Association of Therapeutic Dose of Valproic Acid and Plasma Glycine Levels in Epileptic Patients
By Shakeel Ahmad, Muhammad Aamir, Sobia Irum Kirmani, Zujaja Hina Haroon, Muhammad Usman Munir, Usama Bin KhalidAffiliations
doi: 10.29271/jcpsp.2021.09.1020ABSTRACT
Objectives: To determine the frequency of hyperglycinemia in epileptic patients taking valproic acid (VPA); and the correlation between therapeutic dose of valproic acid and plasma glycine levels in epileptic patients.
Study Design: Observational, cross-sectional study.
Place and Duration of Study: Department of Chemical Pathology and Endocrinology, Armed Forces Institute of Pathology Rawalpindi, in collaboration with Combined Military Hospital, Rawalpindi, from August 2020 to January 2021.
Methodology: Plasma glycine levels were analysed on ion exchange chromatography (IEC)-based instrument, Biochrome 30+ of epileptic patients undergoing treatment with anti-epileptic agents. Therapeutic doses of valproic acid were taken as serum trough levels of valproic acid and analysed on chemiluminescence-based Abbott Architect Plus i1000 SR. Mann-Whitney U-test was applied to compare plasma glycine levels in epileptic patients on valproic acid and those on multiple anti-epileptic agents. Spearman’s correlation was used to correlate plasma glycine levels in epileptic patients with trough levels of valproic acid, duration of treatment and frequency of fits/year.
Results: A total of 77 participants, upto 15 years of age, were enrolled. Plasma glycine levels were significantly raised (p <0.001) in those epileptics who were on valproic acid (monodrug therapy), in comparison with those on multiple anti-epileptic agents. There were significant positive correlations between glycine levels and trough valproic acid levels (r = 0.830), duration of treatment (r = 0.525) and frequency of seizures (r = 0.326).
Conclusion: Epileptic patients treated with valproic acid (VPA) had raised plasma glycine levels, that increased with therapeutic dose of valproic acid and duration of treatment and was associated with increased frequency of fits in those patients.
Key Words: Epilepsy, Seizure, Glycine, Valproic acid.
INTRODUCTION
Epilepsy is a condition of recurrent, unprovoked seizures that affects more than 70 million people worldwide.1 Epilepsy has a huge burden on the healthcare system of a country and affects socio-economic aspects of a society. Epilepsy is diagnosed with either recurrent seizures or a tendency towards recurrent unprovoked seizures i.e. a single seizure accompanied by evidence from clinical, electroencephalographic or neuroimaging tests that an increased risk (at least 60%) exists for future seizures in next one decade. Epileptic seizures are further categorised into generalised, focal and epileptic spasms.2
There are numerous causes of epilepsy due to different underlying dysfunctions of brain.3 Epilepsy presents with variety of symptoms with multiple risk factors; and is associated with strong genetic predisposition.4
Anti-epileptic drugs (AEDs) are the first line treatment of epilepsy. Anti-epileptic drugs therapy leads to fits-free in about 70% of all children with epilepsy.5 Some patients are being treated with single anti-epileptic drug i.e. valproic acid, carbamazepine, levetiracetam, lamotrigine, while some patients require combination therapy with 1 to 3 anti-epileptic drugs with different mechanism of actions. Valproic acid is most commonly used first line AED in treatment of epilepsies including generalised tonic clonic seizures and partial seizures.6 It acts by enhancing gamma-aminobutyric acid (GABA) function only at high concentrations. Valproic acid also increases the synthesis of GABA by stimulating glutamate decarboxylase (GAD).7 Upto 68% epileptic patients treated with valproic acid tend to have raised plasma glycine, due to inhibition of its major metabolic pathway; glycine cleavage system (GCS).8 Glycine is a non-essential amino acid, performs several functions in the central nervous system (CNS), acts both as an inhibitory as well as excitatory neurotransmitter.9 Glycine acts in the processing of motor and sensory information, participates in movement, vision, and audition. Glycine is released in central nervous system along with GABA, which is the main inhibitory amino acid neurotransmitter in humans. Glycine also enhances excitatory neurotransmission by potentiating the action of glutamate at N-methyl-D-aspartate (NMDA) receptors.9 Thus, paradoxical rise of plasma glycine levels can add in excitatory neurotransmission in epileptic patients treated with valproic acid, which can further increase the frequency of fits and will result in failure of achieving fits-free state with a single AED (valproic acid).9
As the treatment response of valproic acid can vary from person to person, frequency of hyperglycinemia and valproic acid therapy response has not been studied in Pakistani population so far. There was a need of carrying out a study that should quantitate these results in our population. Furthermore, by documenting raised plasma glycine levels in epileptic patients treated with valproic acid, will prevent clinicians from misdiagnosis of non-ketotic hyperglycinemia (NKH), which is an inherited metabolic disorder of glycine metabolism in children.10
The aim of the current study was to determine the frequency of raised plasma glycine levels among epileptic patients being treated with valproic acid; and also to correlate plasma glycine levels with therapeutic dose of valproic acid, duration of treatment and frequency of fits.
METHODOLOGY
It was an observational cross-sectional study, conducted from August 2020 to January 2021, at the Department of Chemical Pathology and Endocrinology, Armed Forces Institute of Pathology, Rawalpindi, in collaboration with Combined Military Hospital, Rawalpindi, with prior approval by Ethical Committee.
WHO calculator was used for sample size calculation based on prevalence of epilepsy in Pakistan (0.9%).11 Non-probability convenient sampling technique was used for sampling after the approval of Institution’s Ethical Committee approval. A total of 77 epileptic patients were enrolled in this study, further divided into two sub-groups; group one is on monodrug therapy, i.e. valproic acid (VPA), and group two is on multiple anti-epileptic drugs (Multi-AEDs) therapy. Participants were enrolled after taking informed consent with fulfillment of inclusion criteria that included patients with diagnosed generalised tonic clonic seizure, upto 15 years of age, taking anti-epileptic drugs for duration ≥ 12 months and experiencing fits on and off. Fits-free patients for ≥ 1 year, those taking any amino acid supplements, having history of birth asphyxia, brain stroke, recent surgical procedure, head injury, and alcohol intoxication were excluded from this study. Annual frequency of seizures was noted on pre-designed proforma. For plasma glycine levels, 3.5ml blood sample was drawn in Lithium heparin tubes, centrifuged at 1500rpm for 5 minutes in refrigerated centrifuge and separated plasma was stored at -20C˚ till analysis. Then 100µL of plasma was mixed with 100 µL of 5% Sulfasalicylic acid. Mixture was vortexed and incubated at 4C˚ for 30 minute and ultra-centrifuged at 10000 rpm for 5 minutes. After spin filtering of sample mixture, supernatant was centrifuged at high frequency, 100000 rpm, for 1 minute on micro-centrifuge. Samples were transferred into gas chromatography vials. Plasma glycine was analysed on Biochrome 30+ via ion exchange chromatography (IEC). Commercially prepared control materials were run along with each batch. Whereas, therapeutic doses of valproic acid were taken as serum trough levels of valproic acid and analysed on chemiluminescence-based Abbott Architect Plus i1000 SR.
SPSS version 21 was used for data analysis. The normality of distribution of the data was determined by Shapiro-Wilk test. Data being non-parametric, quantitative variables were expressed as median and IQR. Statistical comparisons were performed using Mann-Whitney U-test. Spearman’s correlation was used to determine correlation between plasma glycine levels and serum trough valproic acid levels, duration of treatment and frequency of fits/year. Significance was set at p <0.05 (95% confidence interval). Linear regression analysis was applied between plasma glycine levels and serum valproic acid levels, treatment duration and frequency of fits.
Table I: Descriptive statistics of quantitative parameters.
|
|
N |
Median IQR |
|
Age (years) |
77 |
3.08 (2.06 - 4.08) |
|
Duration of treatment on valproic acid (months) |
44 |
12 (12 - 34) |
|
Plasma glycine levels on Valproic acid therapy (µmol/l) |
44 |
518.00 (348.50 - 704.50) |
|
Plasma glycine levels taking multiple antiepileptic drugs therapy (µmol/l) |
33 |
306.00 (232.50 - 461.50) |
|
Serum valproic acid levels (µg/ml) |
44 |
77.00 (57.75 – 86.00) |
|
Frequency of fits/year |
44 |
2.00 (2.00 – 3.00) |
Table II: Mann-Whitney U-test.
|
Anti-epileptic drugs |
N |
Plasma glycine levels (µmol/l) |
p-value (Mann Whitney |
|
Median range (25th–75th) |
|||
|
Valproic acid |
44 |
518.00 (348.50 - 704.50) |
<0.001 |
|
Multiple anti-epileptic drugs |
33 |
306.00 (232.50 - 461.50) |
|
|
Total |
77 |
|
RESULTS
A total of 77 patients were enrolled in this study, out of which 45 (58.4%) were males and 32 (41.6%) were females. Forty-four (57.1%) epileptic patients were treated with valproic acid and 33 (42.9%) patients were treated with multiple anti-epileptic drugs. Median age of all participants was 3.08 (2.06 - 4.08) years, while their median plasma glycine level was 421.00 ((281-583.5) µmol/L. Median duration of treatment with valproic acid was 12 (12 - 34) months and median serum valproic acid level was 77(57.75 – 86.00) µg/ml. Median frequency of fits was 2.00 (2.00 – 3.00) fits/year.
Out of 44 epileptic patients on valproic acid treatment, 34 (77.3%) patients had raised plasma glycine levels while 10 (22.7%) had normal glycine levels. Median plasma glycine level in epileptic patients taking valproic acid was 518.00 (IQR 356) µmol/L. While, out of 33 epileptic patients on multi-drug therapy, 14 (42%) patients had raised plasma glycine levels. Median plasma glycine levels in epileptic patients taking multiple anti-epileptic drugs was 306.00 (IQR 229.00) µmol/L.
Mann-Whitney U-test was applied to compare plasma glycine levels of epileptic patients on valproic acid therapy and multiple anti-epileptic drugs therapy. The results
as given in Table II indicated that patients on valproic acid therapy had significantly high levels of plasma glycine levels in comparison with those taking multiple anti-epileptic drugs therapy (p <0.05).
Spearman’s correlation technique was applied to assess the correlation between plasma glycine levels and dose of valproic acid, duration of treatment and frequency of fits. Significant positive correlation was found between plasma glycine levels and serum valproic acid levels (r=0.830), plasma glycine levels and duration of treatment (r=0.525) and plasma glycine levels and frequency of fits (r=0.326).
Linear regression between plasma glycine levels and serum Valproic acid levels, treatment duration and frequency of fits are shown below (Figure 1).
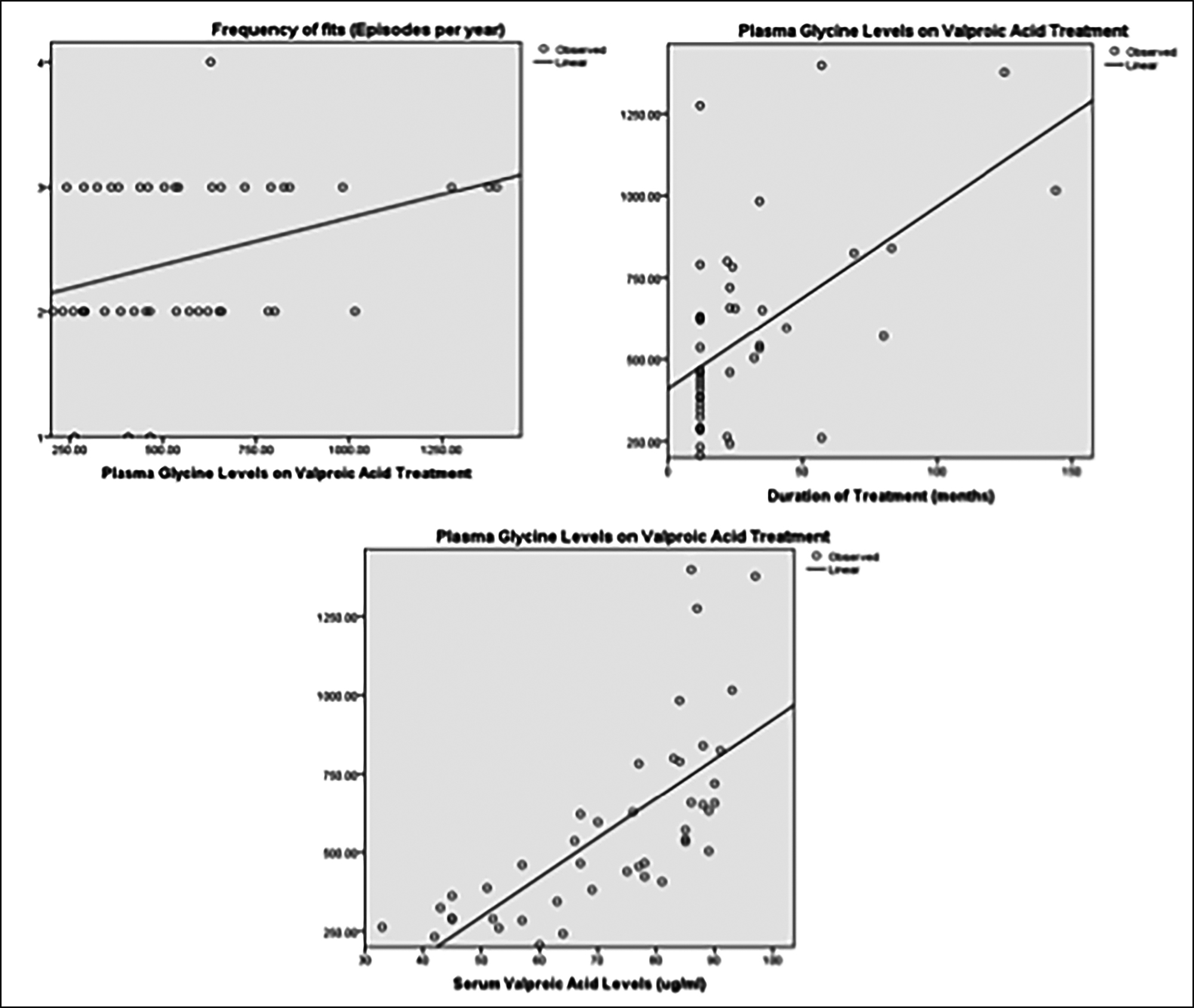 Figure 1: Relationship between plasma glycine levels in patients on valproic acid, and serum valproic acid levels duration of treatment and frequency of fits
Figure 1: Relationship between plasma glycine levels in patients on valproic acid, and serum valproic acid levels duration of treatment and frequency of fits
DISCUSSION
Epilepsy is a chronic disease of the brain, characterised by enduring seizures, unprovoked by any immediate central nervous system insult, and by the neurobiological, cognitive, psychological, and social consequences of seizure recurrences.12 In this study, the plasma glycine levels of patients treated with valproic acid were significantly raised as compared to patients taking multiple anti-epileptic drugs. This can be explained by inhibition of glycine cleavage system at mitochondrial level by valproic acid via valproyl-CoA, as documented by Luis et al.13 or defect in receptor and brain signalling pathway as reported by Rainesalo et al.14 glycine can be formed from serine by a reversible folate-dependent reaction catalysed by the enzymes glycine decarboxylase (GDC) and serine hydroxymethyltransferase (SHMT) known as glycine cleavage system (GCS). GDC and SHMT are both responsible for the inter-conversion of glycine. According to this study, frequency of hyperglycinemia is high in epileptic patients being treated with valproic acid. The present study results are substantiated by earlier studies. A study conducted by Navarro-Quesada et al. revealed that there was a progressive rise in plasma glycine levels in epileptic patients treated with valproic acid.7 In another study, carried out by Gago et al. concluded that there is a significant correlation between plasma amino acid level and valproic acid treatment in epileptic patients.15 Rao et al. study also stated that plasma glycine levels of epileptic patients treated with Valproic acid were raised.16
This study concluded that there was a positive correlation between the dose of valproic acid and plasma glycine levels. Navarro-Quesada et al. study also revealed there was positive correlation between plasma glycine levels and plasma levels of valproic acid.7 This phenomenon is likely attributed to the inhibitory effect of valproic acid on plasma glycine metabolism. Luis et al. has also described the effect of valproic acid on plasma glycine levels metabolism, leading to raised plasma glycine level treated with anti-epileptic drugs.13
The results of this study showed that there was a positive significant correlation between plasma amino acids and duration of valproic acid therapy in epileptic patients. Navarro-Quesada et al. study results also revealed that there was a progressive rise in plasma glycine levels correlating with anti-epileptic drugs therapy.7 This study concluded that there was significant correlation between the plasma glycine levels and frequency of fits in epileptic patients treated with valproic acid. Tahia et al. conducted study on patients with treatment-resistant epilepsy, results showed that there was significant correlation between plasma glycine level and frequency of fit.17 This may be due to multifactorial etiology and genetic variation related to pharmacogenomics.18
Raised glycine levels may precipitate fits, so therapeutic drug monitoring of valproic acid should be performed of epileptic patients taking valproic acid at six months after starting treatment and annually thereafter.19 Metabolic causes and genetic associations of epilepsy must be kept in mind before treating patients with valproic acid for long duration.20
CONCLUSION
Epileptic patients treated with valproic acid had raised plasma glycine levels as compared to epileptic patients treated with multiple anti-epileptic drugs. Plasma glycine levels increased with duration of treatment and dose of valproic acid. It is also associated with increased frequency of fits in epileptic patients.
RECOMMENDATIONS:
Therapeutic drug monitoring of valproic acid and plasma amino acid levels need to be carried out in patients treated for long duration, in order to avoid precipitation of fits. Furthermore, this known association of valproic acid with raised plasma glycine levels prevents clinicians from misdiagnosis of any aminoacidopathy (NKH).21
ETHICAL APPROVAL:
Ethical approval was taken from Armed forces Institute of Pathology Ethical Committee prior to research work.
PATIENTS’ CONSENT:
Written consents were taken from all participants of this study.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
SA, MA: Data collection, data analyses, results, discussion and literature review.
SIK, ZHH: Results, discussion and literature review.
MUM: Results and literature review.
UBK: Data analyses and discussion.
REFERENCES
- Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet 2019; 393(10172):689-701. doi.org/10. 1016/S0140-6736 (18)32596-0.
- Stafstrom CE, Carmant L. Seizures and Epilepsy: An overview for neuroscientists. Cold Spring HarbPerspect Med 2015; 5(6):a022426. doi: 10.1101/cshperspect.a022426.
- Shorvon SD, Andermann F, Guerrini R. The causes of epilepsy: Common and uncommon causes in adults and children. Cambridge: Cambridge University Press. (pp. 1-42). doi:10.1017/CBO9780511921001.003.
- Werner FM, Coveñas R. Classical neurotransmitters and neuropeptides involved in generalized epilepsy: A focus on antiepileptic drugs. Curr Med Chem 2011; 18(32):4933-48. doi: 10.2174/092986711797535191.
- Moosa ANV. Antiepileptic drug treatment of epilepsy in children. Continuum (Minneap Minn) 2019; 25(2):381-407. doi: 10.1212/CON.0000000000000712.
- Hanaya R, Arita K. The new antiepileptic drugs: Their neuropharmacology and clinical indications. Neurol Med Chir (Tokyo) 2016; 56(5):205-20. doi: 10.2176/nmc.ra. 2015 -0344.
- Navarro-Quesada FJ, Fernández L, Vaquero-Abellán M, Marchante-Serrano C, Jiménez C. Evaluation of the effect of long term valproic acid treatment on plasma levels of carnitine, ammonia and amino acids related to the urea cycle in pediatric epileptic patients. Revista De Neurologia 1997; 25(143):pp.1037-44.
- Hernandes MS, Troncone LR. Glycine as a neurotransmitter in the forebrain: A short review. J Neural Transm (Vienna) 2009; 116(12):1551-60. doi: 10.1007/s00702-009-0326-6.
- López-Corcuera B, Geerlings A, Aragón C. Glycine neurotransmitter transporters: An update. Mol Membr Biol 2001; 18(1):13-20. PMID: 11396606.
- Poothrikovil RP, Al Thihli K, Al Futaisi A, Al Murshidi F. Nonketotic hyperglycinemia: Two case reports and review. Neurodiagn J 2019; 59(3):142-151. doi: 10.1080/2164 6821.2019.1645549.
- Khatri IA, Iannaccone ST, Ilyas MS, Abdullah M, Saleem S. Epidemiology of epilepsy in Pakistan: Review of literature. J Pak Med Assoc 2003; 53(12):594-7. PMID: 14765939.
- Beghi E. The epidemiology of epilepsy. Neuroepidemiol 2020; 54(2):185-91. doi: 10.1159/000503831.
- Luís PBM, Ruiter JPN, Aires CCP, Soveral G, de Almeida IT, Duran M, et al. Valproic acid metabolites inhibit dihydrolipoyl dehydrogenase activity leading to impaired 2-oxoglutarate-driven oxidative phosphorylation. Biochimica Biophysica Acta 2007; 1767(9):1126-330. doi.org/10. 1016/j.bbabio.2007.06.007.
- Rainesalo S, Keränen T, Palmio J, Peltola J, Oja SS, Saransaari P. Plasma and cerebrospinal fluid amino acids in epileptic patients. Neurochem Res 2004; 29(1):319-24. doi.org/10.1023/B:NERE.0000010461.34920.0c.
- Castro-Gago M, Rodrigo-Saez E, Novo-Rodriguez I, Camiña MF, Rodriguez-Segade S. Hyperaminoacidemia in epileptic children treated with valproic acid. Child's Nerv Syst 1990; 6(8):434-6. doi.org/10.1007/BF00302087.
- Rao ML, Stefan H, Scheid C, Kuttler AD, Froscher W. Serum amino acids, liver status, and antiepileptic drug therapy in epilepsy. Epilepsia 1993; 34(2):347-54. doi.org/10.1111/j. 1528-1157.1993.tb02420.x.
- Tahia S. Role of plasma amino acids profiles in pathogenesis and prediction of severity in patients with drug resistant epilepsy. Egyptian J Hos Med 2019; 77(1): 4681-7. doi: 10.21608/ejhm.2019.45934.
- Suls A, Mullen SA, Weber YG, Verhaert K, Ceulemans B, Guerrini R, et al. Early-onset absence epilepsy caused by mutations in the glucose transporter GLUT1. Ann Neurol 2009; 66(3):415-9. doi: 10.1002/ana.21724.
- NICE (April 2018). Bipolar disorder. https://www.nice.org.uk/ guidance/cg185.
- Mortensen PB, Kølvraa S, Christensen E. Inhibition of the glycine cleavage system: hyperglycinemia and hyper glycinuria caused by valproic acid. Epilepsia 1980; 21(6):563-9. doi: 10.1111/j.1528-1157.1980.tb04310.x.
- Iqbal M, Prasad M, Mordekar SR. Nonketotic hyper glycinemia case series. J Pediatr Neurosci 2015; 10(4):355-8. doi: 10.4103/1817-1745.174445.