Invasive and Non-invasive Ductal Carcinoma within Malignant Phyllodes Tumour with Axillary Lymph Node Metastases
By Osman Erdogan1, Alper Parlakgumus1, Zeynel Abidin Tas2, Kemal Yener3, Umit Turan3Affiliations
doi: 10.29271/jcpsp.2022.08.S92ABSTRACT
Phyllodes tumours are uncommon breast neoplasms constituting 1-2% of breast malignancies. Metastasis is usually haematogenous, and axillary lymph node dissection is not routinely performed. A phyllodes tumour with concomitant invasive ductal carcinoma (IDC) is even rarer. When IDCor ductal carcinoma in-situ (DCIS) is detected, the management of the condition changes completely. We report a case of a 22-year female presenting with a mass in the right breast and palpable axillary lymph nodes. The pathological examination demonstrated a malignant phyllodes tumour with concomitant IDC and DCIS. The patient elected to have modified radical mastectomy, and the pathological examination showed metastasis in the axillary lymph nodes. The patient was administered appropriate therapy. At the last visit, she did not have the clinical signs of disease. This is the first youngest case of axillary lymph node metastases with both DCIS and IDC on pathological examination in malignant phyllodes tumour.
Key Words: Malignant phyllodes, Invasive ductal carcinoma, Ductal carcinoma in-situ, Lymph node metastasis.
INTRODUCTION
Phyllodes tumours are rarely encountered biphasic tumours and are responsible for 2.5% of all fibroepithelial breast lesions with an epithelial component arranged in clefts surrounded by a hypercellular mesenchymal component. They are akin to fibroadenomas clinically and radiologically but have different histological features. Phyllodes tumours typically have abundant stroma with varying cellularity and mitotic activity. Although they can appear at any age, they are more common in middle-aged women and usually occur in an older age group than fibroadenomas.1 Based on tumour margins and the degree of mitosis, stromal cell differentiation, and stromal overgrowth, they are classified into three types; i.e. malignant, benign or borderline.2
The majority of phyllodes tumours are benign and about 10% are malignant. These tumours greatly vary in size, and although larger tumours are more likely to be malignant, the evaluation of their biological behaviour is based on their histological features. Local recurrence rates of about 15% have been reported and attributed to the extent of excision.3 In phyllodes tumours, malignant transformation generally occurs in the stroma and very rarely in the epithelium. Malignant phyllodes tumours comprise approximately 1‑2% of all the breast tumors.3 Wide resection is considered the primary treatment for phyllodes tumours.4
We described a case of an invasive ductal carcinoma (IDC) with an in-situ component occurring within a malignant phyllodes tumour in a 22-year female. To our knowledge, this is the youngest patient with this type of composite tumour. This type of combination of tumours with lymph node metastases is also the first case in the medical literature.
CASE REPORT
A 22-year female presented to our institution with a hard and well-defined tumour measuring 6-7 cm in size in the right breast. On physical examination, there were enlarged axillary lymph nodes, in which the tumour did not invade the skin and pectoral muscles. Mammography and ultrasonography showed a well-defined tumour in the breast measuring 6×5 cm in dimensions with a regular border and low echogenicity. The patient and members of her family did not have a history of malignancy. Core biopsy showed a biphasic neoplasm with an epithelial component and a stromal element with a provisional diagnosis of phyllodes tumour. Surgical excision was planned for the patient. The pathological examination of the specimen from segmental resection revealed a malignant phyllodes tumour measuring 9×8 cm in size with IDC and high-grade ductal carcinoma in-situ (DCIS) (Figure 1a-c). There was positivity in some parts of the surgical margins. Therefore, a modified radical mastectomy was performed subsequently. The permanent section revealed a 3×2 cm IDC accompanied by high-grade DCIS and metastases in 4 out of 19 lymph nodes (Figure 1d). The immune his to chemical examination demonstrated oestrogen receptor (ER)-positive, progesterone receptor (PR)-positive, and HER-2-neu-positive IDC. The patient received chemotherapy (Adriamycin and cyclophosphamide plus paclitaxel and Herceptin), and local irradiation, followed by tamoxifen for hormone therapy. During her 6-year postoperative follow-up, there was no metastasis or local recurrence.
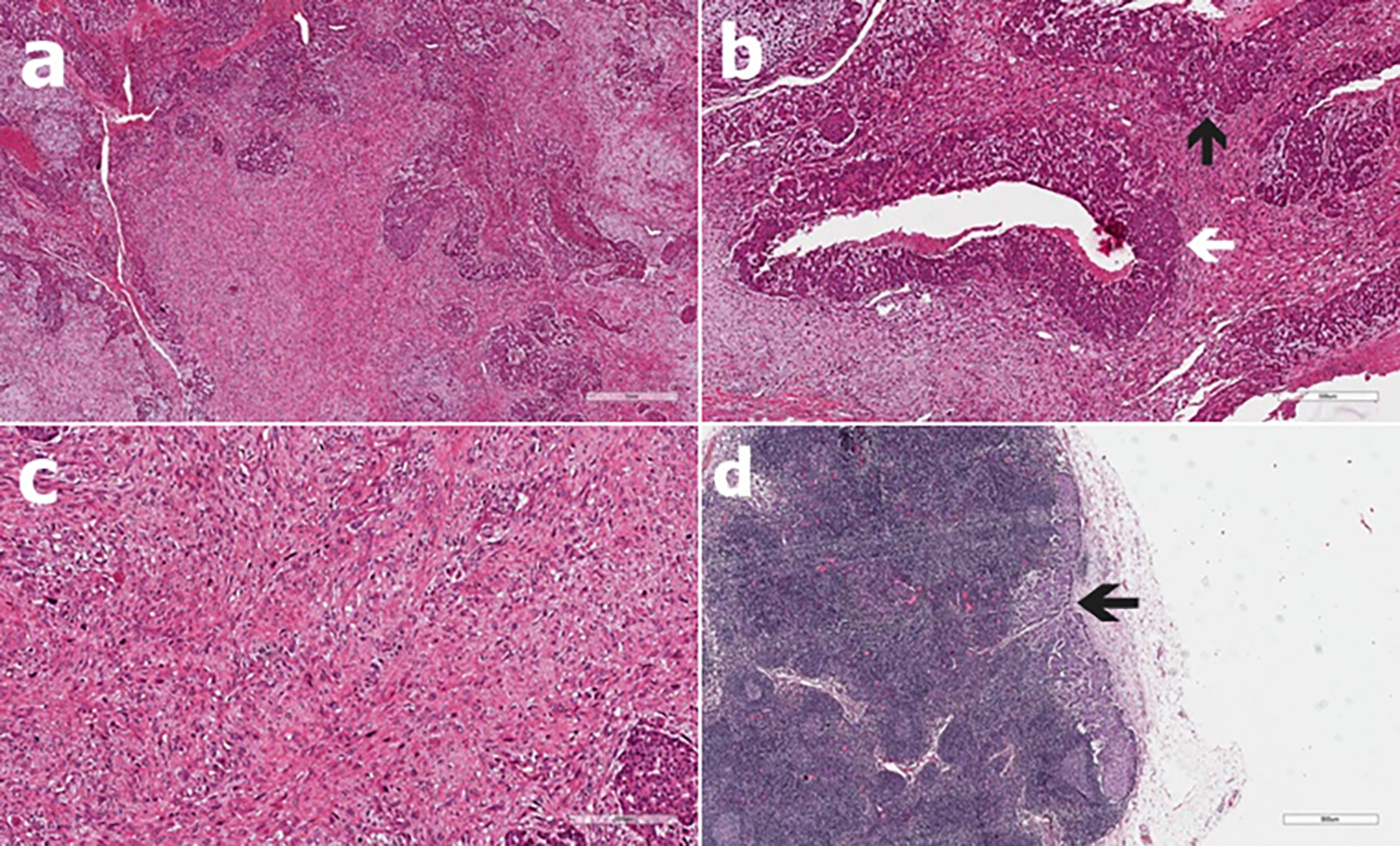 Figure 1: Microscopic photographs of the tumour. (a) Phyllodes tumour with highly cellular stroma and long, slit-like spaces lined by bland epithelia (H&E, ×40). (b) Intraductal (left white arrow) and invasive (up black arrow) carcinoma within the phyllodes tumour (H&E, ×200). (c) Appearance of phyllodes tumour with severe nuclear atypia and brisk mitosis (H&E, ×400). (d) Carcinomatous lymph node metastases with tumor cells (H&E, ×40).
Figure 1: Microscopic photographs of the tumour. (a) Phyllodes tumour with highly cellular stroma and long, slit-like spaces lined by bland epithelia (H&E, ×40). (b) Intraductal (left white arrow) and invasive (up black arrow) carcinoma within the phyllodes tumour (H&E, ×200). (c) Appearance of phyllodes tumour with severe nuclear atypia and brisk mitosis (H&E, ×400). (d) Carcinomatous lymph node metastases with tumor cells (H&E, ×40).
DISCUSSION
Phyllodes tumours are rare breast neoplasms accounting for 1-2 % of benign and malignant breast neoplasms.1,3 They are well-defined, biphasic tumours with two apparent components; i.e. epithelial and stromal. The epithelial component of phyllodes tumours can display various changes, including hyperplasia, epithelial proliferation, or metaplasia. Malignant change is rarely seen in the epithelial component.5 The reported subtypes include non-invasive and invasive ductal and lobular carcinomas, tubular carcinoma, and squamous carcinoma.6
Phyllodes tumours accompanied by foci of carcinoma are not considered a metaplastic carcinoma and they are reported as separate entities.7 Malignant phyllodes tumours with intraductal/invasive carcinoma are different from a metaplastic carcinoma in terms of their behaviour, treatment, and prognosis. Metaplastic carcinoma is a highly aggressive carcinoma because of its sarcomatoid features and frequent metastasis. However, malignant phyllodes have a high rate of recurrence, and metastasis, which is less common.8 Metaplastic carcinomas are almost always negative for hormone receptors.9 Foci of intraductal/invasive carcinoma in malignant phyllodes show variable ER/PR expression, based on their nuclear grades.10 In our case, malignant phyllodes had an epithelial component positive for ER and PR.
Malignant transformation in the epithelial part of phyllodes tumours has been described in approximately 33 cases in a review published in 2016.6 The age of the patients with phyllodes tumours accompanied by carcinoma was reported to range between 26 to 80 years with the most patients older than 50 years.6 The present case is the youngest one reported to have this combination of tumours on presentation. Moreover, to our knowledge, the case presented here is the first youngest one to have axillary lymph node metastases with both DCIS and IDC on pathological examination.
The surgical approach to phyllodes tumours is usually a wide resection with 1-2 cm margins. Adequate margins prevent local recurrences during the excision of phyllodes tumours.6,11 Distant metastases develop in 5%-10% of patients with malignant phyllodes tumours. These tumours usually do not metastasize to axillary lymph nodes. When a phyllodes tumour is accompanied by a carcinoma, lymph node sampling could be performed depending on the features of carcinoma and the type of planned surgery.12
In the literature, there are only two case reports describing the lymph node metastasis of the phyllodes tumour with concomitant IDC and DCIS. The first case, reported by Parfitt et al.,12 was a 26-year woman with a left breast mass found to be a benign phyllodes tumour with nonspecific breast carcinoma and foci of invasive ductal carcinoma in-situ. She underwent axillary lymph node dissection due to palpable axillary nodes, and four metastatic lymph nodes were detected. After the patient received chemoradiotherapy followed by tamoxifen treatment, she had a three-year disease-free survival. The other case, presented by Korula et al. was a 51-year woman with a fixed mass of the left breast and multiple palpable hard nodes in the axilla.9 After trucut biopsy, a modified radical mastectomy was performed. The pathological examination of the obtained specimen revealed DCIS foci within a malignant phyllodes tumour. The invasive component was not included in the sections obtained for pathological examination. Metastatic carcinoma was detected in two lymph nodes. The patient received chemotherapy, radiation, and tamoxifen treatment. During a one-year postoperative follow-up, she did not have any clinical disease.
We elected to perform modified radical mastectomy due to the presence of IDC and clinically palpable nodes. The patient received four sessions of chemotherapy and radiotherapy, followed by tamoxifen. She seemed to have lymph node metastasis from the IDC component within a malignant phyllodes tumour. Lymph node harvesting is not performed to manage phyllodes tumours due to the rarity of axillary metastasis.13 The treatment of the carcinoma component is as important as the malignant phyllodes tumour management.13 The case presented here supports this view as axillary lymph node status provided guidance in predicting prognosis and deciding treatment.
Axillary lymph node metastasis of invasive carcinoma within a malignant phyllodes tumour is a very rare condition. Phyllodes tumours and invasive carcinoma should be treated individually. This case emphasizes the significance of evaluating phyllodes tumours for simultaneous carcinomatous management of the axilla and subsequent treatment options in such rare tumours.
PATIENT’S CONSENT:
Consent for publication was obtained from the patient whose data is included in this manuscript.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
OE: Critical revision, drafting and writing the manuscript.
AP: Approved the final version to be submitted.
ZAT: Pathological interpretation, design, and literature research.
KY: Design and literature research.
UT: Interpretation and critical revision for important intellectual content.
REFERENCES
- Moinfar F. Biphasic tumours. In: Moinfar F, editor. Essentials of diagnostic breast pathology: A practical approach. New York, NY: Springer; 2007; p.320-50.
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH and van de Vijver MJ (eds). WHO Classification of Tumours of the Breast. ed. 4th, Lyon; IARC Press; 2012.
- Lee AHS. Recent developments in the histological diagnosis of spindle cell carcinoma, fibromatosis and phyllodes tumour of the breast. Histopathol 2008; 52(1):45-57. doi: 10.1111/j.1365-2559.2007.02893.x.
- Schwickerath J, Blessing MH and Wolff F. A rare clinical manifestation of a combination tumour of cystosarcoma phyllodes malignum and an intraductal cancer. Geburtshilfe Frauenheilkd 1992; 52(9):557-9. German. doi: 10.1055/s- 2007-1023181.
- Nio Y, Iguchi C, Tsuboi K, Naruyama R. Ductal carcinoma in situ arising within a benign phyllodes tumour: A case report with review of the literature. Oncol Lett 2011; 2(2):223-8. doi: 10.3892/ol.2010.226.
- Wu DI, Zhang H, Guo L, Yan XU, Fan Z. Invasive ductal carcinoma within borderline phyllodes tumour with lymph node metastases. A case report and review of the literature. Oncol Lett 2016; 11(4):2502-6. doi: 10.3892/ ol.2016.4238.
- Korula A, Varghese J, Thomas M, Vyas F, Korula A. Malignant phyllodes tumour with intraductal and invasive carcinoma and lymph node metastasis. Singapore Med J 2008; 49(11):e318-21.
- Lenhard MS, Kahlert S, Himsl I, Ditsch N, Untch M, Bauerfeind I. Phyllodes tumour of the breast: Clinical follow-up of 33 cases of this rare disease. Eur J Obstet Gynecol Reprod Biol 2008; 138(2):217-21. doi: 10.1016/j. ejogrb.2007.08.002.
- Beatty JD, Atwood M, Tickman R, Reiner M. Metaplastic breast cancer: Clinical significance. Am J Surg 2006; 191(5): 657-64. doi: 10.1016/j.amjsurg.2006.01.038.
- Tang P, Wang X, Schiffhauer L, Wang J, Bourne P, Yang Q, et al. Expression patterns of ER - alpha, PR, HER-2/neu, and EGFR in different cell origin subtypes of high grade and non -high grade ductal carcinoma in situ. Ann Clin Lab Sci 2006; 36(2):137-43.
- Macher-Goeppinger S, Marme F, Goeppert B, Penzel R, Schirmacher P, Sinn HP, et al. Invasive ductal breast cancer within a malignant phyllodes tumour: Case report and assessment of clonality. Hum Pathol 2010; 41(2): 293-6. doi: 10.1016/j.humpath.2009.08.006.
- Parfitt J, Armstrong C, O’Malley F, Ross J, Tuck A. In-situ and invasive carcinoma within a phyllodes tumour associated with lymph node metastases. World J Surg Oncol 2004; 2:46. doi: 10.1186/1477-7819-2-46.
- De Rosa G, Ferrara G, Goglia P, Ghicas C, Zeppa P. In situ and microinvasive carcinoma with squamoid differentiation arising in a phyllodes tumour: Report of a case. Tumori 1989; 75(5):514-7.