. 2013; 77: 886-892. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23486165
- Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004; 350: 1953-1959. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15128895
- Grossman W. Evaluation of the systolic and diastolic function of the ventricles and the myocardium. in: Grossman W (ed): Grossman's cardiac catheterization, angiography, and intervention, Volume 1. 7th ed lippincott williams & wilkins. 2006: 315-331.
- Zafrir B, Paz H, Wolff R, Salman N, Merhavi D, et al. Mortality rates and modes of death in heart failure patients with reduced versus preserved systolic function. Eur J Intern Med. 2011; 22: 53-56. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21238894
- Klein AL, Burstow DJ, Tajik AJ, Zachariah PK, Bailey KR, et al. Effects of age on left ventricular dimensions and filling dynamics in 117 normal persons. Mayo Clin Proc. 1994; 69: 212-224. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8133658
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2016; 29: 277-314. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27037982
- Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: The cardiovascular health study. J Am Coll Cardiol. 2001; 37:1042-1048. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11263606
- Redfield MM, Jacobsen SJ, Burnett JC Jr., Mahoney DW, Bailey KR, et al. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003; 289: 194-202. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12517230
- Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011; 124: 24-30. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21670229
- Tschope C, Paulus WJ. Is echocardiographic evaluation of diastolic function useful in determining clinical care? Doppler echocardiography yields dubious estimates of left ventricular diastolic pressures. Circulation. 2009; 120: 810-820. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19720947
- Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: New insights from a combined hemodynamic and doppler echocardiographic study. J Am Coll Cardiol. 1988; 12: 426-440. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3392336
- Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009; 119: 62-70. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19075104
- Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by doppler echocardiography in patients with hypertrophic cardiomyopathy: Correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007; 116: 2702-2708. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18025528
- Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, et al. The cardiovascular disease continuum validated: Clinical evidence of improved patient outcomes: Part i: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006; 114: 2850-2870. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17179034
- Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002; 27: 92-98. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12025467
- van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, et al. Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008; 117: 43-51. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18071071
- Krum H, Elsik M, Schneider HG, Ptaszynska A, Black M, et al. Relation of peripheral collagen markers to death and hospitalization in patients with heart failure and preserved ejection fraction: Results of the I-PRESERVE collagen sub-study. Circ Heart Fail. 2011; 4: 561-568. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21750125
- Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, et al. Mode of death in patients with heart failure and a preserved ejection fraction: Results from the irbesartan in heart failure with preserved ejection fraction study (i-preserve) trial. Circulation. 2010; 121: 1393-1405. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20231531
- Zizek B, Poredos P. Increased left ventricular mass and diastolic dysfunction are associated with endothelial dysfunction in normotensive offspring of subjects with essential hypertension. Blood Press. 2007; 16: 36-44. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17453750
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. J. Heart disease and stroke statistics--2011 update: A report from the American heart association. Circulation. 2011; 123: e18-e209
- Hildebrandt P. Systolic and non-systolic heart failure: Equally serious threats. JAMA. 2006; 296: 2259-2260. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21160056
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. Eur Heart J. 2012; 33: 1787-1847. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22611136
- Fitzgibbons TP, Meyer TE, Aurigemma GP. Mortality in diastolic heart failure: An update. Cardiol Rev. 2009; 17: 51-55. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19367145
- Caudron J, Fares J, Bauer F, Dacher JN. Evaluation of left ventricular diastolic function with cardiac MR imaging. Radiographics. 2011; 31: 239-259. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21257944
- Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005; 111: 774-781. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15699264
- Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation. 2015; 131: 1247-1259. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25637629