Anatomy and Physiology of Metabotropic Glutamate Receptors in Mammalian and Avian Auditory System
*Corresponding Author(s):
Yong LuDepartment Of Anatomy And Neurobiology, Northeast Ohio Medical University, Ohio, United States
Tel:+1 3303256656,
Email:ylu@neomed.edu
Abstract
Glutamate, as the major excitatory neurotransmitter used in the vertebrate brain, activates ionotropic and metabotropic glutamate receptors (iGluRs and mGluRs), which mediate fast and slow neuronal actions, respectively. mGluRs play important modulatory roles in many brain areas, forming potential targets for drugs developed to treat brain disorders. Here, we review studies on mGluRs in the mammalian and avian auditory system. Although anatomical expression of mGluRs in the cochlear nucleus has been well characterized, data for other auditory nuclei await more systematic investigations especially at the electron microscopy level. The physiology of mGluRs has been extensively studied using in vitro brain slice preparations, with a focus on the auditory circuitry in the brainstem. These in vitro physiological studies have demonstrated that mGluRs participate in synaptic transmission, regulate ionic homeostasis, induce synaptic plasticity, and maintain the balance between Excitation and Inhibition (E/I) in a variety of auditory structures. However, the modulatory roles of mGluRs in auditory processing remain largely unclear at the system and behavioral levels, and the functions of mGluRs in auditory disorders remain entirely unknown.
Keywords
ABBREVIATIONS
AC: Auditory Cortex
AVCN: Anteroventral Cochlear Nucleus
CN: Cochlear Nucleus
DCN: Dorsal Cochlear Nucleus
EPSC/P: Excitatory Postsynaptic Current/Potential
GABABR: GABAB Receptor
GIRK: G-Protein- Coupled Inward Rectifier K+
HF: High-Frequency
IC: Inferior Colliculus
IGluR: Ionotropic Glutamate Receptor
IHC: Inner Hair Cell
IPSC/P: Inhibitory Postsynaptic Current/Potential
LF: Low Frequency
LSO: Lateral Superior Live
LTD/P: Long-Term Depression/Potentiation
MF: Middle-Frequency
MGB: Medial Geniculate Body
mGluR: Metabotropic Glutamate Receptor
MNTB: Medial Nucleus of Trapezoid Body
MSO: Medial Superior Olive
mRNA: Messenger Ribonucleic Acid
NA: Nucleus Angularis
NL: Nucleus Laminaris
NM: Nucleus Magnocellularis
OHC: Outer Hair Cell
PVCN: Posteroventral Cochlear Nucleus
SON: Superior Olivary Nucleus
VCN: Ventral Cochlear Nucleus
VGCC: Voltage-Gated Ca2+ Channel
INTRODUCTION
mGluRs were discovered more than 30 years ago [1-3]. To date, eight members of mGluRs have been identified and divided into three major groups (group I: mGluR1 and 5; group II: mGluR2 and 3; and group III: mGluR4, 6, 7, and 8) based on their amino acid sequence, pharmacological properties, and signaling transduction pathways [4]. mGluRs are expressed widely in the peripheral and central nervous system, exhibit a high degree of homology across different animal species, and exert neuromodulatory actions via multiple signaling pathways [5-7]. Group I mGluRs are predominantly expressed at postsynaptic loci and are coupled primarily to Gq/G11 proteins associated with stimulation of the phospholipase C pathway. Group II and III mGluRs are predominantly expressed at presynaptic loci and are coupled to Gi/Go proteins associated with inhibition of the adenylyl cyclase pathway. Because mGluRs play modulatory roles in various physiological and pathological conditions, mGluRs have been implicated in multiple brain disorders [8,9] and drugs targeting mGluRs have been tested via clinical trials to treat schizophrenia [10] and autism [11]. Unfortunately, no successful clinical trials have been yielded yet. While this review is focused on mGluRs in the auditory system of both mammals and birds, readers are referred to several excellent reviews on the general topics of mGluRs [4,6,9,12-16].
It is conceivable that mGluRs are involved in auditory information processing, considering that glutamate is used as the major excitatory neurotransmitter from the peripheral hearing organ (the cochlea) all the way up to the Auditory Cortex (AC), and that mGluRs have been found to be expressed throughout the auditory nervous system. Understanding the anatomy (expression and distribution) and physiology of mGluRs at each level of the auditory system will not only provide a deep understanding of modulatory mechanisms that underlie auditory processing, but may also help design potential therapeutic approaches targeting mGluRs for the treatment of hearing disorders. Here, we will review the anatomical expression and physiology of mGluRs in the mammalian as well as the avian auditory system, with a focus on the subcortical structures.
OVERVIEW OF MGLURS EXPRESSION AND PHYSIOLOGICAL METHODS
Table 1 summarizes the anatomical data on mGluR expression in the mammalian auditory system, based on two primary sources. First, data are available from anatomical studies that examined expression of mGluRs throughout the whole brain [17-22]. Because the auditory system is not the focus, the data on mGluR expression in these studies lack details for mGluRs in auditory structures.
|
Structure |
mGluR Group |
mGluR I |
mGluR II |
mGluR III |
References |
|||||||
|
|
I |
II |
III |
1 |
5 |
2 |
3 |
4 |
6 |
7 |
8 |
|
|
Cochlea |
yes |
n.d. |
yes |
(+) |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
++ |
n.d. |
[34,35] |
|
CN |
||||||||||||
|
AVCN |
Yes |
Yes |
Yes |
++ |
n.d. |
++ |
n.d. |
(+) |
n.d. |
(+) |
n.d. |
[20-30]
|
|
PVCN |
Yes |
Yes |
Yes |
++ |
n.d. |
++ |
n.d. |
(+) |
n.d. |
(+) |
n.d. |
|
|
DCN |
Yes |
Yes |
Yes |
++ |
+/- |
++ |
+? |
(+) |
n.d. |
(+) |
n.d. |
|
|
SOC |
||||||||||||
|
MNTB |
Yes |
Yes |
Yes |
++(#) |
n.d. |
++(§) |
++(§) |
++(†) |
n.d. |
n.d. |
n.d. |
[31,36,37] |
|
LSO |
n.d. |
yes |
n.d. |
n.d. |
n.d. |
+? |
+? |
n.d. |
n.d. |
n.d. |
n.d. |
[38] |
|
Others |
n.d. |
n.d. |
n.d. |
|
|
|
|
|
|
|
|
|
|
IC |
Yes |
n.d. |
Yes |
+ |
+ |
n.d. |
n.d. |
+? |
(+) |
+? |
+? |
[39-41] |
|
MGB |
Yes |
Yes |
n.d. |
++ |
n.d. |
+? |
+? |
n.d. |
n.d. |
n.d. |
n.d. |
[42-44] |
|
AC |
n.d. |
Yes |
n.d. |
n.d. |
n.d. |
+? |
+? |
n.d. |
n.d. |
n.d. |
n.d. |
[33,45] |
n.d.: No data
++: Positive protein expression detected by immunohistochemistry or western blot
(+): Positive mRNA expression detected by RT-PCR
-/+: Weak or no expression
?+: Unknown expression of specific mGluR member
#: Postsynaptic expression only
§: Both presynaptic and postsynaptic expression
†: Presynaptic expression only
An exception is the expression of mGluRs in the Cochlear Nucleus (CN), which has been well studied [20,21,23-30]. Second, the expression of mGluRs in a number of auditory structures are available from physiological studies where anatomical data were used to support the physiology [31-33]. Generally, these studies did not provide in-depth details about the anatomy of mGluRs. Therefore, the anatomical data of mGluRs in the auditory system are far from being complete. One general impression, however, is that mGluRs are extensively expressed in cells at each and all levels of the auditory system. More systematical investigation of the expression of mGluRs in the auditory system is warranted for the future, both at the light and electron microscopy levels.
Physiological studies on mGluRs in the central auditory system have been performed mostly using in vitrobrain slice preparations. The use of such a method allows for stable and reliable intracellular whole-cell recordings for long periods of time [46,47], rending studies of both short- and long-term effects of mGluRs on neuronal properties possible. This approach also allows for application of pharmacological agents at known concentrations, which is important for studying the effects of mGluR agonists and antagonists, considering their concentration dependent specificity [6]. The physiological results reviewed below are mostly obtained using whole-cell recordings. Some of the experiments were also performed using an advanced patch recording method, perforated patch recording. Because perforated patch recording usually can better preserve the intracellular signaling molecules [48-50] it is a critical method when examining the effect of mGluRs in large postsynaptic cells, because under whole-cell recording mode, one could potentially wash out intracellular signaling molecules that are required for G protein-coupled receptors to function [51-53]. The disruption of the signaling pathway would consequently lead to observation of false-negative results. Supporting this notion, we recently observed that activation of mGluRs enhanced high voltage-gated K+ channel currents in timing coding neurons in the auditory brainstem. Critically, the modulation was only detected under perforated patch recording but not under conventional whole-cell recording [54]. This emphasizes the need and importance of using perforated patch recording to examine mGluR effects on postsynaptic properties, especially when negative results are observed by using conventional whole-cell recordings.
MGLURS IN MAMMALIAN PERIPHERAL HEARING ORGAN (THE COCHLEA)
Glutamate is the main excitatory transmitter used at the synapses between hair cells and spiral ganglion neurons, and both iGluRs and mGluRs are expressed in the cochlea [38,55]. Of group I mGluRs, messenger RNA (mRNA) for mGluR1 has been detected in both type I and type II ganglion neurons in mammalian cochlea of the rat and guinea pig [34]. Anatomical data on group II mGluRs in the cochlea are lacking, although a physiological study suggested that group II mGluRs are expressed on the efferent lateral olivocochlear GABAergic fibers in the guinea pig cochlea [56]. Of group III mGluRs, mGluR7, is expressed in both Inner and Outer Hair Cells (IHCs and OHCs), as well as in spiral ganglion cells in mouse and human tissues [35]. The expression of mGluR7 exists at birth (Postnatal day 1, P1) and persists into maturation (P21 and adult) in the mouse cochlea [35].
Of the mGluRs shown to express in the cochlea, physiological evidence for involvement of group I mGluR1 and group III mGluR7 in neurotransmission at the synapses between hair cells and the dendrites of spiral ganglion neurons, as well as mGluR II on the efferent input to IHCs, has been reported. Group I and II mGluRs are involved in modulation of neurotransmission at the lateral olivocochlear efferent system (Figure 1). An excitatory action mediated by group I mGluRs on the afferent terminals of spiral ganglion cells has been demonstrated. Application of DHPG, an agonist for group I mGluRs, increases the spike firing in spiral ganglion neurons [57,58], followed by an increase in intracellular Ca2+ concentration [59]. Consistently, ACPD (Aminocyclopentane-1,3-dicarboxylic acid), an agonist for group I and II mGluRs, produces an inward current under voltage clamp, and causes membrane depolarization and spiking under current clamp in spiral ganglion neurons [59]. These studies indicate that group I mGluRs are located postsynaptically on the IHC afferents of spiral ganglion neurons. The excitatory effects of group I mGluRs in the cochlea generally last longer than those mediated by iGluRs. This suggests that mGluRs do not mediate the fast neurotransmission in the cochlea where a high speed of transmission is essential for transduction of acoustic signals, but instead may be more important for enhancing the cellular excitability of spiral ganglion neurons under high frequency inputs.
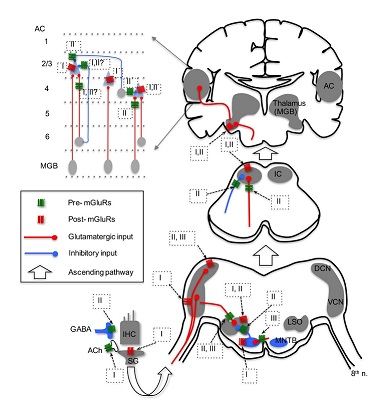
In contrast with postsynaptic excitation by group I mGluRs, modulation by group I and II mGluRs on the efferent system has been reported. Group I mGluRs, specifically mGluR1, enhance acetylcholine release from the efferent terminals, which in turn activate nicotinic receptors and subsequently SK2 channels, resulting in increased inhibition in IHCs [60]. More importantly, this enhanced inhibition in IHCs could be mediated by glutamate released from IHCs themselves, forming a feedback control mechanism on IHC excitability. In vitro microvolume superfusion on guinea pig cochlea preparations shows that activation of group mGluRs, but not group I and III mGluRs, increases dopamine release at the dopaminergic synapses of the lateral olivocochlear system [56]. Consequently, dopamine can activate D2 receptors on IHC afferents, and may lead to protective effects against excitotoxicity caused by excessive glutamate release from the IHCs in response to noise exposure. It is believed that the mechanism for increased dopamine release is via a sequence of modulatory actions at multiple synapses, including activation of group II mGluRs on GABAergic terminals, which suppresses GABA release, leading to disinhibition of dopaminergic terminals innervating IHC afferents [56]. Given the complexity of this hypothetical pathway, physiological recordings such as patch recordings from the IHC afferents are needed to confirm the direct and indirect actions of mGluRs on GABA and dopamine release. Physiological data on group III mGluRs in the cochlea are lacking, although there is evidence that mGluR7 variants are involved in conferring susceptibility to age-related hearing loss [35]. Because mGluR7 is expressed at both pre- and postsynaptic loci at the synapses between hair cells and spiral ganglion neurons [35], the protein may provide protection for ganglion neurons against glutamate excitotoxicity via inhibition of glutamatergic transmission in the cochlea.
Because group I and the other two groups of mGluRs have opposite effects on excitatory transmission at IHC synapses, one would expect that antagonists for group I and agonists for group II or III mGluRs may have the same effect to protect against excitotoxicity. The idea of using antagonism for one group of mGluRs and agonism for other groups of mGluRs to achieve the same purpose reflects the complexity of mGluR mediated actions, and the highly likelihood of cross-talks among different mGluR members [61]. Indeed, even for the same group mGluRs, there seem to be conflicting proposals regarding whether and how they provide protection of the cochlea against acoustic damage. For instance, protective effects by mGluR1 antagonism against pathological conditions were proposed based on the observation that mGluR1 level was up-regulated in response to excitotoxic insults [62]. In contrast, blockage of group I mGluRs in the cochlea reduces the amplitude of compound action potentials in response to loud sound without altering the hearing threshold [59]. This suggests that antagonism, rather than up-regulation of group I mGluRs, may provide protective effects to the cochlea against acoustic trauma.
MGLURS IN MAMMALIAN CENTRAL AUDITORY SYSTEM
Cochlear Nucleus (CN) - Among the auditory structures, the CN, especially the dorsal cochlear nucleus [63], is the most extensively studied in terms of mGluRs expression [64]. Of the two group I subtypes, the expression of mGluR1 in the CN has been detected in mice [26], rats [24,25], guinea pigs [23], and bats [28]. At both the mRNA and protein levels, expression of mGluR1 varies among different divisions of the CN, as well as among different cell types even within the same division [23,24,26]. The overall expression level of mGluR1 is strongest in the DCN, moderate in the Anteroventral CN (AVCN), and weak in the Posteroventral CN (PVCN) [17,25,26,28] Within the DCN of the rodent, mGluR1 is strongly expressed in unipolar brush cells and cartwheel cells [23,30], and is most commonly observed in postsynaptic compartments of DCN neurons [23,24]. Although mGluR1 is absent in granule cells in the molecular and fusiform cell layers [26], it is detected on terminal axons in the molecular layer and small cell shell of the DCN [26], suggesting possible presynaptic actions mediated by mGluR1. Although data on subcellular distribution of mGluRs in the auditory system are largely lacking, a few studies utilizing electron microscopy have reported that mGluR1 is expressed in the basal dendrites (receiving auditory nerve inputs), but not in the apical dendrites (receiving parallel fiber inputs) in DCN fusiform cells [65,66]. In the Ventral CN (VCN), mGluR1 is strongly expressed in globular bushy cells and stellate cells [26]. The expression of mGluR5 is weak or non-detectable in the DCN [24], and its expression in the VCN is not known. Among group II subtypes, mGluR2 expression was detected in the DCN of the rat and mouse [21], with strong expression in the granular domain. The cell types expressing mGluR2 in the DCN seem to be the unipolar brush cells and Golgi cells [29,44] which is similar to expression of mGluR2 in the cerebellum [21]. In addition, expression of mGluR2 was observed in both the soma-dendritic domain and the axonal domain in the DCN [21,44], suggesting that mGluR2 may exert both pre- and postsynaptic modulation on CN neurons. The mGluR2 immunoreactivity is also present in the VCN in sparsely scattered cells [21], which are presumptive stellate cells with thin dendrites. No data are available for the expression of mGluR3 in the CN. Compared to group I and II mGluRs, data are much less available about the expression of group III mGluRs in the CN. A low level of mGluR4 mRNA and a moderate level of mGluR7 mRNA was observed in the CN of the rat [20]. Among the few studies that investigated protein expression of mGluR7 in the CN, results are not consistent. [22] reported positive expression of mGluR7 in the DCN of the rat, whereas [27] did not detect immunoreactive labelling for mGluR7 in the same animal species. Because these studies examine the expression of mGluRs in the whole brain without a focus on the auditory system, firm conclusions on the expression of group III mGluRs in the CN cannot yet be drawn.
While a relatively large number of studies have focused on the expression of mGluRs in the CN (Table 1), only a few studies have examined the physiological roles of mGluRs using in vitro brain slice preparations [29,67-69] (Figure 1). In the CN, one study, using field potential recordings in guinea pig brain slices, discovered that all three groups of mGluRs appear to depress the field responses evoked by electrical stimulation of the parallel fibers in the DCN [67]. Using whole-cell patch-clamp recording from mouse brain slices, [29] found that activation of group II mGluRs in Golgi cells generates a membrane hyperpolarization, which is mediated by the activation of G-Protein-Coupled Inward Rectifier K+ (GIRK) channels. Importantly, a novel long-term plasticity mediated by mGluRs was discovered in the mouse DCN [68], which is unusual for brain stem circuits. In this study, both of group II and III mGluRs, but not group I mGluRs, presumably contribute to the induction of both Long-Term Potentiation (LTP) and Long-Term Depression (LTD) of Excitatory Postsynaptic Currents (EPSCs) in fusiform and cartwheel cells in the DCN. Interestingly, such long-term plasticity is pathway-specific. The plasticity is induced for the parallel fiber multisensory pathway, but not the auditory fiber nerve pathway [68]. Such a bidirectional synaptic plasticity (both LTP and LTD) induced by mGluRs in the same neurons suggest that mGluRs may contribute to auditory learning. In the VCN, even though the expression of mGluR5 is not known and its expression in DCN is weak [24], a study clearly showed that pharmacological activation of group I mGluRs (primarily mGluR5) can depolarize bushy cells in the AVCN, and more importantly tonic activity of group I mGluRs is present [68]. Thus, it is proposed that group I mGluRs facilitate the excitability of bushy cells to counteract presynaptic inhibition mediated by GABAB Receptors (GABABRs) [69].
Studies using in vivo recording approaches to examine the physiological roles of mGluRs in auditory processing are quite few. To date, there is only one in vivo study on mGluRs done in the CN of the cat and gerbil at the systems level [70]. In this study, bidirectional modulation of neuronal firing in the DCN by iontophoretic application of generic mGluR agonists (ACPD or CCG) was observed [70]. The varying effects of mGluRs on the auditory responses of DCN neurons could be attributed to the presence of multiple subtypes of mGluRs on different loci of varying cell types. Further studies combining mGluR drugs that target specific mGluR members and recordings from identified cell types should be performed. The difficulty of distinguishing the anatomically intermingled multiple cell types in the CN may be reduced dramatically with the development of new tools, such as an optogenetic approach that allows activation of specific cell types using light stimuli.
Medial Nucleus of Trapezoid Body (MNTB) - Expression of mGluRs in the MNTB has been reported, with varying receptor locations depending on the subtype of mGluRs. mGluR1 is found to be exclusively expressed in MNTB neurons in the rat [31]. These receptors are presumably located on the postsynaptic membrane that is opposite to presynaptic cannabinoid type 1 receptors [31].
Immunoreactivity for mGluR2/3, members of group II mGluRs, was present at both presynaptic and postsynaptic domains of MNTB neurons in the rat [37]. Group II mGluRs are also present on glial cells exclusively during postnatal development [37]. Among members of group III mGluRs, mGluR4, is identified at the presynaptic glutamatergic terminals innervating MNTB principle neurons in the rat. The mGluR4 expression is highly developmentally regulated, with strong activity in a short period (P7-12) before hearing onset (at around P13) [36]. These results suggest that group II mGluRs and the group III mGluR4 may play a role in the development of synapses in the MNTB. Whether group I mGluRs in the MNTB experience similar developmental change is not known.
Activation of postsynaptic mGluR1 in MNTB neurons induces the release of endocannabinoids, which in return activate type 1 cannabinoid receptors on the presynaptic terminal (calyx of Held), resulting in inhibition of Ca2+ channels and subsequent suppression of glutamate release [31] (Figure 1). Besides mediating such retro-suppression of neurotransmission, activation of group I mGluRs modulates the phosphorylation of Kv3.1b [71], which is a critical factor defining the ability of MNTB neurons to follow high frequency inputs [72]. Physiological function of group II mGluRs in MNTB is not known. However, because group II mGluRs are expressed at both pre- and postsynaptic loci in MNTB [37], it is conceivable that modulation of glutamatergic transmission via actions on both sites exists. Activation of group III mGluRs has been shown to reduce EPSC amplitude in MNTB neurons [73]. Interestingly, blocking the endogenous activity of group III mGluRs with antagonists does not change excitatory synaptic strength. Rather, it lowers release probability and increases the size of the readily releasable pool [73]. This raises an intriguing possibility that, in the auditory brainstem, endogenous activity of mGluRs may change the synaptic state even when modulation of synaptic strength by antagonism of mGluRs is not detectable.
Lateral Superior Olive (LSO) - In the LSO of the rat, group II mGluRs (mGluR2/3) are most highly expressed during early developmental age (P4), and the expression diminishes days after the onset of hearing [32]. The loci of group II mGluRs in the LSO are not known, although physiological data suggest that group II mGluRs are localized at both presynaptic glutamatergic and glycinergic terminals [32,74], as well as postsynaptic loci [75,76]. Consistent with anatomical evidence that the expression of mGluRs peaks during development and diminishes after maturation [32], the physiological role of mGluRs in the LSO has been detected mainly in developing neurons (Figure 1). The application of ACPD, the non-specific mGluR agonist, depolarizes LSO neurons in P8-14 gerbils, and the effect can last ~20 min [75]. Further calcium imaging studies show that activation of group I and II, but not III, mGluRs increases intracellular Ca2+ concentration in developing LSO neurons (P0-4) [76], but the response amplitude declines over the period of hearing development (from P0 to P20) [77]. The group I mGluR-mediated Ca2+ signaling is partially due to Ca2+ influx through transient receptor potential-like channels in response to activation of mGluR1 [78]. The effects of mGluRs observed in these studies [75-77] are attributed to activation of postsynaptic mGluRs, because the parameters measured are properties directly obtained from postsynaptic cells. Modulatory effects of presynaptic mGluRs at LSO synapses have been reported (Figure 1). Modulation by group II mGluRs of the inhibitory glycinergic transmission at the MNTB-LSO synapse has been also reported [32]. It is proposed that glutamate molecules, released from VCN neurons at the LSO, spill over and activate mGluRs on MNTB terminals innervating LSO neurons, producing heterosynaptic modulation. This modulation diminishes gradually in early development and is not detectable just a few days after hearing onset [32], which suggests that mGluRs may play an important role in the development of this synapse. Further, the non-specific mGluR agonist ACPD inhibits Excitatory Postsynaptic Potentials (EPSPs) of LSO neurons in P14-22 rats, and at least group III mGluRs are involved [74]. Overall, mGluRs modulate the function of the LSO in development across multiple animal species.
Inferior Colliculus (IC) - Using Real-Time-PCR method for detection of mRNA for group I mGluRs (mGluR1, 5), it is shown that the expression level of these mGluRs is developmentally down regulated in the rat IC [40] and overlapped with Transient Receptor Potential (TRPC1) channels [41]. All members of group III mGluRs (mGluR 4, 6, 7, and 8) are also detected in the rat IC [39]. Although it is believed that mGluR6 is expressed exclusively in the retina [79], mGluR6 is present in the IC tissues [39]. Consistently, expression of mGluR6 in non-retinal neural tissues has also been reported [80,81]. The IC is a hub of the central auditory system, which receives ascending and descending inputs from many auditory nuclei. In addition, the IC is divided into multiple sub-nuclei, each of which contains multiple cell types with distinct morphological and intrinsic properties. Based on the currently available data, it is largely unknown which areas and cell types in the IC express which mGluRs.
A few studies have examined the physiological role of mGluRs in the IC, with a focus on the central part of the IC (Figure 1). MCPG (Methyl-4-Carboxyphenylglycine), a non-specific antagonist mGluRs, increases the gain in action potential firing of IC neurons [82]. Consistently, antagonism of group II mGluRs also increases firing of IC neurons in vivo [83], suggesting suppression of neuronal excitability by endogenous activity of group II mGluRs. Using whole-cell patch recordings in brains slices, [84] showed that activation of presynaptic group II mGluRs suppresses both EPSCs and Inhibitory Postsynaptic Currents (IPSCs) in IC neurons. In contrast, agonists for group I or III mGluRs do not affect synaptic and intrinsic properties [84]. However, the negative results on group I mGluRs in this study appear to conflict with those reported by other research groups. For example, activation of group I mGluRs induces an increase in intracellular Ca2+ concentration in IC neurons [40], and iontophoretic application of group I mGluR agonists increases firing rate of IC neurons in vivo [83]. However, because iontophoretic application does not allow one to precisely estimate the concentration of drug [85], alternative approaches that apply drugs with known concentration such as pressure ejection may be utilized in future studies. Finally, anticonvulsant actions of mGluRs, especially by group III mGluRs in genetically epilepsy-prone animals have been reported [39,86], raising the possibility that drugs targeting mGluRs could be used to treat audiogenic seizures.
Auditory thalamus (the Medial Geniculate Body-MGB) - There are very limited data on mGluR expression in the MGB from a few papers that examined mGluR expression in the rat brain. Protein expression of the group I mGluR1 was detected in the MGB, and seems to be present at postsynaptic cells only [41,43]. Furthermore, the expression of group II mGluRs is present in the MGB, as detected with an antibody against mGluR2/3 [44]. Very few studies have examined the physiology of mGluRs in the MGB. The application of the non-specific mGluR agonist ACPD causes a depolarizing effect in MGB neurons recorded in slice preparations [87,88]. mGluR-mediated responses in the thalamus were observed via the descending glutamatergic input only [89,90], suggesting that the effect may be mediated via the descending input from the AC, but not from the ascending input from the IC. Because ACPD is a non-specific mGluR agonist, it is unclear which member and group of mGluRs is involved in the response. Based on the concentration of ACPD (50 µM) and its EC50 on mGluRs (105-170 µM for mGluR1, and 5 µM for mGluR2), however, the authors suggested that both mGluR1 and mGluR2 might be involved [87] (Figure 1). It is worth pointing out that while the ascending input from the IC via the lemniscal pathway to MGB neurons does not activate mGluRs, the ascending input from the IC via the non-lemniscal pathway to the dorsal part of the auditory thalamus does involve a mGluR1 component [91]. Further works are required to examine if and how mGluRs modulate synaptic transmission in the auditory thalamus.
Auditory Cortex (AC) - Among three groups of mGluRs, at the light microscopy level, group II mGluRs (mGluR2/3) are expressed in various neocortical regions including the AC, and these mGluRs co-localize with vesicular glutamate transporter 2 [33,45], which suggests the presence of presynaptic group II mGluRs on the excitatory inputs in the AC.
The AC is composed of six structurally distinct layers, and there exist multiple cell types in a single layer. Neurons in the AC receive ascending inputs primarily from the auditory thalamus, and form intracortical circuits among different layers. The function of mGluRs in the AC has also been [92]. Involvement of mGluRs in neuromodulation of the AC circuits is dependent on the input pathways (Figure 1). Bidirectional regulation by mGluRs of synaptic transmission and cellular excitability in pyramidal neurons in layers 2 and 3 has been reported. Presynaptic mGluRs activated by ACPD suppress both EPSPs and Inhibitory Postsynaptic Potentials (IPSPs) in response to stimulation of the synaptic inputs originating from layer 6 of the AC. The involvement of mGluR component in the excitatory responses of pyramidal cells are cell type-dependent, in that a group I mGluR component exists in class 2 cells (modulator neurons) but not in class 1 cells (driver neurons) [93]. Stimulation of the afferents at high frequency (125 Hz) but not low frequency (10 Hz) causes activation of group I mGluRs in class 2 cells [93], consistent with the idea that mGluRs may be activated only under conditions of strong and repetitive sensory inputs. Group II mGluRs also modulate GABAergic input to layers2/3 cells from layer 4 [94]. In addition, activation of postsynaptic group I mGluRs in layers 2 and 3 fast spiking neurons increases cellular excitability [95,96].
Modulation by group I mGluRs of the excitatory responses of pyramidal cells in layer 4 depends on the source of the synaptic inputs. For instance, there is no mGluR component in the EPSPs of layer 4 pyramidal neurons when the thalamocortical pathway to layer 4 is activated [97], however, activation of the intracortical pathway from layer 6 depolarizes layer 4 neurons via group I mGluRs [98]. Interestingly, activation of postsynaptic group II mGluRs by glutamate release from the layer 6 input produces an inhibitory effect in layer 4 cells via activation of downstream target GIRK channels [45]. Therefore, two different mGluRs (group I versus II here) can be activated by the same pathway (e.g., layer 6 input), and generates exactly the opposite effects on cellular excitability, providing bidirectional regulation and thus maintenance of homeostasis of excitability. Similarly, at the synapses formed between the primary and the secondary AC, postsynaptic group I mGluRs facilitate while group II mGluRs inhibit cellular excitability of neurons. This effect occurs only for neurons that express small but not large EPSPs [99], indicating synapse-specific activation of mGluRs. In addition, group II mGluRs strongly inhibit excitatory transmission of the thalamocortical pathway via presynaptic action [33], and modulate GABAergic input to layer 4 [94]. Similarly, involvement of mGluRs in long-term synaptic plasticity in layer 4 neurons is also input pathway dependent. Application of the nonspecific mGluR antagonist MCPG did not affect Long-Term Plasticity (LTP or LTD of field EPSPs) of excitatory transmission from layer 6 to layer 4 cells, suggesting the absence of mGluR involvement in long-term plasticity at this synapse [100]. In contrast, group I mGluRs are required for the induction of LTD and LTP of EPSCs in layers 3 and 4 cells when the thalamocortical pathway is activated [101,102]. No mGluR component is found in the excitatory responses from the thalamic inputs to layers 5 and 6 [103].
Overall, the physiological roles of mGluRs in the AC are complicated, due to that mGluR-mediated modulation depends on the combination of a number of factors including the sources of synaptic inputs, the cell types, and the location of the cells. Future experiments need to take these issues into consideration and tease out the functions of mGluRs at various synapses in order to better understand the roles of mGluR modulation in AC circuits.
MGLURS IN AVIAN AUDITORY BRAINSTEM
The chicken auditory brainstem has been used for decades as an excellent model for studying mechanisms underlying auditory signal processing [104-106]. Because of the specialized anatomy and well-characterized functions, the auditory nuclei in this model system provide a unique opportunity to study mGluR-mediated modulation in specific functional auditory circuits (Figure 2). After entering the brainstem, the auditory nerve (8th n.) branches and innervates the cochlear Nucleus Angularis (NA) and Nucleus Magnocellularis (NM), two subnuclei of the cochlear nucleus in birds. Bilateral projections from the NM innervate the Nucleus Laminaris (NL). The NM and NL are involved in coding temporal information of sound, whereas the NA is primarily involved in coding sound intensity among other features. All three nuclei (NM, NL, and NA) receive feedback inhibition from the ipsilateral Superior Olivary Nucleus (SON), which receives its excitatory inputs from the NL and NA. These auditory nuclei are structurally distinct and easily identified in brain slice preparations. More importantly, the synaptic connections among these nuclei are largely intact in brain slice preparations. These distinct features make the avian auditory brainstem an excellent model to study the physiological roles of mGluRs in the CNS. In addition, the avian auditory brainstem provides a more feasible model as compared to the mammalian system, because of the relatively high homogeneity of cell types and the ease of identification of cells in slice preparations.
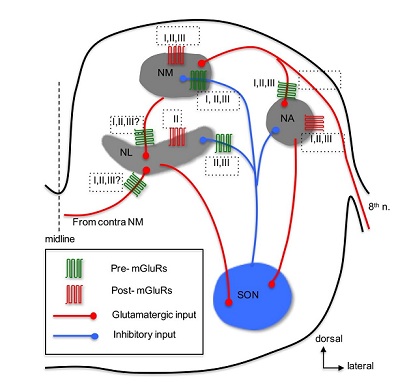
Anatomical expression of mGluRs in the avian auditory system has been reported in a few studies. Immunohistochemistry has revealed expression of group I mGluRs (both mGluR1 and mGluR5) in NM neurons [107], group II mGluRs in NM [108], NL [109,110], and groups and III mGluRs in NA neurons [111]. There are yet no anatomical data identifying presynaptic versus postsynaptic loci of mGluRs in the avian auditory brainstem. About 10 years after mGluRs discovered, Rubel and colleagues [112] discovered that activation of mGluRs increases phosphatidylinositol metabolism in NM neurons. Following up this study, mGluR-mediated regulation of Ca2+ signaling in NM neurons was extensively studied primarily by the same research group [113-120]. The key findings from these studies are that mGluRs, especially group I mGluRs, play critical roles in regulating Ca2+ signaling and maintaining Ca2+ homeostasis and cell survival in NM neurons [121]. Recently, we extended these previous studies by examining synaptic activity-induced Ca2+ signaling and found that glutamate release can induce an increase of intracellular Ca2+ concentration by activation of mGluRs in NM neurons [122]. Maintenance and survival of NM neurons depend on their afferent excitatory input from the auditory nerve [104]. Another parallel line of research by Hyson and colleagues has focused on mGluR-mediated regulation of protein synthesis in NM neurons. They found that deprivation of the auditory nerve input disrupts protein synthesis in NM neurons [123]. Further studies have shown that this activity-dependent regulation of protein synthesis relies on activity of mGluRs [124,125], but not involve iGluRs [126]. Specifically, group I and II mGluRs are required to maintain protein synthesis in NM neurons, because blocking either group I or II mGluRs eliminates activity-dependent regulation of ribosomes in vitro [127,128] as well as in vivo [129]. Together, these studies demonstrate the regulatory roles of mGluRs in Ca2+ signaling and protein synthesis, suggesting potential neuroprotective effects of mGluRs under both normal and abnormal hearing conditions.
1. NM- Previously a Ca2+ imaging study found that activation of postsynaptic mGluRs modulated Ca2+influx via Voltage-Gated Ca2+ Channels (VGCCs) in NM neurons [113]. Consistently, by using electrophysiological approaches that allow higher resolution in monitoring subtle changes in membrane Ca2+ currents, [130] characterized VGCC types using various blockers specific for different subtypes of VGCCs, and found that NM neurons possess both low- and high-threshold VGCCs, with N-type channels being dominant. Activation of mGluRs with agonists for selectively targeting individual subtypes of mGluRs reduces VGCC current [130], indicating that multiple mGluRs are present on postsynaptic NM neurons and are involved in regulating Ca2+ influx through membrane VGCCs.
Besides modulation of intrinsic properties by postsynaptic mGluRs, regulation of synaptic properties via presynaptic mGluRs has been commonly observed in the CNS [131]. Indeed, a series of studies examining mGluR-mediated modulation of synaptic transmission in the chicken auditory brainstem have been undertaken, with a focus on mGluR-mediated heteroreceptor modulation of the inhibitory inputs to NM and NL. The inhibitory input, primarily from the ipsilateral SON in the avian auditory brainstem neurons, bears an unusual feature in that it is depolarizing but potently inhibitory in NM [132-134] NL [135], and NA [136] neurons. The depolarized reversal potential of the inhibitory transmission in these neurons makes it possible that overdriving of the inhibitory afferent can generate GABA-induced action potentials [133,135,136]. Because such unusual spiking activity could disrupt phase-locking fidelity of the timing coding neurons in response to their excitatory inputs, multiple mechanisms have been discovered to prevent GABA-induced spiking. For example, regulation of the inhibitory synaptic strength in NM neurons is achieved partially by intrinsic low-threshold Kv conductance [134], by a feedback mechanism via presynaptic GABABRs [137], and by heterosynaptic inhibition of GABA release at NM through activation of presynaptic mGluRs [138]. Based on these results, we proposed “a dual modulation model” stating that mGluRs exert a tonic modulation of GABA release in NM neurons, while GABABRs provide a feedback mechanism limiting over-activation of the inhibitory system.
Interestingly, we discovered a novel form of heterosynaptic long-term synaptic plasticity, mGluR II-mediated Long-Term Depression (LTD) of GABAergic transmission in NM neurons [108]. This novel form of plasticity could serve several potential physiological functions, such as contribution to synaptogenesis and synaptic refinement during development, or in dendritic retraction after afferent deprivation. In mature animals, such LTD is proposed to be able to prevent spikes induced by over-activation of the GABAergic input, ensuring high fidelity of phase-locking and precise temporal processing of sounds. Unusually, mGluRs did not act as autoreceptors to modulate the excitatory transmission in NM [108,139]. However, whether there is mGluR-mediated modulation of EPSCs in Low-Frequency (LF) neurons needs to be further examined because these LF neurons are different from Middle/High-Frequency (MF/HF) neurons in terms of cellular morphology and physiology [140-142], and LF neurons have been generally excluded in our previous recordings for consistency of sampled cell populations.
While in vitro studies have established functional roles of mGluRs in modulation of intrinsic and synaptic properties in NM neurons, in vivo studies examining roles of mGluRs in temporal coding in NM neurons are completely lacking. Based on our observation that mGluRs control the inhibitory strength in NM neurons to prevent generation of GABA-induced spike that are not phase-locked to the excitatory input from the auditory nerve, we propose that mGluRs enhance the phase-locking fidelity of NM neurons and consequently improve coincidence detection in NL neurons.
NL - Given the similarities in neuronal properties between NM and NL neurons, it is not surprising that “the dual-modulation model” proposed for NM neurons also applies to NL neurons. Indeed, we confirmed this prediction by showing that both GABABRs and mGluRs modulate inhibitory input in NL neurons [135,143]. The difference in modulation between NM and NL is that group II and III mGluRs, but not group I mGluRs, are involved in the modulation at NL. Although we discovered a mGluR II-LTD in NM neurons, whether group II mGluRs induce LTD of GABA release in NL awaits further investigation. Although strong, the dual neuromodulation of the synaptic inhibition in NL neurons did not change the temporal profile of IPSPs evoked by stimulation of the afferent inputs at high rates [144]. This is critical for the tonic GABAergic inhibition to function as a gain control mechanism in sharpening coincidence detection window in NL neurons. Indeed, GABABRs reduce tonic inhibition via presynaptic modulation of GABA release [145], and we predict that mGluRs have similar effects on tonic inhibition because they, like GABABRs, reduce GABA release via presynaptic actions [135]. Taken together, we propose that mGluR-mediated modulation of synaptic inhibition in NL is highly potent and dynamic, ensuring proper inhibitory strength in binaural hearing processing.
Modulation of the excitatory transmission by mGluRs in NL neurons awaits further clarification, because the reported results from different research groups are not consistent. We found that neither GABABRs nor mGluRs modulated EPSCs in neurons sampled primarily from the MF and HF regions of NL in late embryos [135]. Okuda et al.,[109] showed that mGluRs (mainly group II and possibly group III) suppressed EPSCs of NL neurons in chick hatchlings in a graded manner along the frequency axis of NL, with a strong modulation occurred in LF neurons, less modulation detected in MF neurons, and even smaller modulation observed in HF neurons. Consistent with the physiology of mGluRs, a graded expression of group II mGluRs along the frequency axis of NL was observed [109]. The authors reasoned that the discrepancy between the two studies is due to the use of animals of different age, and they showed that the expression of mGluRs in late embryos was weak. Our more recent results showed that groups I and II but not III mGluRs suppressed glutamatergic transmission predominantly in LF NL neurons (data not shown). Identity of mGluRs modulating the excitatory input to NL needs further investigation.
Synaptic excitation in NL neurons is tonotopically distributed in a graded manner, with EPSCs becoming faster and larger with increasing tuning frequency [146,147]. Consistently, synaptic inhibition appears to be distributed in a graded manner, with fast IPSCs and minimal tonic inhibition in LF neurons but much slow IPSCs and strong tonic inhibition in MF/HF neurons [145,148,149]. Based on these findings, we hypothesized that neuromodulation is tonotopically distributed to improve synaptic integration at particular sound frequencies. Specifically, we propose that mGluR-mediated modulation of synaptic transmission in NL is tonotopically distributed in a manner complementary to the tonotopic distribution of synaptic excitation and inhibition. In other words, mGluRs modulate synaptic excitation in LF neurons and the modulation rapidly diminishes in MF/HF neurons, whereas mGluRs exert a graded modulation of synaptic inhibition across the frequency axis in NL. This complementary mode of neuromodulation could maintain a balance of synaptic excitation and inhibition in neurons across the frequency axis. Equally important, we recently reported tonotopically distributed modulation by mGluRs of intrinsic properties of NL neurons [54]. Using perforated patch clamp recordings, we found that activation of group II mGluRs enhanced the high threshold voltage-gated potassium (Kv) currents in NL neurons. The enhancement was also induced by synaptically released glutamate. The modulation was frequency-coding region dependent, being pronounced in low frequency neurons. The intracellular mechanism involved the Gβγ signaling pathway leading to activation of phospholipase C and protein kinase C. In perforated current-clamp recordings, the modulation strengthened membrane outward rectification, sharpened action potentials, and improved the ability of NL neurons to follow high frequency inputs, suggesting that group II mGluRs provides a feedforward modulatory mechanism to regulate temporal processing under the condition of heightened synaptic inputs.
In vivo physiological experiments examining the roles of mGluRs in the NL are of great significance but currently completely lacking. Based on the previous in vitro studies, mechanisms underlying mGluR-mediated modulation on coincidence detection and ITD coding in NL are likely to be highly dependent on frequency regions. In LF neurons, mGluRs may improve coincidence detection by sharpening the excitatory responses and regulating the strength of inhibitory input, whereas in MF/HF neurons, mGluRs may improve ITD coding primarily via controlling the inhibitory synaptic strength. It is plausible that mGluRs provide a gain control mechanism for ITD coding, resulting in neuronal adaptation in sound localization, similar to the GABABRs modulatory function in the MSO [150].
NA - In contrast to the relatively and extensively studied mGluR modulation in the avian timing coding pathway, i.e., NM and NL, the physiology of mGluRs in the intensity coding pathway starting with the NA are less understood. The majority of NA neurons are believed to be multi-functional including encoding intensity of sounds [151], and have large dynamic range (the range of sound intensity within which a neuron fires spikes between 5% and 95% of its maximal spike rate), suggesting of high sensitivity of NA neurons to changes in sound intensity [152]. Our recent study [111] showed that groups II and III mGluRs suppressed, while group I mGluRs enhanced the cellular excitability of non-onset firing NA neurons. Activation of all three groups of mGluRs with their respective agonists inhibited the glutamatergic transmission in NA neurons, in a cell type-independent manner and via presynaptic actions. Given that mGluRs are likely to be activated in response to repetitive stimuli at high intensity, mGluRs may play a role in shaping non-monotonic input-output functions in the NA. Therefore, mGluRs may improve intensity coding in NA neurons and consequently enhance the precision for sound localization using interaural intensity difference as a cue in higher order auditory nuclei.
In summary, postsynaptic mGluRs regulate Ca2+ signaling and protein synthesis in NM neurons and presynaptic mGluRs modulate synaptic transmission in NM and NL in a frequency region dependent manner. While modulation by mGluRs on synaptic inhibition is largely similar between NM and NL, modulation on synaptic excitation is more complex. Further, mGluRs in the avian auditory system have been intensively studied using in vitro methods, it is imperative to test how mGluRs affect auditory processing at the systems level using in vivo physiological methods.
Taken together, mGluRs in the avian auditory brainstem, similar to that in the mammalian lower auditory brainstem, modulate neuronal properties through both pre and postsynaptic mechanisms. However, a meaningful comparison of mGluR modulation between the avian auditory brainstem neurons (in NM and NL) and their mammalian counterparts (AVCN bushy cells and MSO) cannot yet be formed, because there are little data on mGluRs for mammalian bushy cells, and literally there are no data on mGluRs for the MSO. This again points to the need for further in-depth investigation of mGluRs in the auditory system across different animal models.
CONCLUDING REMARKS
In this article, we mainly reviewed the anatomy and physiology of mGluRs at cellular level in both mammalian and avian auditory systems. It remains a huge challenge to investigate the modulatory effects and mechanisms of mGluRs in particular cell types, especially at the system and behavior levels. Strategies utilizing an array of approaches in multiple animal model systems are required in order to tackle these issues and shed light on the roles of mGluRs in auditory processing. Many fundamental questions regarding the essential roles of mGluRs in auditory processing remain unanswered. Do mGluRs play critical roles in the development of auditory circuits? How do mGluRs modulate auditory processing at the systems level? Are mGluRs tonotopically distributed in the mammalian auditory system? If so, do they play a role in frequency tuning? Do mGluRs provide protection against hearing loss? Do mGluRs play potential roles in auditory disorders, such as tinnitus and deafness? Future investigations are warranted to tackle these important questions.
CONFLICT OF INTEREST
The author declares no competing financial interests.
ACKNOWLEDGEMENTS
The authors thank the current and former laboratory members for their great contributions. This work was supported by National Institute on Deafness and other Communication Disorders Grant R01 DC016054 (YL).
REFERENCES
- Sladeczek F, Pin JP, Recasens M, Bockaert J, Weiss S (1985) Glutamate stimulates inositol phosphate formation in striatal neurones. Nature 317: 717-719.
- Nicoletti F, Iadarola MJ, Wroblewski JT, Costa E (1986) Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: Developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A 83: 1931-1935.
- Nicoletti F, Wroblewski JT, Novelli A, Alho H, Guidotti A, et al. (1986) The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci 6: 1905-1911.
- Niswender CM, Conn PJ (2010) Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295-322.
- Ferraguti F, Shigemoto R (2006) Metabotropic glutamate receptors. Cell Tissue Res 326: 483-504.
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, et al. (2011) Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 60: 1017-1041.
- Tharmalingam S, Burns AR, Roy PJ, Hampson DR (2012) Orthosteric and allosteric drug binding sites in the caenorhabditis elegans mgl-2 metabotropic glutamate receptor. Neuropharmacology 63: 667-674.
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, et al. (2005) Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov 4: 131-144.
- Krystal JH, Mathew SJ, D’Souza DC, Garakani A, Gunduz-Bruce H, et al. (2010) Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs 24: 669-693.
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, et al. (2007) Activation of mGluR2/3 receptors as a new approach to treat schizophrenia: A randomized phase 2 clinical trial. Nat Med 13: 1102-1107.
- Oberman LM (2012) mGluR antagonists and GABA agonists as novel pharmacological agents for the treatment of autism spectrum disorders. Expert Opin Investig Drugs 21: 1819-1825.
- Cartmell J, Schoepp DD (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75: 889-907.
- Pinheiro PS, Mulle C (2008) Presynaptic glutamate receptors: Physiological functions and mechanisms of action. Nat Rev Neurosci 9: 423-436.
- Olive MF (2009) Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev 2: 83-98.
- Lodge D, Tidball P, Mercier MS, Lucas SJ, Hanna L, et al. (2013) Antagonists reversibly reverse chemical LTD induced by group I, group II and group III metabotropic glutamate receptors. Neuropharmacology 74: 135-146.
- Bruno V, Caraci F, Copani A, Matrisciano F, Nicoletti F, et al. (2017) The impact of metabotropic glutamate receptors into active neurodegenerative processes: A “dark side” in the development of new symptomatic treatments for neurologic and psychiatric disorders. Neuropharmacology 115: 180-192.
- Shigemoto R, Nakanishi S, Mizuno N (1992) Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: An in situ hybridization study in adult and developing rat. J Comp Neurol 322: 121-135.
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N (1993) Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience 53: 1009-1018.
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N (1993) Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: An in situ hybridization study. J Comp Neurol 335: 252-266.
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N (1995) Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol 360: 555-570.
- Ohishi H, Neki A, Mizuno N (1998) Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: An immunohistochemical study with a monoclonal antibody. Neurosci Res 30: 65-82.
- Bradley SR, Rees HD, Yi H, Levey AI, Conn PJ (1998) Distribution and developmental regulation of metabotropic glutamate receptor 7a in rat brain. J Neurochem 71: 636- 645.
- Wright DD, Blackstone CD, Huganir RL, Ryugo DK (1996) Immunocytochemical localization of the mGluR1α metabotropic glutamate receptor in the dorsal cochlear nucleus. J Comp Neurol 364: 729-745.
- Petralia RS, Wang YX, Zhao HM, Wenthold RJ (1996) Ionotropic and metabotropic glutamate receptors show unique postsynaptic, presynaptic, and glial localizations in the dorsal cochlear nucleus. J Comp Neurol 372: 356-383.
- Petralia RS, Wang YX, Singh S, Wu C, Shi L, et al., (1997) A monoclonal antibody shows discrete cellular and subcellular localizations of mGluR1α metabotropic glutamate receptors. J Chem Neuroanat 13: 77-93.
- Bilak SR, Morest DK (1998) Differential expression of the metabotropic glutamate receptor mGluR1α by neurons and axons in the cochlear nucleus: In situ hybridization and immunohistochemistry. Synapse 28: 251-270.
- Kinoshita A, Shigemoto R, Ohishi H, van der Putten H, Mizuno N (1998) Immunohistochemical localization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: A light and electron microscopic study. J Comp Neurol 393: 332-352.
- Kemmer M, Vater M (2001) Cellular and subcellular distribution of AMPA-type glutamate receptor subunits and metabotropic glutamate receptor 1α in the cochlear nucleus of the horseshoe bat (Rhinolophus rouxi). Hear Res 156: 128-142.
- Irie T, Fukui I, Ohmori H (2006) Activation of GIRK channels by muscarinic receptors and group II metabotropic glutamate receptors suppresses Golgi cell activity in the cochlear nucleus of mice. J Neurophysiol 96: 2633-2644.
- Dino MR, Mugnaini E (2008) Distribution and phenotypes of unipolar brush cells in relation to the granule cell system of the rat cochlear nucleus. Neuroscience 154: 29-50.
- Kushmerick C, Price GD, Taschenberger H, Puente N, Renden R, et al. (2004) Retroinhibition of presynaptic Ca2+ currents by endocannabinoids released via postsynaptic mGluR activation at a calyx synapse. J Neurosci 24: 5955-5965.
- Nishimaki T, Jang IS, Ishibashi H, Yamaguchi J, Nabekura J (2007) Reduction of metabotropic glutamate receptor-mediated heterosynaptic inhibition of developing MTNB-LSO inhibitory synapses. Eur J Neurosci 26: 323-330.
- Lee CC, Sherman SM (2012) Intrinsic modulators of auditory thalamocortical transmission. Hear Res 287: 43-50.
- Safieddine S, Eybalin M (1995) Expression of mGluR1α mRNA receptor in rat and guinea pig cochlear neurons. Neuroreport 7: 193-196.
- Friedman RA, Van Laer L, Huentelman MJ, Sheth SS, Van Eyken E, et al. (2009) GRM7 variants confer susceptibility to age-related hearing impairment. Hum Mol Genet 18: 785-796.
- Elezgarai I, Benítez R, Mateos JM, Lázaro E, Osorio A, et al. (1999) Developmental expression of the group III metabotropic glutamate receptor mGluR4a in the medial nucleus of the trapezoid body of the rat. J Comp Neurol 411: 431-440.
- Elezgarai I, Bilbao A, Mateos JM, Azkue JJ, Benítez R, et al. (2001) Group II metabotropic glutamate receptors are differentially expressed in the medial nucleus of the trapezoid body in the developing and adult rat. Neuroscience 104: 487-498.
- Niedzielski AS, Safieddine S, Wenthold RJ (1997) Molecular analysis of excitatory amino acid receptor expression in the cochlea. Audiol Neurootol 2: 79-91.
- Yip PK, Meldrum BS, Rattray M (2001) Elevated levels of group-III metabotropic glutamate receptors in the inferior colliculus of genetically epilepsy-prone rats following intracollicular administration of L-serine-O-phosphate. J Neurochem 78: 13-23.
- Martinez-Galan JR, Perez-Martinez FC, Juiz JM (2012) Signalling routes and developmental regulation of group I metabotropic glutamate receptors in rat auditory midbrain neurons. J Neurosci Res 90: 1913-1923.
- Valero ML, Caminos E, Juiz JM, Martinez-Galan JR (2015) TRPC1 and metabotropic glutamate receptor expression in rat auditory midbrain neurons. J Neurosci Res 93: 964-972.
- Martin LJ, Blackstone CD, Huganir RL, Price DL (1992) Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron 9: 259-270.
- Fotuhi M, Sharp AH, Glatt CE, Hwang PM, von Krosigk M, et al. (1993) Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci 13: 2001-2012.
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ (1996) The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience 71: 949-976.
- Lee CC, Sherman SM (2009) Glutamatergic inhibition in sensory neocortex. Cereb Cortex 19: 2281-2289.
- Dingledine R, Dodd J, Kelly JS (1980) The in vitro brain slice as a useful neurophysiological preparation for intracellular recording. J Neurosci Methods 2: 323-362.
- Trussell L (1999) Recording and analyzing synaptic currents and synaptic potentials. Curr Protocols Neurosci.
- Horn R, Marty A (1988) Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92: 145-159.
- Kyrozis A, Reichling DB (1995) Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods 57: 27-35.
- Fan JS, Palade P (1998) Perforated patch recording with beta-escin. Pflugers Arch 436: 1021-1023.
- Trussell LO, Jackson MB (1987) Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci 7: 3306-3316.
- Vargas G, Yeh TY, Blumenthal DK, Lucero MT (1999) Common components of patchclamp internal recording solutions can significantly affect protein kinase A activity. Brain Res 828: 169-173.
- Thomas D, Kiehn J, Katus HA, Karle CA (2004) Adrenergic regulation of the rapid component of the cardiac delayed rectifier potassium current, Ikr, and the underlying hERG ion channel. Basic Res Cardiol 99: 279-287.
- Hamlet WR, Lu Y (2016) Intrinsic plasticity induced by group II metabotropic glutamate receptors via enhancement of high-threshold Kv currents in sound localizing neurons. Neuroscience 324: 177-190.
- Eybalin M (1993) Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol Rev 73: 309-373.
- Doleviczényi Z, Halmos G, Répássy G, Vizi ES, Zelles T, et al. (2005) Cochlear dopamine release is modulated by group II metabotropic glutamate receptors via GABAergic neurotransmission. Neurosci Lett 385: 93-98.
- Kleinlogel S, Oestreicher E, Arnold T, Ehrenberger K, Felix D (1999) Metabotropic glutamate receptors group I are involved in cochlear neurotransmission. Neuroreport 10: 1879-1882.
- Oestreicher E, Arnold W, Felix D (2002) Neurotransmission of the cochlear inner hair cell synapse – implications for inner ear therapy. Adv Otorhinolaryngol 59: 131-139.
- Peng BG, Li QX, Ren TY, Ahmad S, Chen SP, et al. (2004) Group I metabotropic glutamate receptors in spiral ganglion neurons contribute to excitatory neurotransmissions in the cochlear. Neuroscience 123: 221-230.
- Ye Z, Goutman JD, Pyott SJ, Glowatzki E (2017) mGluR1 enhances efferent inhibition of inner hair cells in the developing rat cochlea. J Physiol 595: 3483-3495.
- Di Menna L, Joffe ME, Iacovelli L, Orlando R, Lindsley CW, et al. (2018) Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 128: 301-313.
- Puel JL, Saffiedine S, Gervais d'Aldin C, Eybalin M, Pujol R (1995) Synaptic regeneration and functional recovery after excitotoxic injury in the guinea pig cochlea. C R Acad Sci [III] 318: 67-75.
- Mugnaini E, Warr WB, Osen KK (1980) Distribution and light microscopic features of granule cells in the cochlear nuclei of cat, rat, and mouse. J Comp Neurol 191: 581-606.
- Petralia RS, Rubio ME, Wang YX, Wenthold RJ (2000) Differential distribution of glutamate receptors in the cochlear nuclei. Hear Res 147: 59-69.
- Rubio ME, Wenthold RJ (1997) Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron 18: 939-950.
- Rubio ME, Wenthold RJ (1999) Differential distribution of intracellular glutamate receptors in dendrites. J Neurosci 19: 5549-5562.
- Molitor SC, Manis PB (1997) Evidence for functional metabotropic glutamate receptors in the dorsal cochlear nucleus. J Neurophysiol 77: 1889-1905.
- Fujino K, Oertel D (2003) Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci U S A 100: 265-270.
- Chanda S, Xu-Friedman MA (2011) Excitatory modulation in the cochlear nucleus through group I metabotropic glutamate receptor activation. J Neurosci 31: 7450-7455.
- Sanes DH, McGee J, Walsh EJ (1998) Metabotropic glutamate receptor activation modulates sound level processing in the cochlear nucleus. J Neurophysiol 80: 209-217.
- Song P, Kaczmarek LK (2006) Modulation of Kv3.1b potassium channel phosphorylation in auditory neurons by conventional and novel protein kinase C isozymes. J Biol Chem 281: 15582-15591.
- Johnston J, Forsythe ID, Kopp-Scheinpflug C (2010) Going native: Voltage-gated potassium channels controlling neuronal excitability. J Physiol 588: 3187-3200.
- Billups B, Graham BP, Wong AY, Forsythe ID (2005) Unmasking group III metabotropic glutamate autoreceptor function at excitatory synapses in the rat CNS. J Physiol 565: 885-896.
- Wu SH, Fu XW (1998) Glutamate receptors underlying excitatory synaptic transmission in the rat’s lateral superior olive studied in vitro. Hear Res 122: 47-59.
- Kotak VC, Sanes DH (1995) Synaptically evoked prolonged depolarizations in the developing auditory system. J Neurophysiol 74: 1611-1620.
- Ene FA, Kullmann PH, Gillespie DC, Kandler K (2003) Glutamatergic calcium responses in the developing lateral superior olive: Receptor types and their specific activation by synaptic activity patterns. J Neurophysiol 90: 2581-2591.
- Ene FA, Kalmbach A, Kandler K (2007) Metabotropic glutamate receptors in the lateral superior olive activate TRP-like channels: Age- and experience-dependent regulation. J Neurophysiol 97: 3365-3375.
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, et al. (2003) Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426: 285-291.
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, et al. (1993) Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem 268: 11868-11873.
- Faden AI, Ivanova SA, Yakovlev AG, Mukhin AG (1997) Neuroprotective effects of group III mGluR in traumatic neuronal injury. J Neurotrauma 14: 885-895.
- Ghosh PK, Baskaran N, van den Pol AN (1997) Developmentally regulated gene expression of all eight metabotropic glutamate receptors in hypothalamic suprachiasmatic and arcuate nuclei--a PCR analysis. Brain Res Dev Brain Res 102: 1-12.
- Miko IJ, Sanes DH (2009) Transient gain adjustment in the inferior colliculus is serotonin- and calcium-dependent. Hear Res 251: 39-50.
- Voytenko SV, Galazyuk AV (2011) mGluRs modulate neuronal firing in the auditory midbrain. Neurosci Lett 492: 145-149.
- Farazifard R, Wu SH (2010) Metabotropic glutamate receptors modulate glutamatergic and GABAergic synaptic transmission in the central nucleus of the inferior colliculus. Brain Res 1325: 28-40.
- Stone TW (1985) Microiontophoresis and pressure ejection. In: Smith AD (ed.). IBRO Handbook Series: Methods in the Neurosciences, Wiley.
- Tang E, Yip PK, Chapman AG, Jane DE, Meldrum BS (1997) Prolonged anticonvulsant action of glutamate metabotropic receptor agonists in inferior colliculus of genetically epilepsy-prone rats. Eur J Pharmacol 327: 109-115.
- Tennigkeit F, Schwarz DW, Puil E (1999) Effects of metabotropic glutamate receptor activation in auditory thalamus. J Neurophysiol 82: 718-729.
- Schwarz DW, Tennigkeit F, Puil E (2000) Metabotropic transmitter actions in auditory thalamus. Acta Otolaryngol 120: 251-254.
- McCormick DA, von Krosigk M (1992) Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci U S A 89: 2774-2778.
- Eaton SA, Salt TE (1996) Role of N-methyl-D-aspartate and metabotropic glutamate receptors in corticothalamic excitatory postsynaptic potentials in vivo. Neuroscience 73: 1-5.
- Lee CC, Sherman SM (2010) Topography and physiology of ascending streams in the auditory tectothalamic pathway. Proc Natl Acad Sci U S A 107: 372-377.
- Sherman SM (2014) The function of metabotropic glutamate receptors in thalamus and cortex. Neuroscientist 20: 136-149.
- Viaene AN, Petrof I, Sherman SM (2011) Synaptic properties of thalamic input to layers 2/3 and 4 of primary somatosensory and auditory cortices. J Neurophysiol 105: 279-292.
- Liu T, Petrof I, Sherman SM (2014) Modulatory effects of activation of metabotropic glutamate receptors on GABAergic circuits in the mouse cortex. J Neurophysiol 111: 2287-2297.
- Bandrowski AE, Aramakis VB, Moore SL, Ashe JH (2001) Metabotropic glutamate receptors modify ionotropic glutamate responses in neocortical pyramidal cells and interneurons. Exp Brain Res 136: 25-40.
- Bandrowski AE, Moore SL, Ashe JH (2002) Activation of metabotropic glutamate receptors by repetitive stimulation in auditory cortex. Synapse 44: 146-157.
- Lee CC, Sherman SM (2008) Synaptic properties of thalamic and intracortical inputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol 100: 317-326.
- Lee CC, Sherman SM (2009) Modulator property of the intrinsic cortical projection from layer 6 to layer 4. Front Syst Neurosci 3: 3.
- Covic EN, Sherman SM (2011) Synaptic properties of connections between the primary and secondary auditory cortices in mice. Cereb Cortex 21: 2425-2441.
- Watanabe K, Kamatani D, Hishida R, Kudoh M, Shibuki K (2007) Long-term depression induced by local titanic stimulation in the rat auditory cortex. Brain Res 1166: 20-28.
- Blundon JA, Bayazitov IT, Zakharenko SS (2011) Presynaptic gating of postsynaptically expressed plasticity at mature thalamocortical synapses. J Neurosci 31: 16012-16025.
- Chun S, Bayazitov IT, Blundon JA, Zakharenko SS (2013) Thalamocortical long-term potentiation becomes gated after the early critical period in the auditory cortex. J Neurosci 33: 7345-7357.
- Viaene AN, Petof I, Sherman SM (2011) Synaptic properties of thalamic input to the subgranular layers of primary somatosensory and auditory cortices in the mouse. J Neurosci 31: 12738-12747.
- Rubel EW, Hyson RL, Durham D (1990) Afferent regulation of neurons in the brain stem auditory system. J Neurobiol 21: 169-196.
- Grothe B (2003) New roles for synaptic inhibition in sound localization. Nat Rev Neurosci 4: 540-550.
- Burger RM, Fukui I, Ohmori H, Rubel EW (2011) Inhibition in the balance: Binaurally coupled inhibitory feedback in sound localization circuitry. J Neurophysiol 106: 4-14.
- Zirpel L, Janowiak MA, Taylor DA, Parks TN (2000) Developmental changes in metabotropic glutamate receptor-mediated calcium homeostasis. J Comp Neurol 421: 95-106.
- Tang ZQ, Liu YW, Shi W, Dinh EH, Hamlet WR, et al. (2013) Activation of synaptic group II mGluRs induces long-term depression at GABAergic synapses in CNS neurons. J Neurosci 33: 15964-15977.
- Okuda H, Yamada R, Kuba H, and Ohmori H (2013) Activation of metabotropic glutamate receptors improves the accuracy of coincidence detection by presynaptic mechanisms in the nucleus laminaris of the chick. J Physiol 591: 365-378.
- Lu Y (2014) Metabotropic glutamate receptors in auditory processing. Neuroscience 274: 429-445.
- Shi W, Lu Y (2017) Metabotropic glutamate and GABA receptors modulate cellular excitability and glutamatergic transmission in chicken cochlear nucleus angularis neurons. Hear Res 346: 14-24.
- Zirpel L, Nathanson NM, Rubel EW, Hyson RL (1994) Glutamate-stimulated phosphatidylinositol metabolism in the avian cochlear nucleus. Neurosci Lett 168: 163-166.
- Lachica EA, Rübsamen R, Zirpel L, Rubel EW (1995) Glutamatergic inhibition of voltageoperated calcium channels in the avian cochlear nucleus. J Neurosci 15: 1724-1734.
- Lachica EA, Kato BM, Lippe WR, Rubel EW (1998) Glutamatergic and GABAergic agonists increase [Ca2+]i in avian cochlear nucleus neurons. J Neurobiol 37: 321-337.
- Kato BM, Lachica EA, Rubel EW (1996) Glutamate modulates intracellular Ca2+ stores in brain stem auditory neurons. J Neurophysiol 76: 646-650.
- Zirpel L, Rubel EW (1996) Eighth nerve activity regulates intracellular calcium concentration of avian cochlear nucleus neurons via a metabotropic glutamate receptor. J Neurophysiol 76: 4127-4139.
- Zirpel L, Lachica EA, Rubel EW (1995) Activation of a metabotropic glutamate receptor increases intracellular calcium concentrations in neurons of the avian cochlear nucleus. J Neurosci 15: 214-222.
- Zirpel L, Lippe WR, Rubel EW (1998) Activity-dependent regulation of [Ca2+]i in avian cochlear nucleus neurons: Roles of protein kinases A and C and relation to cell death. J Neurophysiol 79: 2288-2302.
- Kato BM, Rubel EW (1999) Glutamate regulates IP3-type and CICR stores in the avian cochlear nucleus. J Neurophysiol 81: 1587-1596.
- Zirpel L, Parks TN (2001) Zinc inhibition of group I mGluR-mediated calcium homeostasis in auditory neurons. J Assoc Res Otolaryngol 2: 180-187.
- Rubel EW, Fritzsch B (2002) Auditory system development: Primary auditory neurons and their targets. Annu Rev Neurosci 25: 51-101.
- Wang LC, Tang ZQ, Lu Y (2012) Synaptic activity-induced Ca2+ signaling in avian cochlear nucleus magnocellularis neurons. Neurosci Res 72: 129-139.
- Hyson RL, Rubel EW (1989) Transneuronal regulation of protein synthesis in the brainstem auditory system of the chick requires synaptic activation. J Neurosci 9: 2835-2845.
- Hyson RL (1998) Activation of metabotropic glutamate receptors is necessary for transneuronal regulation of ribosomes in chick auditory neurons. Brain Res 809: 214-220.
- Carzoli KL, Hyson RL (2014) Activation of metabotropic glutamate receptors regulates ribosomes of cochlear nucleus neurons. PLoS One 9: 111243.
- Hyson RL (1997) Transneuronal regulation of ribosomes after blockade of ionotropic excitatory amino acid receptors. Brain Res 749: 61-70.
- Nicholas AH, Hyson RL (2004) Group I and II metabotropic glutamate receptors are necessary for the activity-dependent regulation of ribosomes in chick auditory neurons. Brain Res 1014: 110-119.
- Call CL, Hyson RL (2016) Activity-dependent regulation of calcium and ribosomes in the chick cochlear nucleus. Neuroscience 316: 201-208.
- Carzoli KL, Hyson RL (2011) In vivo analysis of the role of metabotropic glutamate receptors in the afferent regulation of chick cochlear nucleus neurons. Hear Res 272: 49-57.
- Lu Y, Rubel EW (2005) Activation of metabotropic glutamate receptors inhibits high voltage-gated calcium channel currents of chicken nucleus magnocellularis neurons. J Neurophysiol 93: 1418-1428.
- Schoepp DD (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299: 12-20.
- Hyson RL, Reyes AD, Rubel EW (1995) A depolarizing inhibitory response to GABA in brainstem auditory neurons of the chick. Brain Res 677: 117-126.
- Lu T, Trussell LO (2001) Mixed excitatory and inhibitory GABA-mediated transmission in chick cochlear nucleus. J Physiol 535: 125-131.
- Monsivais P, Rubel EW (2001) Accommodation enhances depolarizing inhibition in central neurons. J Neurosci 21: 7823-7830.
- Tang ZQ, Gao H, Lu Y (2009) Control of a depolarizing GABAergic input in an auditory coincidence detection circuit. J Neurophysiol 102: 1672-1683.
- Kuo SP, Bradley LA, Trussell LO (2009) Heterogeneous kinetics and pharmacology of synaptic inhibition in the chick auditory brainstem. J Neurosci 29: 9625-9634.
- Lu Y, Burger RM, Rubel EW (2005) GABA(B) receptor activation modulates GABAA receptor-mediated inhibition in chicken nucleus magnocellularis neurons. J Neurophysiol 93: 1429-1438.
- Lu Y (2007) Endogenous mGluR activity suppresses GABAergic transmission in avian cochlear nucleus magnocellularis neurons. J Neurophysiol 97: 1018-1029.
- Otis TS, Trussell LO (1996) Inhibition of transmitter release shortens the duration of the excitatory synaptic current at a calyceal synapse. J Neurophysiol 76: 3584-3588.
- Fukui I, Ohmori H (2004) Tonotopic gradients of membrane and synaptic properties for neurons of the chicken nucleus magnocellularis. J Neurosci 24: 7514-7523.
- Fukui I, Sato T, Ohmori H (2006) Improvement of phase information at low sound frequency in nucleus magnocellularis of the chicken. J Neurophysiol 96: 633-641.
- Oline SN, Burger RM (2014) Short-term synaptic depression is topographically distributed in the cochlear nucleus of the chicken. J Neurosci 34: 1314-1324.
- Tang ZQ, Lu Y (2012) Development of GPCR modulation of GABAergic transmission in chicken nucleus laminaris neurons. PloS One 7: 35831.
- Lu Y (2009) Regulation of glutamatergic and GABAergic neurotransmission in the chick nucleus laminaris: Role of N-type calcium channels. Neuroscience 164: 1009-1019.
- Tang ZQ, Dinh EH, Shi W, Lu Y (2011) Ambient GABA-activated tonic inhibition sharpens auditory coincidence detection via a depolarizing shunting mechanism. J Neurosci 31: 6121-6131.
- Sanchez JT, Wang Y, Rubel EW, Barria A (2010) Development of glutamatergic transmission in binaural auditory neurons. J Neurophysiol 104: 1774-1789.
- Slee SJ, Higgs MH, Fairhall AL, Spain WJ (2010) Tonotopic tuning in a sound localization circuit. J Neurophysiol 103: 2857-2875.
- Tang ZQ, Lu Y (2012) Two GABAA responses with distinct kinetics in a sound localization circuit. J Physiol 590: 3787-3805.
- Yamada R, Okuda H, Kuba H, Nishino E, Ishii TM, et al., (2013) The cooperation of sustained and phasic inhibitions increases the contrast of ITD-tuning in low-frequency neurons of the chick nucleus laminaris. J Neurosci 33: 3927-3938.
- Stange A, Myoga MH, Lingner A, Ford MC, Alexandrova O, et al., (2013) Adaptation in sound localization: From GABA(B) receptor-mediated synaptic modulation to perception. Nat Neurosci 16: 1840-1847.
- Köppl C, Carr CE (2003) Computational diversity in the cochlear nucleus angularis of the barn owl. J Neurophysiol 89: 2313-2329.
- Warchol ME, Dallos P (1990) Neural coding in the chick cochlear nucleus. J Comp Physiol A 166: 721-734.
Citation: Zheng-Quan Tang, Lu Y (2018) Anatomy and Physiology of Metabotropic Glutamate Receptors in Mammalian and Avian Auditory System. Trends Anat Physiol 1: 001.
Copyright: © 2018 Zheng-Quan Tang, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.