-
PDF
- Split View
-
Views
-
Cite
Cite
Kylie Hill, Kevin R. Gain, Sean W. McKay, Christine Nathan, Eli Gabbay, Effects of High-Intensity Inspiratory Muscle Training Following a Near-Fatal Gunshot Wound, Physical Therapy, Volume 91, Issue 9, 1 September 2011, Pages 1377–1384, https://doi.org/10.2522/ptj.20100241
Close - Share Icon Share
Severe injuries sustained during combat may classify individuals as undeployable for active service. It is imperative that every effort is made to optimize physical function following such injuries.
A 38-year-old man sustained a gunshot wound during armed combat. The bullet entered via the left axilla and exited from the right side of the abdomen, resulting in severe thoracic and abdominal injuries. Five months later, he continued to describe severe dyspnea on exertion. During a cardiopulmonary exercise test on a cycle ergometer, he achieved a maximum rate of oxygen uptake of 2,898 mL·min−1 (114% predicted) and maximum power of 230 W (114% predicted). His maximum forced inspiratory flow was 5.95 L·s−1, and inspiratory reserve volume at test end was ∼80 mL. The test was terminated by the patient due to dyspnea that was too severe to tolerate. Video fluoroscopy demonstrated impaired right hemidiaphragm function. The main goals of therapy were to reduce dyspnea on exertion and to enable return to full work duties. A program of high-intensity, interval-based threshold inspiratory muscle training (IMT) was undertaken.
An average of 5 sessions of IMT were completed each week for 10 weeks. During a repeat cardiopulmonary exercise test, the patient achieved a similar power and maximum rate of oxygen uptake. His maximum forced inspiratory flow increased by 48% to 8.83 L·s−1, and he was limited by leg fatigue.
High-intensity IMT was safe and well tolerated. It was associated with improvements in maximum forced inspiratory flow and changed the locus of symptom limitation during high-intensity exercise from dyspnea to leg fatigue.
Severe injuries sustained during armed combat may classify individuals as undeployable for active service for the remainder of their military careers.1 It is imperative that every effort be made to optimize physical function following such injuries. The purpose of this case report is to describe the use of high-intensity inspiratory muscle training (IMT) as part of the rehabilitation of an individual who sustained a near-fatal thoracoabdominal gunshot wound. Specifically, this patient was referred to our clinical service 5 months following his injury, as he described dyspnea during exercise that was too severe to tolerate. Although he reported being limited by dyspnea since the time of his injury, it had previously been attributed to generalized deconditioning as a consequence of his convalescence. However, as this symptom had not subsided despite participation in a comprehensive program of whole-body exercise, his health care team decided it warranted further investigation, and the patient was referred for review by a respirologist.
Data collected during a cardiopulmonary exercise test (CPET) indicated a marked reduction in his maximum forced inspiratory flow and an abnormal breathing pattern during exercise (see “Cardiopulmonary Exercise Testing” section). In people with chronic obstructive pulmonary disease, inspiratory muscle training (IMT) performed using a threshold loading device has been demonstrated to increase maximum inspiratory flow2,3 and confer reductions in dyspnea during activity.4 Therefore, we hypothesized that a program of high-intensity IMT might increase maximum forced inspiratory flow and reduce dyspnea on exertion for this patient. The patient provided informed consent for the publication of this report.
Case Description: Patient History and Review of Systems
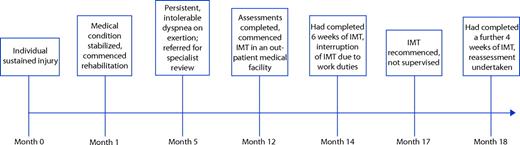
The patient was a 38-year-old lifelong nonsmoker of average height and weight (height=1.70 m, weight=71 kg, body mass index=24.6 kg·m−2). Prior to the injury, the patient had undergone annual medical reviews by military medical personnel and was in excellent health. The bullet had entered his left axilla and exited from the right side of his abdomen, causing severe injuries to both lungs, which resulted in pneumothoraces, extensive subcutaneous emphysema, bibasal atelectasis, and pleural effusions. He also sustained a laceration to the left lobe of his liver and a grazed pancreatic capsule. He developed a pulmonary embolus 9 days following this injury. The time course of events related to his recovery is summarized in Figure 1.
Medical management comprised insertion of bilateral intercostal catheters and multiple abdominal laparotomies. He was intubated and ventilated for 7 days. Once medically stable and discharged home (4 weeks postinjury), rehabilitation was commenced. Rehabilitation comprised a graduated program of daily cardiorespiratory conditioning exercises, including swimming, stationary cycling, and walking, which was progressed to running. Upper- and lower-limb resistance training in addition to core stability exercises were delayed for 3 months following injury to allow adequate time for wound healing. Five months following his injury, his program of cardiorespiratory conditioning exercise continued to be limited by severe dyspnea. Specifically, he reported severe dyspnea during a 2.4-km run. The main goals of physical therapy were to reduce dyspnea during exercise and facilitate return to full work duties.
Clinical Impression
The patient sustained life-threatening injuries to his lungs and liver. The history of intolerable dyspnea during exercise suggested a possible ventilatory limitation resulting from injury to the diaphragm. Baseline tests comprised: (1) high-resolution computerized tomography (CT) of his thorax, (2) chest fluoroscopy, (3) a pulmonary angiogram, (4) laboratory-based CPET using an electronically braked cycle ergometer, (5) spirometry, and (6) measurement of maximum inspiratory pressure (Pimax). These tests were undertaken to: (1) identify the nature and resolution of the injuries, (2) examine diaphragm movement during respiration, (3) exclude ongoing pulmonary thromboembolic disease, (4) determine ventilatory or cardiac limitations to exercise, (5) exclude causes of airflow limitation such as asthma, and (6) quantify the pressure-generating capacity of the inspiratory pump muscles, respectively. The time from referral to our clinical service (month 5; see Fig. 1) to completion of all baseline tests (month 12; see Fig. 1) was 7 months.
Examination
High-Resolution CT and Pulmonary Angiogram (Month 7)
The high-resolution CT scan revealed a small, encysted pneumothorax within the medial aspect of the interlobar fissure, with a small volume of pulmonary atelectasis or scarring in the posterobasal segment of the right lower lobe. These changes were believed to represent a traumatic pneumatocele in the area of pulmonary contusion and were unlikely to account for his symptoms. The pulmonary angiogram was normal.
Chest Fluoroscopy (Month 11)
This test demonstrated impaired right hemidiaphragm function, with evidence of reduced excursion at rest and paradoxical excursion on forced inspiration.
Cardiopulmonary Exercise Testing (Month 11)
This assessment comprised continuous 12-lead electrocardiography and breath-by-breath analysis of respired gas (MedGraphics CPXD cardiopulmonary exercise system*). The patient breathed through a mouthpiece with the nose occluded by a noseclip. Prior to the test, a maximum flow-volume loop was performed by the patient. The exercise protocol comprised 3 minutes of resting data collection and 3 minutes of unloaded pedaling, followed by a continuous ramp increase in power (20 W·min−1) until symptom limitation. Breath-by-breath flow-volume loops were recorded continuously during the test, along with inspiratory capacity every 2 minutes. These measures permit an appreciation of the extent to which flows (ie, inspiratory and expiratory) elicited during exercise encroach upon the maximum available to the patient.5 Furthermore, they allow changes in end-expiratory lung volume (EELV) during exercise to be measured.5 Specifically, a decrease in inspiratory capacity (ie, the volume of air that can be inspired at the end of a normal exhalation) and inspiratory reserve volume (IRV) (ie, the volume of air that can be inspired at the end of a normal inspiration) are indicative of hyperinflation.6
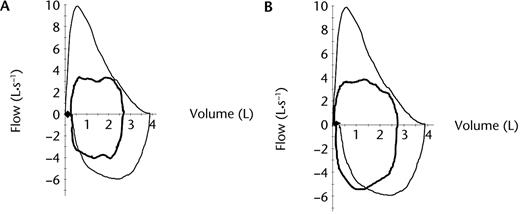
To optimize his performance, the patient chose to cycle from a standing position during the final 30 seconds of the test. As this is not part of the usual CPET protocol, end-test measures for this assessment are reported at 2 time points: (1) immediately prior to when he chose to stand and (2) voluntary cessation due to symptom limitation with the patient in a standing position (Tab. 1). His exercise flow-volume loops at these time points demonstrate: (1) encroachment of inspiratory flow on his maximum forced inspiratory flow recorded at rest, (2) mild expiratory airflow limitation, and (3) an IRV of ∼250 mL (Fig. 2A) that reduced to ∼80 mL (Fig. 2B). Breathing reserve, calculated as the difference between his maximum voluntary ventilation (MVV) measured over 12 seconds and minute ventilation measured at the point he achieved his maximum rate of oxygen uptake, was 58 L·min−1. The patient rated the severity of dyspnea and leg fatigue using the 0 to 10 Borg category ratio scale, where 0 represents no dyspnea or leg fatigue and 10 represents a maximal experience of dyspnea or leg fatigue.7 On test completion, he rated his dyspnea as 10 and leg fatigue as 5 (ie, severe).
Results of Baseline and Posttraining Cardiopulmonary Exercise Testsa
| Variable . | Baseline . | Posttraining . | |||
|---|---|---|---|---|---|
| Rest . | End Test (% Predicted) in Sittingb . | End Test (% Predicted) in Standing . | Rest . | End Test (% Predicted) in Sitting . | |
| Power (W) | 0 | 208 (103%) | 230 (114%) | 0 | 224 (111%) |
| ⩒o2 (mL·min−1) | 259 | 2,591 (102%) | 2,898 (114%) | 182 | 2,751 (102%) |
| ⩒co2 (mL·min−1) | 234 | 3,090 (98%) | 3,685 (117%) | 139 | 3,137 (94%) |
| ⩒E (L·min−1) | 8.5 | 80 (51%)c | 107 (68%)c | 6.2 | 81 (53%)c |
| Breathing frequency (breaths·min−1) | 10 | 33 | 37 | 12 | 29 |
| Tidal volume (L) | 0.86 | 2.43 | 2.90 | 0.53 | 2.75 |
| Inspiratory capacity (L) | 2.56 | 2.73 | 2.73 | 2.82 | 3.19 |
| Inspiratory duty cycle (%) | 30 | 39 | 43 | 25 | 34 |
| Spo2 (%) | 98 | 96 | 96 | 98 | 95 |
| Heart rate (bpm) | 80 | 182 (100%) | 182 (100%) | 60 | 170 (93%) |
| Variable . | Baseline . | Posttraining . | |||
|---|---|---|---|---|---|
| Rest . | End Test (% Predicted) in Sittingb . | End Test (% Predicted) in Standing . | Rest . | End Test (% Predicted) in Sitting . | |
| Power (W) | 0 | 208 (103%) | 230 (114%) | 0 | 224 (111%) |
| ⩒o2 (mL·min−1) | 259 | 2,591 (102%) | 2,898 (114%) | 182 | 2,751 (102%) |
| ⩒co2 (mL·min−1) | 234 | 3,090 (98%) | 3,685 (117%) | 139 | 3,137 (94%) |
| ⩒E (L·min−1) | 8.5 | 80 (51%)c | 107 (68%)c | 6.2 | 81 (53%)c |
| Breathing frequency (breaths·min−1) | 10 | 33 | 37 | 12 | 29 |
| Tidal volume (L) | 0.86 | 2.43 | 2.90 | 0.53 | 2.75 |
| Inspiratory capacity (L) | 2.56 | 2.73 | 2.73 | 2.82 | 3.19 |
| Inspiratory duty cycle (%) | 30 | 39 | 43 | 25 | 34 |
| Spo2 (%) | 98 | 96 | 96 | 98 | 95 |
| Heart rate (bpm) | 80 | 182 (100%) | 182 (100%) | 60 | 170 (93%) |
⩒o2 = rate of oxygen uptake, ⩒co2 = rate of carbon dioxide production, ⩒E = minute ventilation, Spo2 = arterial oxygen saturation. Inspiratory duty cycle represents the proportion of total respiratory cycle time spent in inspiration. At peak exercise, breathing frequency should be <60 breaths·min −1; tidal volume usually plateaus at 50% to 60% of vital capacity (a threefold to fivefold increase from resting measures); inspiratory capacity should increase by 0.50 to 1.0 L from rest; there should be minimal, if any, change in Spo220; and inspiratory duty cycle remains stable at approximately 45%.27
Measurements in this column refer to those recorded during the baseline test immediately prior to the point at which the patient chose to stand on the pedals to cycle. Note that the patient did not stand on the pedals during the posttraining test.
Percentage predicted was calculated using the measured maximum voluntary ventilation as the maximum value.
Results of Baseline and Posttraining Cardiopulmonary Exercise Testsa
| Variable . | Baseline . | Posttraining . | |||
|---|---|---|---|---|---|
| Rest . | End Test (% Predicted) in Sittingb . | End Test (% Predicted) in Standing . | Rest . | End Test (% Predicted) in Sitting . | |
| Power (W) | 0 | 208 (103%) | 230 (114%) | 0 | 224 (111%) |
| ⩒o2 (mL·min−1) | 259 | 2,591 (102%) | 2,898 (114%) | 182 | 2,751 (102%) |
| ⩒co2 (mL·min−1) | 234 | 3,090 (98%) | 3,685 (117%) | 139 | 3,137 (94%) |
| ⩒E (L·min−1) | 8.5 | 80 (51%)c | 107 (68%)c | 6.2 | 81 (53%)c |
| Breathing frequency (breaths·min−1) | 10 | 33 | 37 | 12 | 29 |
| Tidal volume (L) | 0.86 | 2.43 | 2.90 | 0.53 | 2.75 |
| Inspiratory capacity (L) | 2.56 | 2.73 | 2.73 | 2.82 | 3.19 |
| Inspiratory duty cycle (%) | 30 | 39 | 43 | 25 | 34 |
| Spo2 (%) | 98 | 96 | 96 | 98 | 95 |
| Heart rate (bpm) | 80 | 182 (100%) | 182 (100%) | 60 | 170 (93%) |
| Variable . | Baseline . | Posttraining . | |||
|---|---|---|---|---|---|
| Rest . | End Test (% Predicted) in Sittingb . | End Test (% Predicted) in Standing . | Rest . | End Test (% Predicted) in Sitting . | |
| Power (W) | 0 | 208 (103%) | 230 (114%) | 0 | 224 (111%) |
| ⩒o2 (mL·min−1) | 259 | 2,591 (102%) | 2,898 (114%) | 182 | 2,751 (102%) |
| ⩒co2 (mL·min−1) | 234 | 3,090 (98%) | 3,685 (117%) | 139 | 3,137 (94%) |
| ⩒E (L·min−1) | 8.5 | 80 (51%)c | 107 (68%)c | 6.2 | 81 (53%)c |
| Breathing frequency (breaths·min−1) | 10 | 33 | 37 | 12 | 29 |
| Tidal volume (L) | 0.86 | 2.43 | 2.90 | 0.53 | 2.75 |
| Inspiratory capacity (L) | 2.56 | 2.73 | 2.73 | 2.82 | 3.19 |
| Inspiratory duty cycle (%) | 30 | 39 | 43 | 25 | 34 |
| Spo2 (%) | 98 | 96 | 96 | 98 | 95 |
| Heart rate (bpm) | 80 | 182 (100%) | 182 (100%) | 60 | 170 (93%) |
⩒o2 = rate of oxygen uptake, ⩒co2 = rate of carbon dioxide production, ⩒E = minute ventilation, Spo2 = arterial oxygen saturation. Inspiratory duty cycle represents the proportion of total respiratory cycle time spent in inspiration. At peak exercise, breathing frequency should be <60 breaths·min −1; tidal volume usually plateaus at 50% to 60% of vital capacity (a threefold to fivefold increase from resting measures); inspiratory capacity should increase by 0.50 to 1.0 L from rest; there should be minimal, if any, change in Spo220; and inspiratory duty cycle remains stable at approximately 45%.27
Measurements in this column refer to those recorded during the baseline test immediately prior to the point at which the patient chose to stand on the pedals to cycle. Note that the patient did not stand on the pedals during the posttraining test.
Percentage predicted was calculated using the measured maximum voluntary ventilation as the maximum value.
(A) Baseline flow-volume loops at rest (outer black line) and immediately prior to test completion when the patient chose to stand on the pedals to cycle (inner bold black line). ♦=inspiratory reserve volume immediately prior to test completion (∼250 mL). (B) Baseline flow-volume loops at rest (outer black line) and immediately prior to test completion with patient standing on the pedals to cycle (inner bold black line). ♦: inspiratory reserve volume immediately prior to test completion (∼80 mL).
Spirometry (Month 11) and Maximum Inspiratory Pressure (Pimax) (Month 12)
All measurements were made with the patient seated and wearing a noseclip using the MedGraphics CPXD system. Spirometric results are summarized in Table 2. Both forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were reduced from his preinjury measurements of 4.32 and 5.17 L, respectively. To ascertain whether the airflow limitation observed during the CPET was reversible with a bronchodilator (ie, asthma), spirometry was repeated 20 minutes following administration of a short-acting beta-2 agonist. There was minimal bronchodilator response in spirometric measures, suggesting the airflow limitation was fixed. Maximum inspiratory pressure was defined as the highest inspiratory (ie, negative) pressure generated against an occluded airway from residual volume.8 To account for improvements resulting from familiarization,9 the patient was instructed to perform 4 maximum inspiratory maneuvers. The best Pimax was equal to −122 cm H2O (98% predicted).10
Results of Baseline and Posttraining Spirometrya
| Variable . | Baseline . | Posttraining . |
|---|---|---|
| Measured Value (% Predicted) . | Measured Value (% Predicted) . | |
| FVC (L) | 3.90 (88%) | 4.14 (94%) |
| FEV1 (L) | 3.29 (89%) | 3.21 (87%) |
| FEV1/FVC (%) | 84 (106%) | 78 (97%) |
| Maximum forced inspiratory flow (L·s−1) | 5.95 | 8.83 |
| Variable . | Baseline . | Posttraining . |
|---|---|---|
| Measured Value (% Predicted) . | Measured Value (% Predicted) . | |
| FVC (L) | 3.90 (88%) | 4.14 (94%) |
| FEV1 (L) | 3.29 (89%) | 3.21 (87%) |
| FEV1/FVC (%) | 84 (106%) | 78 (97%) |
| Maximum forced inspiratory flow (L·s−1) | 5.95 | 8.83 |
FVC = forced vital capacity, FEV1 = forced expiratory volume in 1 second. Predicted values were derived from Hankinson et al.28
Results of Baseline and Posttraining Spirometrya
| Variable . | Baseline . | Posttraining . |
|---|---|---|
| Measured Value (% Predicted) . | Measured Value (% Predicted) . | |
| FVC (L) | 3.90 (88%) | 4.14 (94%) |
| FEV1 (L) | 3.29 (89%) | 3.21 (87%) |
| FEV1/FVC (%) | 84 (106%) | 78 (97%) |
| Maximum forced inspiratory flow (L·s−1) | 5.95 | 8.83 |
| Variable . | Baseline . | Posttraining . |
|---|---|---|
| Measured Value (% Predicted) . | Measured Value (% Predicted) . | |
| FVC (L) | 3.90 (88%) | 4.14 (94%) |
| FEV1 (L) | 3.29 (89%) | 3.21 (87%) |
| FEV1/FVC (%) | 84 (106%) | 78 (97%) |
| Maximum forced inspiratory flow (L·s−1) | 5.95 | 8.83 |
FVC = forced vital capacity, FEV1 = forced expiratory volume in 1 second. Predicted values were derived from Hankinson et al.28
Clinical Impression
A diagnosis was made of right hemidiaphragm dysfunction and mild fixed airflow limitation. This diagnosis was based on the findings of chest fluoroscopy and flow-volume loops and was consistent with the sensation of severe dyspnea during exercise. Inspiratory flow measured at symptom limitation during exercise was 91% of his maximum forced inspiratory flow recorded at rest, suggesting that the power of his inspiratory muscles was compromised.11 End-test measurements of IRV, collected both in sitting and standing, had diminished to a level previously demonstrated to elicit severe dyspnea in patients with fixed airflow obstruction.6 The patient did not report any pain on inspiration during any of the tests. Based on the results of earlier work in patients with chronic obstructive pulmonary disease, we hypothesized that high-intensity IMT would increase maximum inspiratory flow and reduce dyspnea2,3 and thereby facilitate this patient's return to full duty at work.
Intervention
The patient was instructed to undertake the IMT while sitting, wearing a noseclip. The first session commenced with a 1-minute warm-up at a load equivalent to 15% of his measured Pimax. Thereafter, the load used throughout the first training session was increased to 30% of his Pimax, as earlier work has demonstrated that this is the minimum load capable of inducing training-related gains in inspiratory muscle function.12 An interval-based training protocol was used characterized by 2 minutes of loaded breathing, interspersed with 1 minute of rest. This 3-minute sequence was repeated 7 times so that each training session was 21 minutes in duration.13,14 Threshold loads were chosen to train the inspiratory muscles, as the load imposed is largely independent of the breathing pattern used by the patient,15 thereby allowing the training to be undertaken with minimal supervision. Furthermore, threshold loads necessitate muscular contractions that maximize inspiratory flow and optimize the power of the inspiratory muscles.3,11,15 An interval-based program was selected, as it allows very high training loads to be achieved, thereby optimizing the magnitude of training-related benefits in inspiratory muscle function and relief of dyspnea.14 Additional details of this training program have been published elsewhere.16 The patient was instructed to stop immediately if he experienced any sharp pain on inspiration.
Given the severity of the patient's injuries and the persistence of a small pneumothorax, training during the first 6 weeks was performed in an outpatient medical clinic. Over this period, heart rate and arterial oxygen saturation (Spo2) were measured continuously via pulse oximetry and finger probe. The patient was instructed to reduce the training load if Spo2 decreased to ≤90%. During the first 2 training sessions, a physical therapist monitored respiratory rate for evidence of hypoventilation. Two different devices were used for IMT. For the first 4 weeks, a Respironics Threshold IMT device† was used. The manufacturer's spring, which allowed the imposition of training loads between −7 and −41 cm H2O, was replaced with one of identical dimensions but higher tension, which allowed the imposition of loads of between −14 and −103 cm H2O. The properties of the new spring have been reported elsewhere.14 This modification served 2 purposes. First, as the patient's baseline Pimax was −122 cm H2O, the new spring was needed to ensure the device could impose loads of ≥30% of his Pimax. Second, it represented a risk minimization strategy by preventing the patient from attempting training loads that exceeded 85% of his baseline Pimax during the first 4 weeks of training. At the end of the first 4 weeks, the patient was changed to the POWERbreathe Classic (Sports),‡ an IMT device capable of imposing training loads between −10 and −250 cm H2O. At the time these devices were changed, the patient was using the highest load that could be imposed using the modified Respironics Threshold IMT device, which represented an intensity equivalent to approximately 84% of his baseline Pimax.
Although the load used for the first training session was prescribed at a percentage of his Pimax measured at baseline, loads were progressed according to the magnitude of symptoms elicited during loaded breathing.16 Specifically, the patient was instructed to select training loads that elicited an effort equivalent to 4 on the 0 to10 Borg scale (ie, “somewhat hard”).7 Following completion of the first training session, he was instructed to adjust the load used for the 1-minute warm-up to 50% of the highest load achieved during the preceding training session.16
The patient completed an average of 5 sessions of IMT per week for 6 weeks, separated by a 3-month interruption due to work duties, followed by a further 4 weeks of training immediately prior to reassessment. During this 3-month interruption, no specific or supervised exercise training was undertaken. Earlier work has demonstrated that 3 months following the cessation of IMT, there is a tendency for Pimax to remain greater than pretraining values.17 Therefore, although training-related gains would have diminished during the 3-month interruption, it is unlikely that all measures would have returned to pretraining values. During the initial 6 weeks of training, the patient was supervised during the IMT sessions, and his adherence to training was excellent. Following this period, although he performed the IMT in the absence of direct supervision, he was highly motivated to return to full active service, and his self-reported adherence to the therapy remained excellent.
Throughout the period of IMT, the patient continued with his rehabilitation program (see “Case Description: Patient History and Review of Systems” section) at the highest intensity he could manage without inducing intolerable dyspnea (ie, breathlessness that was too severe to tolerate). These exercises were not undertaken with direct supervision.
Outcome
Heart Rate and Spo2 During IMT
Inspiratory muscle training elicited minimal increase in heart rate and mild transient desaturation. Specifically, the largest increase and peak heart rate achieved during any IMT session were 29 bpm and 108 bpm, respectively. The largest decrease and nadir Spo2 achieved during any IMT session were 6% and 92%, respectively. The patient did not report any pain or dizziness during the sessions.
Reassessment Procedures and Findings
Reassessment was undertaken 6 months following initiation of the IMT program. The results are summarized in Tables 1 and 2.
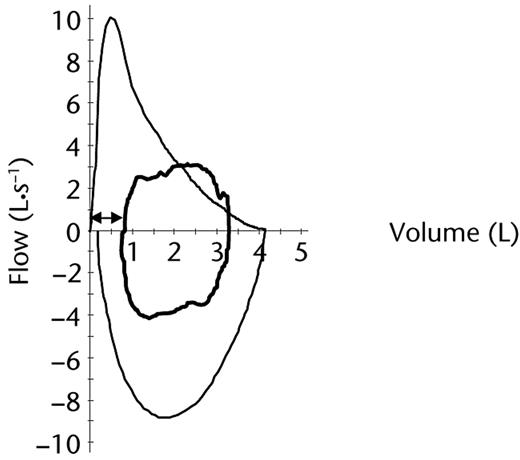
Maximum power achieved during the CPET was 224 W (111% predicted).18 Due to intolerable leg fatigue, the patient felt he could not stand on the pedals at the end of the test this time, a factor that is likely to explain the small decrease in the maximum rate of oxygen uptake on test completion relative to baseline results (Tab. 1). At the point he achieved his maximum rate of oxygen uptake, breathing reserve was 73 L·min−1 and his exercise flow-volume loops revealed an inspiratory flow equivalent to 46% of his maximum forced inspiratory flow recorded at rest together with an IRV of approximately 750 mL (Fig. 3). He rated the severity of dyspnea and leg fatigue as 7 and 10, respectively.
Posttraining flow-volume loops at rest (outer black line) and immediately prior to test completion (inner bold black line). ⇿=inspiratory reserve volume immediately prior to test completion (∼750 mL).
A repeat high-resolution CT scan performed 2 months following initiation of IMT demonstrated complete resolution of the pneumothorax. Scarring in the posterobasal segment was still evident and believed to represent fibrosis.
Discussion
To our knowledge, this is the first case-report describing the use of high-intensity, interval-based threshold IMT to facilitate resolution of severe dyspnea on exertion, initiated 12 months following a thoracoabdominal gunshot wound. The important findings of this case report were: (1) despite a normal aerobic exercise capacity, this patient used an inefficient breathing strategy during exercise, which was detected using exercise flow-volume loops and was consistent with impaired right hemidiaphragm function; (2) IMT, when properly supervised, was feasible, safe, and well-tolerated; (3) IMT produced a large increase in maximum forced inspiratory flow; and (4) the locus of symptom limitation during exercise changed from the dyspnea to leg fatigue.
Changes in the Flow-Volume Loops
Aside from the results of the chest fluoroscopy, the most striking abnormalities observed during baseline testing were the end-exercise encroachment of inspiratory flow on maximum forced inspiratory flow (Fig. 2B) and the reduction in IRV. In individuals who are healthy, inspiratory flow during exercise reaches between 50% and 70% of the maximum forced inspiratory flow measured during a resting flow-volume loop.19 Examination of Figure 2B revealed that during baseline testing, inspiratory flows during exercise approximated the maximum forced inspiratory flow previously recorded at rest. A reduction in maximum forced inspiratory flow, even in the presence of preserved Pimax, is suggestive of compromised inspiratory muscle power11 and is consistent with right hemidiaphragm dysfunction.
How Did the Patient's Reduction in Inspiratory Flow Affect the Breathing Strategy Adopted During Exercise?
In health, there is adequate reserve in inspiratory and expiratory flow rates to shift the increased tidal volumes necessary during exercise.20 Given the reserve in expiratory flow, increased tidal volumes are afforded, at least in part, by a decrease in EELV produced by recruitment of the abdominal muscles.21 That is, during exercise, individuals who are healthy initiate inspiration from a lung volume below functional residual capacity. This is an efficient breathing strategy, as it reduces inspiratory muscle work by utilizing chest wall recoil to facilitate inspiratory flow and optimizes the mechanical advantage of the diaphragm.21
In our patient, the somewhat scooped appearance of the expiratory limb (Figs. 2A and 2B) suggested a mild expiratory flow limitation, most likely as a consequence of fibrotic scar tissue. As expiratory flow could not be increased further, with increased tidal volumes, greater time was needed for complete expiration within each respiratory cycle. A corollary is that for a given respiratory frequency, the time available for inspiration was reduced, which necessitated an increase in inspiratory flow. During baseline testing, the lack of reserve in his maximum forced inspiratory flow (Fig. 2B) constrained the extent to which he could: (1) reduce inspiratory time, (2) lengthen expiratory time, and, therefore, (3) utilize a decrease in EELV to meet the tidal volume necessary during exercise.
How Does a Reduction in Inspiratory Flow Translate Into Intolerable Dyspnea?
In our patient, the reduced maximum forced inspiratory flow meant that he was able to reduce EELV during exercise by only 170 mL. The increased tidal volume required during exercise was met largely by utilizing inspiratory capacity until the IRV was diminished to approximately 80 mL. The limited capacity to reduce EELV and subsequent reduction in end-exercise IRV increased the elastic load borne by the inspiratory muscles,6 and the inspiratory effort associated with generating tidal volumes was perceived as inappropriate. This process, known as neuromechanical disassociation, has been postulated to give rise to dyspnea.22
How Did IMT Help?
Following training, maximum inspiratory flow recorded at rest had increased by 2.88 L·s−1 (ie, 48%), and the proportion of total respiratory cycle time available for expiration on completion of the CPET had increased from 57% to 66%. These increases facilitated the tidal volumes necessary during exercise to be met to a greater extent by decreasing EELV and reduced the reliance on utilizing inspiratory capacity. Compared with the baseline test, the decrease in end-exercise EELV following IMT was 200 mL greater and IRV was increased by approximately 700 mL, translating into reduced elastic load on the inspiratory muscles and reduced dyspnea.6 The longer time available for expiration also optimizes blood flow to the inspiratory muscles, which is likely to minimize their fatigue during exercise.23
Compared with measurements collected during the baseline CPET immediately prior to when the patient chose to stand, during the reassessment following IMT, the patient demonstrated an increase in maximum power (208 versus 224 W), peak rate of oxygen uptake (2,591 versus 2,791 mL·min−1), and peak rate of carbon dioxide production (3,090 versus 3,137 mL·min−1), suggesting greater aerobic exercise capacity. These findings may reflect that the reduced elastic load on the inspiratory muscles served to redistribute blood flow to the quadriceps muscles, thereby optimizing exercise capacity.24,25 Notably, at this time point, although minute ventilation was similar (80 versus 81 L·min−1), his breathing pattern was characterized by greater tidal volumes and lower respiratory rate. These changes are consistent with those seen during loaded breathing tasks following a program of IMT.3
Limitations
The limitations of this case report reflect that the management of this patient was guided by clinical needs rather than a research protocol. Following the results of the CPET after IMT, the patient declined further assessment (eg, Pimax). The decision was made not to pursue these measures, as he had met his goals for therapy and further assessment would not have changed his clinical management. This is a shortcoming of our case report and, together with the limitations inherent in a case report, we accept that the changes described cannot be confidently attributed to the IMT program.
As a constant power CPET was not undertaken, we cannot comment on the change in breathing pattern variables at identical work rates. Furthermore, pressure-volume loops26 would have provided more information regarding the mechanical limitations resulting from reduced flow during the baseline CPET. The other important limitation relates to the 3-month interruption during his training period and the lack of supervision of all IMT sessions by a physical therapist, both of which were unavoidable.
Nevertheless, given that IMT was introduced 12 months following the injury, at which point his progress had reached a plateau with other rehabilitation strategies, it seems reasonable to attribute the increased maximum forced inspiratory flow recorded at rest and change in the locus of symptom limitation during exercise from the dyspnea to leg fatigue to the introduction of IMT. These data should be seen as hypothesis-generating.
Clinical Implications and Future Research
The flow-volume loops recorded during the CPET allowed us to identify inefficiencies in breathing strategy and respiratory mechanics that were responsible for this patient's intolerable dyspnea during exercise. Notably, the measurement of Pimax was of no diagnostic value and was used only to prescribe the initial load for IMT. These findings suggest that static measures of the pressure-generating capacity of the inspiratory pump muscles convey little information about how they operate under dynamic conditions such as exercise. In this patient, chest fluoroscopy and the CPET with flow-volume loops provided the most useful diagnostic information. A program of high-intensity IMT should be considered for individuals who demonstrate inspiratory flows during exercise that approximate the maximum inspiratory flow recorded at rest. Further study is needed to confirm the role of IMT in people with diaphragm injury.
Medical Graphics Corp, 350 Oak Grove Pkwy, St Paul, MN 55127.
Philips Home Healthcare Solutions, 65 Epping Rd, North Ryde, New South Wales 2113, Australia.
Sportstek Physical Therapy Supplies Pty Ltd, 6 Park Rd, Oakleigh, Victoria 3166, Australia.
References
Author notes
All authors provided concept/idea/research design, writing, facilities/equipment, and consultation (including review of manuscript before submission). Dr Gain, Mr McKay, Ms Nathan, and Dr Gabbay provided data collection. Dr Hill, Dr Gain, and Ms Nathan provided data analysis. Mr McKay provided the patient and institutional liaisons.




Comments