Research Article
Removal of Chromium from Aqueous Solution by Thermally Treated Mgal Layered Double Hydroxide

Enkhtur Otgonjargal1, Byambasuren Nyamsuren1, Enkhtuul Surenjav1, Gunchin Burmaa1, Jadambaa Temuujin2, and Dashkhuu Khasbaatar1,3*
1Laboratory of Inorganic Chemistry, Institute of Chemistry and Chemical Technology of Mongolian Academy of Science, Peace Avenue, Ulaanbaatar 13330, Mongolia
2Laboratory of Materials Science and Technology, Institute of Chemistry and Chemical Technology of Mongolian Academy of Science, Peace Avenue, Ulaanbaatar 13330, Mongolia
3Department of Chemical and Biological Engineering, School of Engineering and Applied Sciences, National University of Mongolia, P.O.Box 46A/257, Ulaanbaatar, Mongolia
*Address for Correspondence: Dashkhuu Khasbaatar, Laboratory of Inorganic Chemistry, Institute of Chemistry and Chemical Technology of Mongolian Academy of Science, Peace Avenue, Ulaanbaatar 13330, Mongolia, Tel.: +976-75754400-1198; E-mail: d_khasbaatar@seas.num.edu.mn
Dates: Submitted: 21 December 2016; Approved: 09 January 2017; Published: 11 January 2017
How to cite this article: Otgonjargal E, Nyamsuren B, Surenjav E, Burmaa G, Temuujin J, et al. Removal of Chromium from Aqueous Solution by Thermally Treated Mgal Layered Double Hydroxide. Ann Civil Environ Eng. 2017; 1: 001-008. DOI: 10.29328/journal.acee.1001001
Copyright License: © 2017 Otgonjargal E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Layered double hydroxide; Thermal treatment; Adsorption; Chromium
ABSTRACT
MgAl based layered double hydroxide (MgAl-LDH) was used as adsorbent for the removal of chromium oxyanion from an aqueous solution. MgAl-LDH was synthesized successfully using co-precipitation method, and was characterized by X-Ray Diffractometer (XRD), Scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX). MgAl-LDH was thermally treated for improving the chromium adsorption. Samples were treated at 220°C and 450°C. A negligible difference of total chromium adsorption capacities was observed between MgAl-LDH000 and MgAl-LDH220 as 12.56 mg/g and 11.01 mg/g. The maximum chromium adsorption capacity of MgAl-LDH was 88.07 mg/g at 500g/l chromium concentration for MgAl-LDH which has been thermally treated at 450°C (MgAl-LDH450). The results indicated that memory effects of thermally treated MgAl-LDH at certain temperatures were retained and enhanced chromium removal efficiency.
INTRODUCTION
One of the most toxic heavy metals contaminants of concern is the hexavalent chromium (Cr) in drinking water due to natural and anthropogenic activity. Chromium contamination is present in the effluents produced during the electroplating, leather tanning, cement, mining, dyeing and photography industries [1,2].
According to the World Health Organization (WHO) guidelines, the maximum concentration limitation for Cr is 0.05 mg/L in drinking water [3]. Cr (VI) is highly mobile and is considered acutely as toxic and mutagenic for most organisms; in humans its main effects are on skin, liver, kidney and respiratory organs, resulting in a variety of diseases such as dermatitis, hepatic and renal tubular necrosis, bronchitis and bronchogenic carcinoma [4]. Moreover, hexavalent chromium is more toxic than trivalent chromium. This is due to easy reduction, liability, movability and entry into the cell via surface uptake pathway of hexavalent chromium. On the other hand, trivalent chromium is inert and undergoes precipitation in biological pH resulting nontoxicity of trivalent chromium [5,6].
Recently developed layered double hydroxides (LDHs), known as hydrotalcite-like compounds, are a class of synthetic two dimensional nanostructured anionic clays whose structure can be closely related to that of brucite, Mg(OH)2 [7]. Layered double hydroxide can be represented by the general formula [M2+1-xM3+x(OH)2]x+(Anˉ)x/nmH2O, where M2+ and M3+ are divalent and trivalent cations, respectively; x is the M2+/M3+ molar ratio and Anˉ is an anion. Because of unique physicochemical properties, rolling host of anionic species, and inexpensive, LDH can be used many applications such as sorbents, catalyst precursors, medicine stabilizers and ionic conductors. They have positively charged surface when it is in aqueous solution. Thus, negatively charged contaminants are easily adsorbed on them. Not only adsorption but also intercalation space can play dominant roll to trapping negatively charged anions [8]. Due to the exchangeability of the interlayer anions and the high charge density of the sheets, LDH has paid attention in several studies as sorbents to remove harmful anions such as arsenate [9], phosphate [10], and fluoride [11].
Thermal treatment as an efficient modification method is favorable to enhance surface defect of derivatives and beneficial for inner reaction. Calcinated LDHs (layered double oxide, defined as LDO) under a certain temperature can maintain “memory effect” in aqueous solutions [12].
In this study, MgAl layered double hydroxide (MgAl-LDH) was thermally treated at 220°C and 450°C. These two temperatures were main alteration of structure of MgAl-LDH. Thus, three types of LDH including uncalcined MgAl-LDH, calcined at 220°C and 450°C were selected to study on adsorption of chromium from aqueous solution. Therefore, we investigated what kind of change would be happened especially structure of the three sorbents after adsorption of chromium on the sorbents.
MATERIALS AND METHODS
Materials
Magnesium chloride hexahydrate (MgCl2·6H2O), Aluminum chloride hexahydrate (AlCl3·6H2O), sodium hydroxide (NaOH), sodium carbonite (Na2CO3) and sodium dichromate dihydrate (Na2Cr2O7·2H2O) with analytical grade were used for the synthesis of the LDH and were purchased from Sigma Aldrich Co., Ltd, and used without further purification. Deionized water was used throughout the synthesis and treatment processes.
Synthesis of the MgAl-LDH and Thermal treatment
MgAl-LDH adsorbent was synthesized using traditional co-precipitation method [13]. In a typical procedure, 24.1 g of MgCl2·6H2O (0.198 M) and 9.6 g of AlCl3·6H2O (0.0662 M) were dissolved in 600 cm3 distilled water (Solution 1). Solution 2 was prepared by dissolving 31.8 g of Na2CO3 (0.3 M) in 400 cm3 distilled water. Solution 1 and 2 was added into 1 L distilled water under constant stirring at a temperature of 25°C (at ambient temperature), then the pH of the mixed solution was kept at 10 by adding 1 M NaOH. The mixture was aged at 60°C for 4 h. Then the precipitate was separated by a filter paper and washed with distilled water. The wet solid was dried at 50°C for 24 h to obtain the MgAl-LDH. The thermal treatment of MgAl-LDH was carried out in an oven at 220°C and 450°C temperatures for 4 hours in air. 10 g of the MgAl-LDH was taken for thermal treatment. After thermal treatment, mass losses of the MgAl-LDH at 220°C and 450°C were 18% and 37.5%, respectively.
Adsorption Experiments
Stock solution of Cr (VI) was prepared by dissolving Na2Cr2O7·2H2O in deionized water. Adsorption kinetics for removing chromium was studied at the time range from 0.5 minutes to 1440 minutes with constant concentration of chromium at 100 mg/l for MgAl-LDH with no-thermal treatment (MgAl-LDH000) and MgAl-LDH with thermal treatment at 220°C (MgAl-LDH220). For MgAl-LDH with thermal treatment at 450°C (MgAl-LDH450) was used chromium concentration of 500 mg/l. All experiments were performed under constant stirring of 200 rpm. Adsorption isotherms were conducted at 25°C with the initial pH of 7.0, and the adsorbent dose was kept as 5 g/l. The pH of solution was adjusted to the desired values by adding a little amount of HCl, and NaOH solutions with 0.1 N concentration.
Measurements
X-ray diffraction patterns were recorded using CuK α radiation (n=1.5418 Ǻ) on a Philips PW1800 diffractometer operating at 40 kV and 40 mA with 0.25° divergence slit, 0.5° anti-scatter slit, between 1.5 and 20° (2θ) at a step size of 0.0167°. A scanning electron microscope (SEM, S-3400 N, Hitachi, Tokyo, Japan) equipped with energy dispersive X-ray (EDX) was used. pH values were measured with digital pH meter (pH300, Hanna Instruments, Italy). Thermogravimetric and differential thermal analyses (TGA and DTA) were carried out using a TG/DTA7300 (Hitachi Exstar) at the temperature range of 25-970°C with heating rate of 5°C/min in air flow (100 cm3/min). Chromium concentrations in the solution were determined by UV-Visible spectrophotometry method at 450 nm. BH-1200 muffle furnace was used to thermal treatment of the MgAl-LDH.
RESULTS AND DISCUSSION
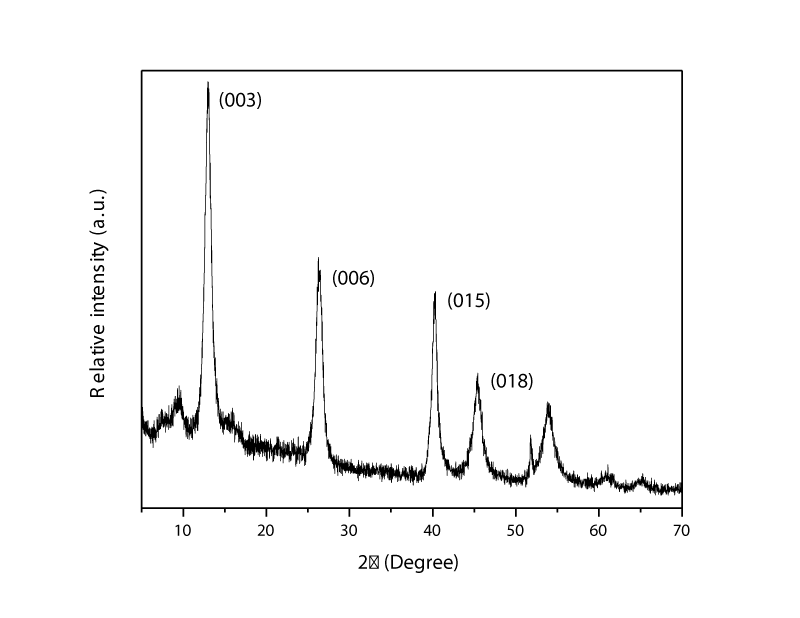
XRD of MgAl-LDH000: Synthesized MgAl-LDH was characterized with XRD which is shown in Figure 1. It shows three LDHs sharp and symmetric peaks at lower 2Ө as (003) and (006), which are characteristic of a lamellar material.
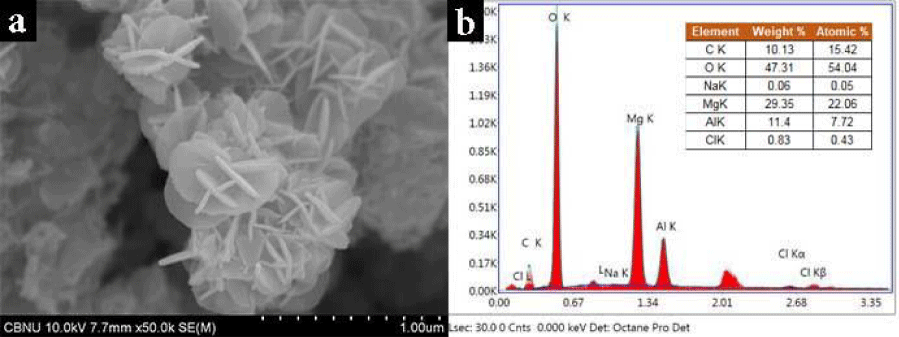
SEM image SEM-EDX analysis: The morphologies of MgAl-LDH000 were characterized by SEM EDX. As shown in Figure 2, a typical hydrotalcite-like structure was exhibited and the orderliness was clearly. The MgAl-LDH000 was observed as homogenously distributed nano particles with layered double structure. A distance between layered double structure was measured around 20-30 nm. The ratio of metals is an important parameter for characterizing MgAl-LDH000 compounds and the molar ratio of Mg to Al was 2.57 which is consistent with the initial ratio of Mg/Al.
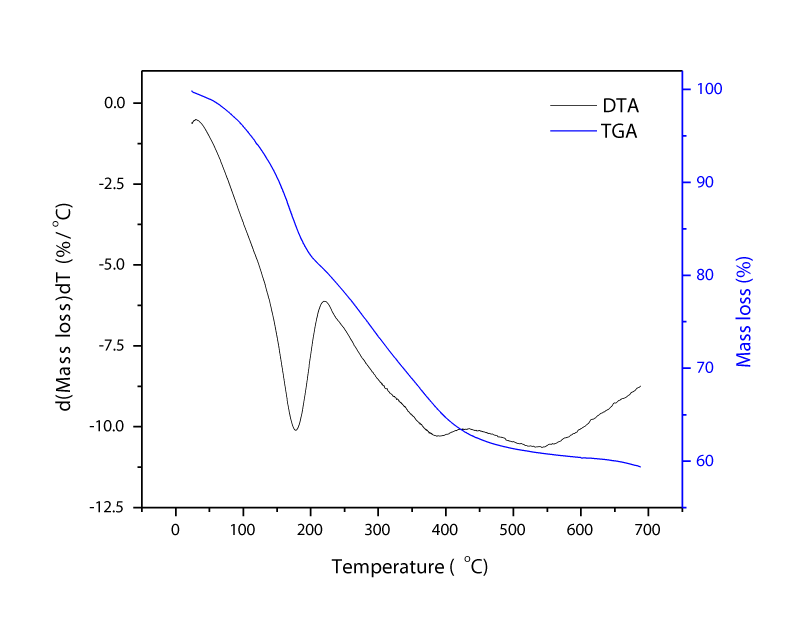
DTA and TGA analysis of MgAl-LDH000: Figure 3 shows TGA and DTA pattern of MgAl-LDH. On the DTA curve, the first endothermic peak occurred at 182°C and it ceased at 223°C. A sharp falling TGA curve up to 205°C represented loss of interlayer and adsorbed water. The second endothermic process was occurred at 393°C and it ended at 440°C. The slope of the TGA curve was decreased until 440°C after the endothermic process. The decomposition of interlayer hydroxyl and carbonate anions occurred at the range of 223-440°C [13,14]. Thermal treatment is one of the favorable methods to enhance surface defect of derivatives and inner reaction. Thermally treated MgAl-LDH under specific temperature can retain “memory effect” in aqueous solutions. Moreover, MgAl-LDH can be readily transferred to the conforming mixed oxide by heating to a certain temperatures which typically involve dehydration at 220°C, dihydroxylation, and decomposition of anions at 450°C:
1. Dehydration (100-220°C): [M21-xM3x(OH)2]x+(An-)x/n.mH2O→[M21-xM3x(OH)2]x+(An-)x/n
2. Dehydroxylation (350-450°C): [M21-xM3x(OH)2]x+(An-)x/n →[M21-xM3xO]x+(An-)x/n
3. Decomposition of anion (420-450°C): [M21-xM3xO]x+(An-)x/n→M21-xM3xO1+x/2(BOy)
Where BOy) denotes the species of decomposed anion [15,7]. According to the DTA and TGA data, physico-chemical characteristics of MgAl-LDH were changed at 220°C and 450°C temperatures. Therefore, MgAl-LDH was thermally treated at 220°C and 450°C.
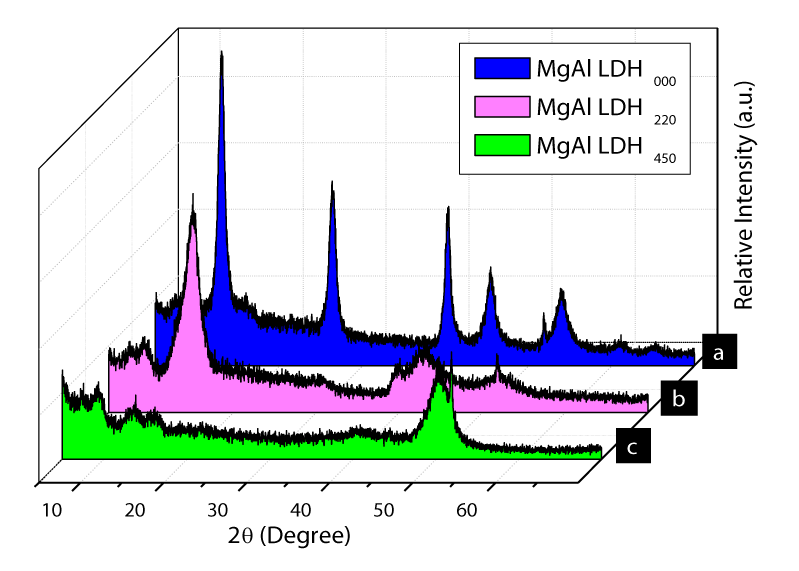
XRD of MgAl-LDH000, MgAl-LDH, and MgAl-LDH450: The Figure 4 shows XRD patterns of the synthesized and thermally treated LDHs. The Figure 4a is the typical pattern of hydrotalcite-like pattern. In the case of MglAl-LDH220, considerable changes were observed for MglAl-LDH220 and MglAl-LDH450. An intensive and sharp reflection of the (003) planes at 12.9° 2θ value for MgAl-LDH000 shifted to 15.3° for MglAl-LDH220. On the other hand, a broad asymmetric reflection at higher 2θ value of MgAl-LDH000 was disappeared for MgAl-LDH220 due to heating up to 220°C and removal of interlayer hydrides. For MglAl-LDH450, the well-defined diffraction peaks of the MgAl-LDH000 were replaced by broad peaks, therefore representing a poor long-range ordered phase. A nanocrystalline material with very tiny nanoparticles or even an amorphous phase was corresponded to these broad peaks [12].
Adsorption study of Cr (VI) on the MgAl-LDH000, MgAl-LDH220, and MgAl-LDH450
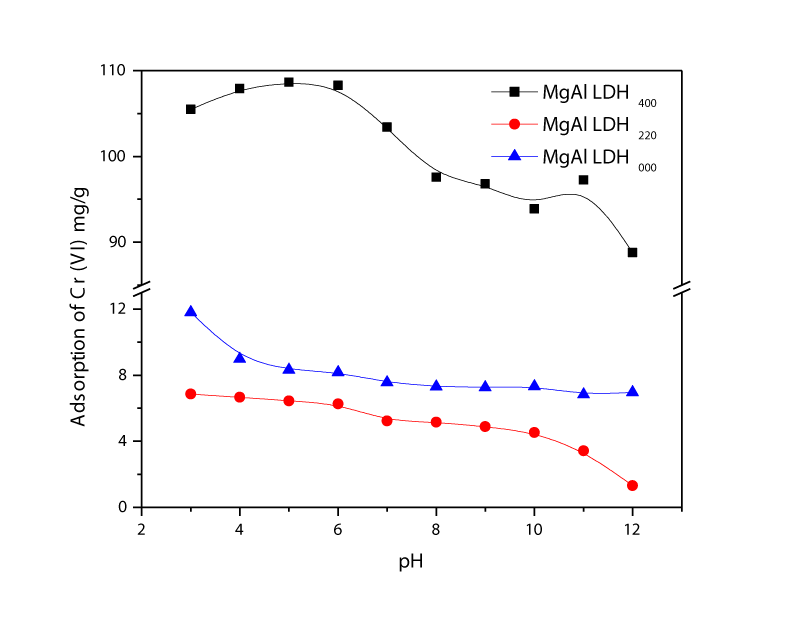
Influence of pH on Chromium uptake: Figure 5 shows the quantitative analysis of chromium adsorbed on the MgAl-LDH000, MgAl-LDH220, and MgAl-LDH450 at increasing pH levels. The difference in the curves may be explained by the inherent chemical and physical properties of adsorbents. As the pH decreased, the adsorbent showed a more pronounced reactivity with chromium. In an acidic medium, the adsorbent was highly protonated and this characteristic increased the attracting effect of oxyanions due to the lack of negative charges. Chromium adsorption was found to be significantly influenced by the solution pH. One gram of MgAl-LDH450 at pH 5.0 was adsorbed 108.7 mg of chromium in one L aqueous solution which represents the maximum adsorption. However, chromium adsorption of MgAl-LDH220 was less than that of MgAl-LDH000. It could be explained that the shrinkage of gaps between sheets of MgAl oxide planes. The maximum adsorptions of MgAl-LDH000 and MgAl-LDH220 at pH3 were 11.79 mg/g and 6.85 mg/g, respectively. Adsorption capacity MgAl-LDH450 was around 9 times higher than the value of MgAl-LDH000. It was explained by the main anion exchange mechanism involved in the sorption of chromium on the adsorbent. MgAl-LDH can remove anions from solution by three different mechanisms such as adsorption on external surface, intercalation by anion exchange and intercalation by reconstruction of calcined material [16]. It meant to be that the crystal structure of MgAl-LDH450 was destructed to amorphous nano type material at 450°C and MgAl double oxide may be obtained. Reconstruction and recrystallization of MgAl-LDH450 was taken place due to the memory effect of LDH [7] when chromium adsorption was happened.
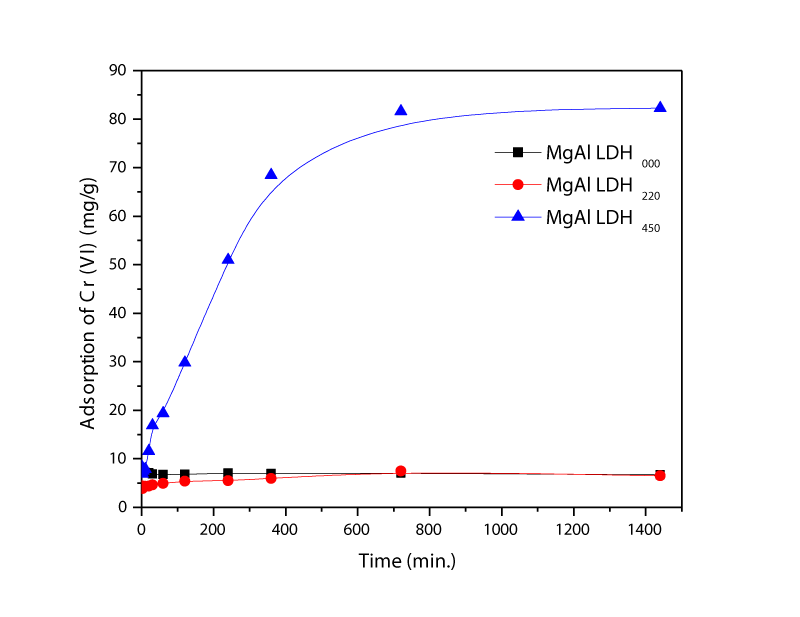
Figure 6 shows the adsorption kinetics of Chromium for an initial solution pH of 7.0 measured with the three adsorbents. It reveals that the adsorption rate sharply increased up to 360 minutes and then almost stabilized up to 700 minutes. Further, the equilibrium was occurred for MgAl-LDH450. Adsorption rate for MgAl-LDH220 and MgAl-LDH000 increased in few seconds and reached to equilibrium. However, adsorption of the latter adsorbents was very low compared with MgAl-LDH450.
Figure 6: Effect of the MgAl-LDH000, MgAl-LDH220, and MgAl-LDH450 on the adsorption kinetics of Cr (VI).
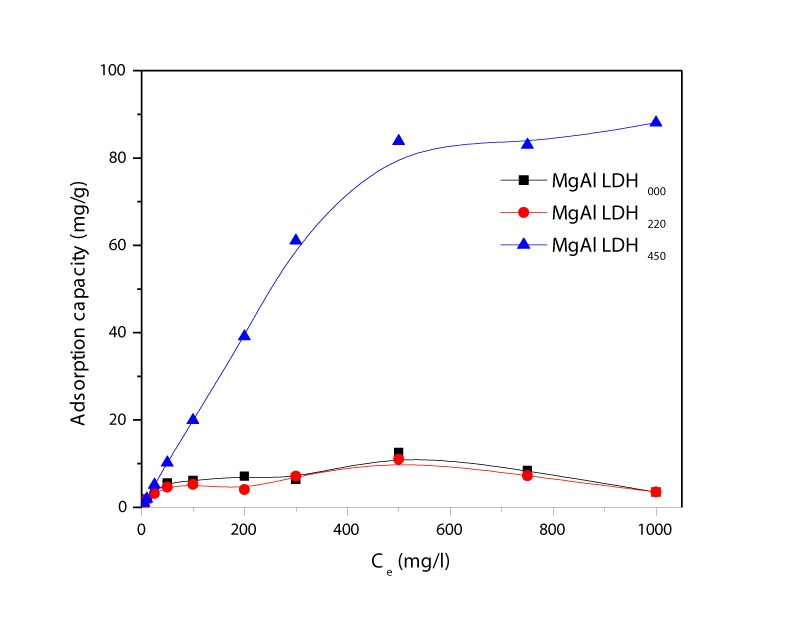
Figure 7 shows adsorption capacities of three adsorbents when the concentration of chromium increases at pH 7. There was no clear difference observed between MgAl-LDH000, and MgAl-LDH220. The adsorption of chromium on both adsorbents were increased to 11 mg/g when chromium concentration in the solution reaches 500 mg/l. After increasing chromium concentration over 500 mg/l, adsorption was decreased possibly due to desorption process that might be happened simultaneously. In the case of MgAl-LDH450, adsorption capacity was dramatically increased until the chromium concentration in the solution reached to 500 mg/l. Thus, the equilibrium was taken place even the concentration increased to 1000 mg/l. The total capacities of MgAl-LDH000, MgAl-LDH220, and MgAl-LDH450 are 12.56 mg/g, 11.01 mg/g, and 88.07 mg/g, respectively.
Figure 7: Adsorption capacities of MgAl-LDH000, MgAl-LDH000, and MgAl-LDH450 depending on chromium concentration in solution.
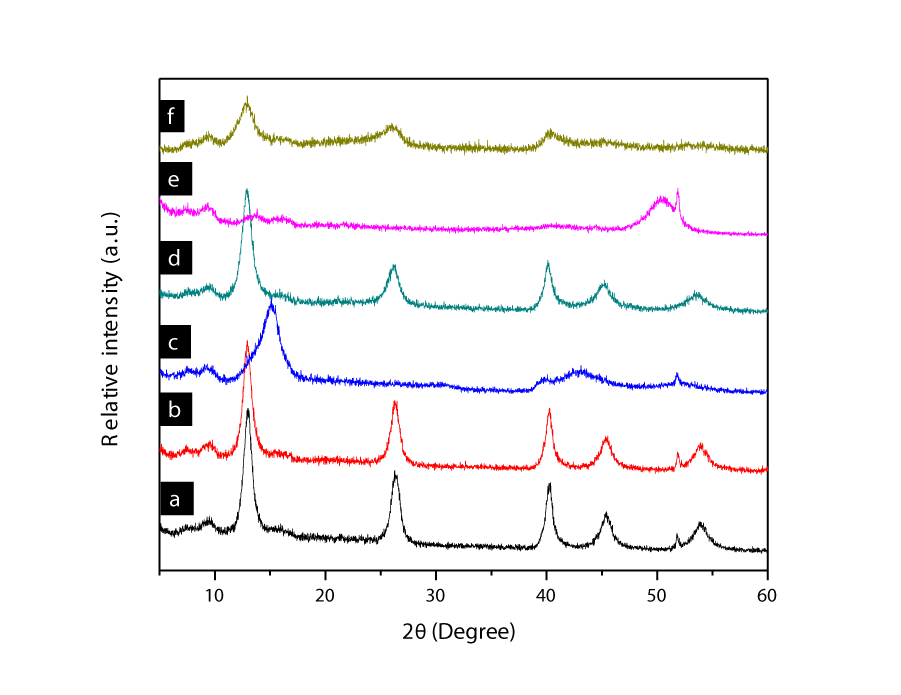
The certain changes in structures of the three adsorbents were occurred after chromium adsorption. Figure 8 represents XRD patterns of the three adsorbents and chromium adsorbed on. There is almost no difference between Figure 8(a) and Figure 8(b). A broad sharp peak of MgAl-LDH220 on 15.22o was shifted to 12.94° and this peak was exactly overlapped on (003) reflection plane of MgAl-LDH. Thus, all the XRD peaks of MgAl-LDH220 with Cr were fitted to the peaks of MgAl-LDH000. It revealed that the thermally treated MgAl-LDH at 220°C was destructed and dehydrated of its structure at some certain degree. The memory effect of MgAl-LDH was strong to reconstruct the basic structure when it was dipped in solution and no matter how much oxyanion in the solution. In the case of MgAl-LDH450 with Cr, memory effect was affected to create the original structure of MgAl-LDH. Carbonates and hydroxyl groups of MgAl-LDH450 were lost in main structure. Therefore, reconstruction of MgAl-LDH450 was not that good, although, some peaks arisen up on XRD pattern of the MgAl-LDH450 with Cr at degrees of 13.04°, 26.1°, and 40.46°, which represented rearranged crystals of MgAl-LDH.
Figure 8: XRD patterns of (a) MgAl-LDH000, (b) MgAl-LDH000 with Cr, (c) MgAl-LDH220, (d) MgAl-LDH220 with Cr, (e) MgAl-LDH450, (f) MgAl-LDH450 with Cr.
The present research indicates that the thermal treatment of MgAl-LDH at certain temperatures can be improved the chromium adsorption from the aqueous solution up to 8 times.
CONCLUSION
MgAl-LDH was synthesized by simple co-precipitation method. The synthesis of hydrocalcite like compound was confirmed by XRD. The morphology of the MgAl-LDH was identified with SEM-EDX and represents homogenously distributed nano particles. The thickness of synthesized MgAl-LDH was around 20-30 nm. Thermal treatment of MgAl-LDH was performed at 220°C, and 450°C and it represents partially decomposed structure. The adsorption capacities of MgAl-LDH000, MgAl-LDH220, and MgAl-LDH450 were 12.56 mg/g, 11.01 mg/g, and 88.07 mg/g at pH 7, respectively. Memory effect was still in MgAl-LDH450 sample, although, MgAl-LDH was deeply destructed to amorphous nano material such as MgAl double oxide.
REFERENCES
- Sarkar M, Rahman AKML, Bhoumik NC. Remediation of chromium and copper on water hyacinth (E. crassipes) shoot powder. Water Resources and Industry. 2017; 17: 1-6. Ref.: https://goo.gl/tD3l3n
- Rathnayake SI, Martens WN, Xi Y, Frost RL, Ayoko GA. Remediation of Cr (VI) by inorganic-organic clay. J Colloid Interface Sci. 2017; 490: 163-173. Ref.: https://goo.gl/J4czGz
- WHO. Guidelines for Drinking-water Quality. In. WHO Press Switzerland 2006.
- Goyer MAM. Advances in Modern Toxicology: Toxicology of Trace Elements. John Wiley & Sons. New York. 2: 1977.
- Saha R, Nandi R, Saha B. Sources and toxicity of hexavalent chromium. Journal of Coordination Chemistry 2011; 64: 1782-1806. Ref.: https://goo.gl/TXVVdm
- Saha B, Orvig C. Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coordination Chemistry Reviews. 2010; 254: 2959-2972. Ref.: https://goo.gl/RBCx4n
- Zou Y, Wang X, Wu F, Yu S, Hu Y, et al. Controllable Synthesis of Ca-Mg-Al Layered Double Hydroxides and Calcined Layered Double Oxides for the Efficient Removal of U(VI) from Wastewater Solutions. ACS Sustainable Chemistry & Engineering. 2016; 5: 1173-1185. Ref.: https://goo.gl/WSj7uz
- Khitous M, Salem Z, Halliche D. Effect of interlayer anions on chromium removal using Mg-Al layered double hydroxides: Kinetic, equilibrium and thermodynamic studies. Chinese Journal of Chemical Engineering. 2016; 24: 433-445. Ref.: https://goo.gl/qM21tA
- Wang S, Gao B, Li Y, Zimmerman AR, Cao X. Sorption of arsenic onto Ni/Fe layered double hydroxide (LDH)-biochar composites. RSC Advances. 2016; 6: 17792-17799. Ref.: https://goo.gl/sQEroZ
- Everaert M, Warrinnier R, Baken S, Gustafsson JP, Vos DD, et al. Phosphate-Exchanged Mg-Al Layered Double Hydroxides: A New Slow Release Phosphate Fertilizer. ACS Sustainable Chemistry & Engineering. 2016; 4: 4280-4287. Ref.: https://goo.gl/Ba2NZ3
- Zhang T, Li Q, Xiao H, Lu H, Zhou Y. Synthesis of Li-Al Layered Double Hydroxides (LDHs) for Efficient Fluoride Removal. Industrial & Engineering Chemistry Research. 2012; 51: 11490-11498. Ref.: https://goo.gl/GoWiHm
- Ferreiraa OP, Alvesa OL, Gouveiab DX, Souza Filhob AG, de Paiva JAC, et al. Thermal decomposition and structural reconstruction effect on Mg-Fe-based hydrotalcite compounds. Journal of Solid State Chemistry. 2004; 177: 3058-3069. Ref.: https://goo.gl/nYy526
- Klemkaite K, Prosycevas I, Taraskevicius R, Khinsky A, Kareiva A. Synthesis and characterization of layered double hydroxides with different cations (Mg, Co, Ni, Al), decomposition and reformation of mixed metal oxides to layered structures. Central European Journal of Chemistry. 2011; 9: 275-282. Ref.: https://goo.gl/1tt917
- Zhou J, Su Y, Zhang J, Xu X, Zhao J, et al. Distribution of OH bond to metal-oxide in Mg3−xCaxFe-layered double hydroxide (x=0-1.5): Its role in adsorption of selenate and chromate. Chemical Engineering Journal. 2015; 262: 383-389. Ref.: https://goo.gl/5b8euu
- Xu ZP, Zhang J, Adebajoa MO, Zhangc H, Zhou C. Catalytic applications of layered double hydroxides and derivatives. Applied Clay Science. 2011; 53: 139-150. Ref.: https://goo.gl/7N5j4l
- Koilraj P, Srinivasan K. High Sorptive Removal of Borate from Aqueous Solution Using Calcined ZnAl Layered Double Hydroxides. Industrial & Engineering Chemistry Research. 2011; 50: 6943-6951. Ref.: https://goo.gl/ZpTjfR