Combined Value of Serum miR-124, TNF-α and IL-1β for Vulnerable Carotid Plaque in Acute Cerebral Infarction
By Linghui Wang, Lei XuAffiliations
doi: 10.29271/jcpsp.2020.04.385ABSTRACT
Objective: To analyse correlation between serum miR-124, TNF-α and IL-1β levels and carotid vulnerable spots in acute cerebral infarction (ACI) patients, and explore the value of above biomarkers in diagnosing carotid artery plaques in ACI patients.
Study Design: Descriptive study.
Place and Duration of Study: Department of Neurology, Affiliated Hospital of Shandong Medical College, China, from January 2018 to November 2019.
Methodology: A total of 160 ACI patients with carotid atherosclerosis were divided into 73 cases of stable plaque group and 87 cases of vulnerable plaque group based on the results of carotid ultrasound. Receiver operating characteristic (ROC) curve was used to evaluate efficacy of serum miR-124, TNF-α and IL-1β used alone or in combination to diagnose carotid vulnerable plaques in ACI.
Results: Serum miR-124 relative expression, TNF-α and IL-1β levels in vulnerable plaque group were higher than those in stable plaque group (all p<0.001). Serum miR-124 in vulnerable plaque group was positively correlated with TNF-α and IL-1β levels (r = 0.976, p<0.001; and r = 0.974, p <0.001). AUC for miR-124, TNF-α, and IL-1β combined in diagnosis of vulnerable carotid plaques was 0.853 (95% CI: 0.790-0.915), with a sensitivity of 82.80%, and a specificity of 78.90%. The AUC diagnosed by miR-124, TNF-α, and IL-1β was greater than all the AUCs diagnosed by three indicators alone.
Conclusion: Serum miR-124, TNF-α and IL-1β can be used as indicators for early auxiliary diagnosis of vulnerable carotid plaques in patients with ACI, and combination of the three has the best diagnostic efficacy.
Key Words: Acute cerebral infarction (ACI) Serum, miR-124, TNF-α, IL-1β, Vulnerable carotid plaque.
INTRODUCTION
As a cerebrovascular disease, acute cerebral infarction (ACI) features brain tissue necrosis caused by a sudden interruption of the blood supply.1,2 It is characterised by a high mortality and disability rate, and there is currently no effective treatment. Vulnerable atherosclerotic plaques have a tendency to rupture and are prone to secondary thrombus or fissure.3,4 It is one of the main causes of ACI. The 2016 ESC/EAS guidelines classified carotid unstable plaques as extremely high risk in the risk classification of cerebral infarction.5 Therefore, early identification of unstable plaques and early intervention with appropriate measures are of great significance for reducing disability and mortality as well as improving the prognosis of ACI. High resolution computed tomography angiography and digital subtraction angiography are imaging diagnostic methods for detecting vulnerable plaques.6,7
However, they have not been widely used in underdeveloped countries due to high expenses. For this reason, the search for serum-sensitive biomarkers for the diagnosis of vulnerable carotid plaques in ACI patients has attracted increasing attention.
Atherosclerosis is a disease of chronic inflammation.8,9 Some studies have pointed out that serum inflammatory factors TNF-α and IL-1β play an important role in the formation, development and rupture of atherosclerotic plaques.10,11 Given their tissue specificity, relative stability and abundance, miRNAs are generally considered as potential biomarkers for tissue damage.12,13 The miR-124 is a brain tissue-specific microRNA involved in brain neurodevelopment. A study has shown that miR-124 can be used as a potential biomarker for early detection of brain injury.14 It has been found that miR-124 is closely related to the formation of unstable atherosclerotic plaques.15 However, the effect of combined assessment with serum miR-124, TNF-α and IL-1β for vulnerable carotid plaque in patients with ACI has not been reported yet.
The purpose of this study was to analyse the correlation between serum miR-124, TNF-α and IL-1β levels and carotid vulnerable spots in ACI patients, and explore the value of the three and their combined use in diagnosing carotid artery plaques in ACI patients.
METHODOLOGY
This descriptive study was conducted at the Department of Neurology, Affiliated Hospital of Shandong Medical College, China, from January 2018 to November 2019. This study was approved by the Hospital Ethics Committee. A total of 160 ACI patients with carotid atherosclerosis were enrolled as research subjects. Inclusion criteria were that ACI patients who were first diagnosed and confirmed by cranial MRI or CT; course of disease <24 hours; patients who were confirmed to have carotid atherosclerotic plaques by carotid ultrasound; and patients who signed informed consent for participating in the study. Exclusion criteria were that patients with cerebral hemorrhage and transient cerebral ischemia diseases; patients with infection, brain trauma and other diseases; patients with malignant tumors; patients with severe systemic diseases such as heart, liver and kidney; patients with a history of trauma or surgery within 3 months; and patients whose ultrasound result did not clearly show the carotid arteries, such as obese or dyskinetic patients.
Criterion for carotid atherosclerotic plaques was that the localised intima-media thickness of the carotid artery ≥1.5 mm. Plaques can be classified into lipid, fibrous, calcified; and mixed plaques based on their pathological morphologies on the carotid ultrasound. Among them, fibrous and calcified plaques were stable plaques. Lipid plaques and mixed plaques are vulnerable plaques. Three experienced ultrasonographers jointly judged the nature of carotid atherosclerotic plaques, and the judgment results were determined based on agreement from more than two ultrasonographers. The 160 ACI patients with carotid atherosclerosis were enrolled and divided into 73 cases of stable plaque group; and 87 cases of vulnerable plaque group based on the results of carotid ultrasound.
Table I: Comparison of serum biomarkers between the two groups.
|
Index |
Stable plaque group (n=73) |
Vulnerable plaque group (n=87) |
p-value |
|
miR-124 relative expression |
2.99 ±0.69 |
3.76 ±0.44 |
<0. 001 |
|
TNF-α(ng/L) |
3.58 ±0.75 |
4.43 ±0.56 |
<0. 001 |
|
IL-1β (ng/L) |
3.37 ±0.70 |
4.16 ±0.46 |
<0. 001 |
On the day of admission, 10 mL venous blood was collected through the cubital vein, and the serum was centrifuged for biomarker detection. Serum TNF-α and IL-1β were detected by ELISA using human TNF-α and IL-1β ELISA kit (Cusabio Biotech, Wuhan, China), respectively. The expression level of serum miR-124 was detected by qRT-PCR using miR-124 qRT-PCR Kit (Zeye Biotech, Shanghai, China). The reaction conditions were 95°C for 10 min, 1 cycle; and 95°C for 10s and 60°C for 30s, 45 cycles. Upstream primer for miR-124 was 5'-GCTAAGGCACGCGGTG-3', and downstream primer 5'-GTGCAGGGTCCGAGGT-3'. Upstream primer for internal reference U6 was 5'-CTCGCTTCGGCAGCACA-3 'and downstream primer 5'-AACGCTTCACGAATTTGCGT-3'. The relative expression of miR-124 was calculated using 2-ΔΔCt.
SPSS version 25 was used to analyse data. Qualitative variables were expressed as frequencies and percentages. Quantitative variables were expressed as means and standard deviations and tested by independent-sample t-test. Pearson's correlation approach was used to analyse the correlation. Receiver operating characteristic (ROC) curve was used to evaluate the efficacy of serum miR-124, TNF-α and IL-1β used alone or in combination to diagnose carotid vulnerable plaques in patients with ACI. The p-value <0.05 was taken as significant.
RESULTS
Among the 160 patients, 92 were males (57.50%) and 68 were females (42.50%), and they were aged 51-75 (61.47 ±3.28) years, with a disease course of 1-21 (12.83 ±1.56) hours. The serum miR-124 relative expression, TNF-α and IL-1β levels in the vulnerable plaque group were higher than those in the stable plaque group (all p <0.001, Table I). The serum miR-124 in the vulnerable plaque group was positively correlated with the TNF-α and IL-1β levels (r = 0.976, p <0.001; r = 0.974, p <0.001, Figure 1).
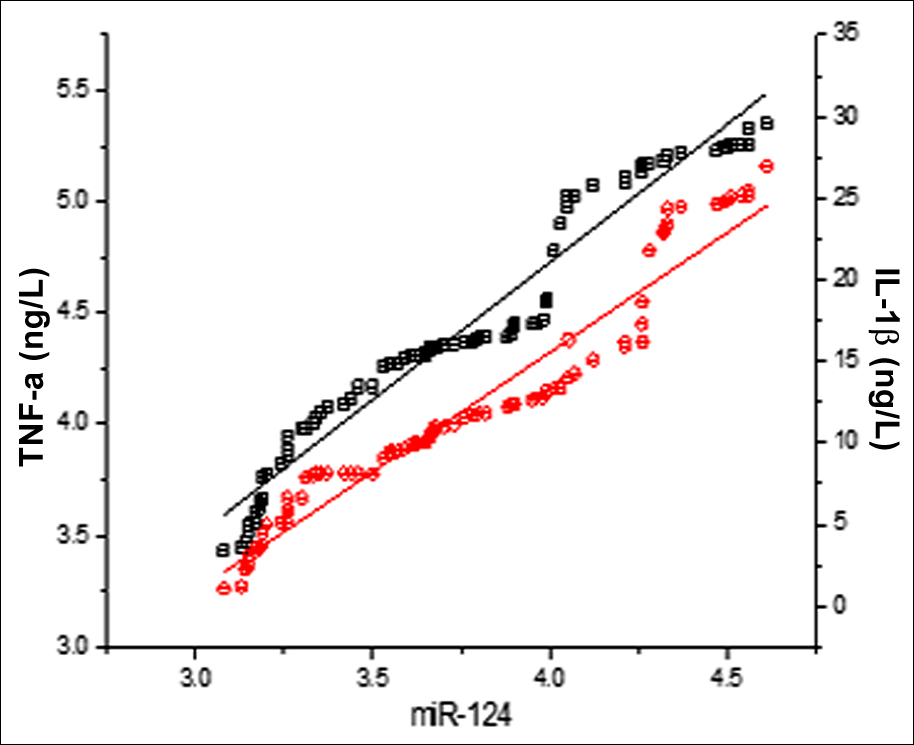 Figure 1: The serum miR-124 in the vulnerable plaque group was positively correlated with the levels of TNF-α (black line, r = 0.976, p <0.001) and IL-1β (red line, r = 0.974, p <0.001).
Figure 1: The serum miR-124 in the vulnerable plaque group was positively correlated with the levels of TNF-α (black line, r = 0.976, p <0.001) and IL-1β (red line, r = 0.974, p <0.001).
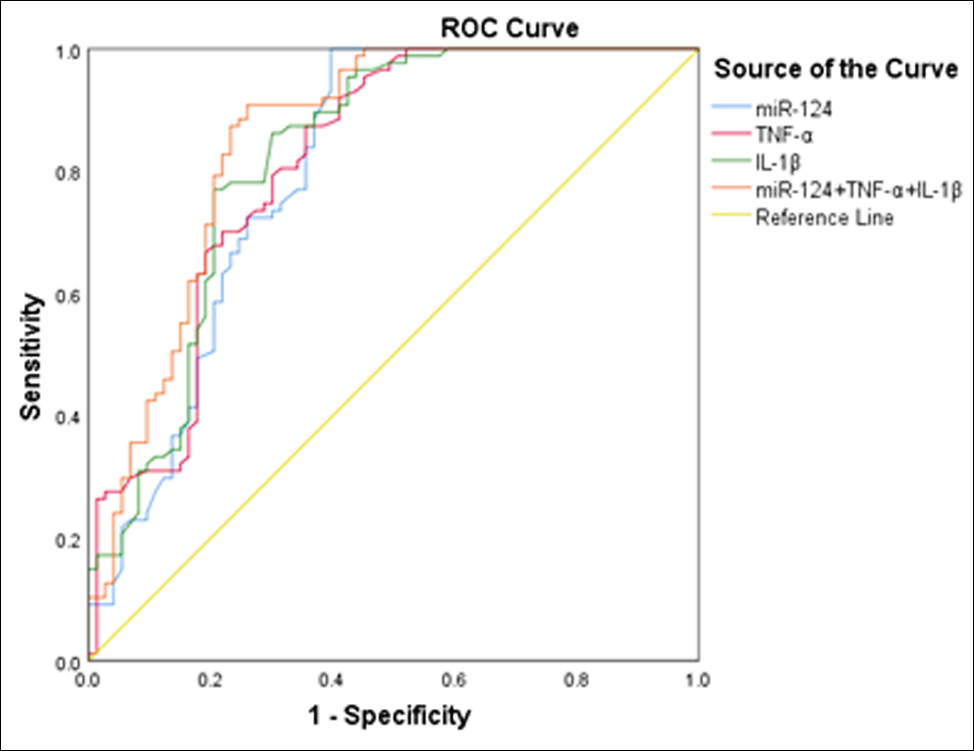 Figure 2: ROC curve of serum miR-124, TNF-α and IL-1β used alone and in combination for diagnosis of carotid vulnerable plaques in patients with ACI.
Figure 2: ROC curve of serum miR-124, TNF-α and IL-1β used alone and in combination for diagnosis of carotid vulnerable plaques in patients with ACI.
AUC of miR-124 alone for carotid vulnerable plaque diagnosis was 0.802 (95% CI: 0.729-0.875), with a sensitivity of 72.40% and a specificity of 74.00%. AUC of TNF-α alone for carotid vulnerable plaque diagnosis was 0.813 (95% CI: 0.744-0.882), with a sensitivity of 72.40% and a specificity of 74.00%. AUC of IL-1β alone for carotid vulnerable plaque diagnosis was 0.822 (95% CI: 0.754- 0.891), with a sensitivity of 77.00% and a specificity of 79.50% (Figure 2).
The AUC for miR-124, TNF-α, and IL-1β combined in the diagnosis of vulnerable carotid plaques was 0.853 (95% CI: 0.790-0.915), with a sensitivity of 82.80% and a specificity of 78.90%. The AUC diagnosed by miR-124, TNF-α, and IL-1β was greater than all the AUCs diagnosed by the three indicators alone.
DISCUSSION
At present, consensus has been reached on early detection of high-risk vulnerable plaques and active treatment of plaque stability.16,17 Ultrasound plays an important role in analysing the stability of carotid plaques.18,19 However, the ultrasound examination results are easily affected by the experience of the ultrasonographer, the characteristics of patients (obesity, dyskinesia, etc.) and other factors. Therefore, it is of great value to combine ultrasound with blood biochemical indicators to assess the degree of plaque stability in clinical practice.
A study showed that inflammation was an important factor of plaque vulnerability and destabilisation.20 TNF-α and IL-1β are important pro-inflammatory factors. Previous study in animals have suggested that atherosclerotic aortas of mice expressed significantly higher IL-1β and TNF-α.21 miRNA-124 can serve as a novel biological marker for early diagnosis of AMI. Serum miR-124 is a promising biomarker for diagnosing acute ischemic stroke and evaluating degree of damage caused by ischemic injury.22 This study analysed the serum levels of miR-124, TNF-α and IL-1β in ACI patients with carotid atherosclerosis. The results showed that the levels of the above indicators in the vulnerable plaque group were significantly higher than those in the stable plaque group. Moreover, the serum level of miR-124 in the vulnerable plaque group was positively correlated with the TNF-α and IL-1β levels. It is speculated that high levels of miR-124, TNF-α and IL-1β can accelerate the transition from stable plaques to vulnerable plaques, indicating that the levels of miR-124, TNF-α and IL-1β can be used as serological indicators for clinical prediction of vulnerable plaques. Sheedy et al. confirmed that IL-1β, closely related to large lipid necrosis core of atherosclerotic plaques and thin fiber caps, is the marker for vulnerable plaques.23 This provides solid support for the results of this study.
Further analysis of the ROC curve revealed that the area under the curve, the sensitivity and specificity of the combined diagnosis of miR-124, TNF-α and IL-1β were larger than those of the above indicators used alone. The reason why the combined detection improves the sensitivity and specificity of diagnosis and is of high diagnostic value may be that miR-124, TNF-α and IL-1β are all closely related to the plaques and represent the different pathological characteristics of unstable plaques, and they have good diagnostic efficacy for plaques at different stages.
For ACI patients with stable plaques, vigilance should be raised if high levels of miR-124, TNF-α and IL-1β appear. Follow-up ultrasound detection should be conducted for early detection of transitional vulnerable plaques and timely treatment of plaque stability, in order to avoid further cerebrovascular accidents. It should be noted that being a single-centre study, this study has a relatively small sample size. In the next step, the author will combine multiple centres to expand the sample size of the cases and further explore the combined value of miR-124, TNF-α and IL-1β for the diagnosis of vulnerable carotid plaques in ACI patients.
CONCLUSION
Serum miR-124, TNF-α and IL-1β can be used as indicators for early auxiliary diagnosis of vulnerable carotid plaques in patients with ACI, and the combination of the three has the best diagnostic efficacy.
ETHICAL APPROVAL:
This study was conducted with the approval from the Ethics Committee of Affiliated Hospital of Shandong Medical College, China.
PATIENTS’ CONSENT:
All patients signed a document of informed consent.
CONFLICT OF INTEREST:
Authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
LW: Acquired data, drafted the manuscript, and contributed substantially to its revision.
LX: Drafted the manuscript, and read and approved the final manuscript.
REFERENCES
- Akutagawa N, Sadashima S, Nakagaki H, Nagano S, Yoshimura T. Intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator (rt-PA) therapy for acute cerebral infarction in a patient with ANCA-associated vasculitis. Rinsho Shinkeigaku 2017; 57:454-6.
- Zheng M, Wang X, Yang J, Ma S, Wei Y, Liu S. Changes of complement and oxidative stress parameters in patients with acute cerebral infarction or cerebral hemorrhage and the clinical significance. Exp Ther Med 2020; 19:703-9.
- Bing R, Driessen RS, Knaapen P, Dweck MR. The clinical utility of hybrid imaging for the identification of vulnerable plaque and vulnerable patients. J Cardiovasc Comput Tomogr 2019; 13:242-7.
- Xu R, Zhang Y, Gao X, Wan Y1, Fan Z. High-sensitivity CRP (C-Reactive protein) is associated with incident carotid artery plaque in chinese aged adults. Stroke 2019; 50:1655-60.
- Enrico A, Federica F. Clinical relevance of biomarkers for the identification of patients with carotid atherosclerotic plaque: potential role and limitations of cysteine protease legumain. Atherosclerosis 2017; 257:248-9.
- Wintermark M, Jawadi SS, Rapp JH, Tihan T, Tong E, Glidden DV, et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol 2008; 29: 875-82.
- Small GR, Chow BJW. CT imaging of the vulnerable plaque. Curr Treat Options Cardiovasc Med 2017; 19:92.
- Soeki T, Sata M. Inflammatory biomarkers and athero-sclerosis. Int Heart J 2016; 57:134-9.
- Krolikoski M, Monslow J, Puré E. The CD44-HA axis and inflammation in atherosclerosis: A temporal perspective. Matrix Biol 2019; 78-79:201-18.
- Liu Z, Lerman LO, Tang H, Barber C, Wan L, Hui MM, et al. Inflammation imaging of atherosclerosis in Apo-E-deficient mice using a (99m)Tc-labeled dual-domain cytokine ligand. Nucl Med Biol 2014; 41:785-92.
- Jing X, Chen SS, Jing W, Tan Q, Yu MX, Tu JC. Diagnostic potential of differentially expressed Homer1, IL-1β, and TNF-α in coronary artery disease. Int J Mol Sci 2014; 16:535-46.
- Koenig EM, Fisher C, Bernard H, Wolenski FS, Gerrein J, Carsillo M, et al. The beagle dog MicroRNA tissue atlas: identifying translatable biomarkers of organ toxicity. BMC Genomics 2016; 17:649.
- Gusar VA, Timofeeva AV, Zhanin IS, Shram SI, Pinelis VG. Estimation of time-dependent microrna expression patterns in brain tissue, leukocytes, and blood plasma of rats under photochemically induced focal cerebral ischemia. Mol Biol (Mosk) 2017; 51:683-95.
- Mirzaei H, Momeni F, Saadatpour L, Sahebkar A, Goodarzi M, Masoudifar A, et al. MicroRNA: Relevance to stroke diagnosis, prognosis, and therapy. J Cell Physiol 2018; 233:856-65.
- Liang WJ, Zhou SN, Shan MR, Wang XQ, Zhang M, Chen Y, et al. AMPKα inactivation destabilises atherosclerotic plaque in streptozotocin-induced diabetic mice through AP-2α/ miRNA-124 axis. J Mol Med (Berl) 2018; 96:403-12.
- Madjid M, Willerson JT, Casscells SW. Intracoronary thermo-graphy for detection of high-risk vulnerable plaques. J Am Coll Cardiol 2006; 47(8 Suppl):C80-5.
- Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, Kerwin WS, et al. The vulnerable, or high-risk, atherosclerotic plaque: Non-invasive MR imaging for characterization and assessment. Radiology 2007; 244:64-77.
- Zamani M, Skagen K, Scott H, Lindberg B, Russell D, Skjelland M. Carotid plaque neovascularization detected with superb microvascular imaging ultrasound without using contrast media. Stroke 2019; 50:3121-27.
- Loizou C, Petroudi S, Pantziaris M, Nicolaides A, Pattichis C. An integrated system for the segmentation of atherosclerotic carotid plaque ultrasound video. IEEE Trans Ultrason Ferroelectr Freq Control 2014; 61:86-101.
- Dickson BC, Gotlieb AI. Towards understanding acute destabilization of vulnerable atherosclerotic plaques. Cardiovasc Pathol 2003; 12:237-48.
- Liu Z, Lerman LO, Tang H, Barber C, Wan L, Hui MM, et al. Inflammation imaging of atherosclerosis in Apo-E-deficient mice using a (99m)Tc-labeled dual-domain cytokine ligand. Nucl Med Biol 2014; 41:785-92.