Abstract
Keywords
Aminoguanidine Acylhydrazone Antibacterial activity Drug-resistance bacterial Molecular docking
Introduction
With the increase of bacteria resistance, various drug-resistant bacteria are constantly being discovered. Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), multi-drug resistant Escherichia coli, and multi-drug resistant Pseudomonas aeruginosa, causing lethal diseases worldwide and great difficulties in the treatment of community-acquired and nosocomial infections (1-4), severely threatened global public health and resulted in high economic costs (5). A possible solution is to research and develop novel antibiotics with the new structure, target, and mechanism of action for the unmet needs to control the infections caused by resistant bacteria, which is always the core of attention for medicinal chemists (6).
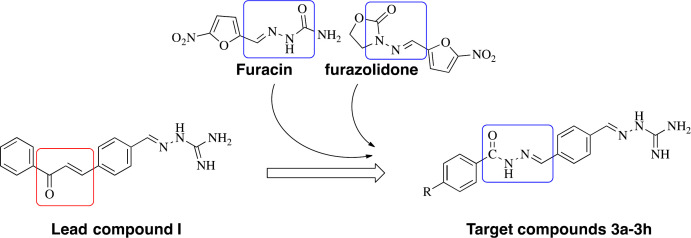
Aminoguanidine derivatives have recently got the attention of pharmaceutical chemists because of their diverse range of biological properties, including anticancer, antibacterial, antifungal, anti-inflammatory activities, and so on (7-10). In our previous work to find new antibacterial agents, several aminoguanidine derivatives were designed and synthesized, and their antibacterial potential was established (10-13). A series of aminoguanidine derivatives containing a chalcone moiety (Compound I, Figure 1) were found with potent antibacterial activity against Gram-positive strains, Gram-negative strains, and clinical isolates of multidrug-resistant (11).
Acylhydrazones have also received considerable concerns because they possess a broad range of pharmacological properties, particularly antibacterial and anticancer activities (14-16). Acylhydrazone derivatives can easily form multiple hydrogen bonds with the proteins of microorganisms to increase the binding force of the receptor. Therefore, acylhydrazone compounds have been extensively researched to find new antimicrobial agents. Furacin and furazolidone, as representatives of clinical drugs containing the acylhydrazone moiety, play an important role in treating infections (17, 18). Recently, this scaffold was found to have important therapeutic targets on β-ketoacyl-acyl carrier protein synthase III (FabH) enzyme (19). FabH has an important role in the catalysis of branched-chain fatty acids, both in gram-positive and gram-negative bacteria. However, there are no significant homologous proteins in humans (20).
Based on the above information, in this study, the structure-based design was employed to obtain novel molecules using compound I as the lead compound, in which the modification was focused on changing the unsaturated ketone to acylhydrazones with the expectation that the C=N group has a better binding with FabH. Thus, a series of new aminoguanidine derivatives containing an acylhydrazone moiety were synthesized, characterized, and screened for their antibacterial activity. Docking studies were carried out to determine the type of interactions between ligand and FabH, viz. hydrogen bonding and hydrophobic interactions.
Experimental
Instruments and Reagents
The reagents and solvents were purchased from Aladdin (Shanghai, China) or Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) and were used as received. Melting points were determined in open capillary tubes and are uncorrected. Reaction courses were monitored by thin-layer chromatography on silica gel-precoated F254 plates (Merck, Darmstadt, Germany). Developed plates were examined with UV lamps (254 nm). Nuclear magnetic resonance spectroscopy was performed on an AV-300 spectrometer (Bruker, Zurich, Switzerland) operating at 300 MHz for 1H and 75 MHz for 13C and using DMSO-d6 as solvent and tetramethylsilane as the internal standard. A MALDI‐TOF/ TOF mass spectrometer (Bruker Daltonik, Germany) was used to measure high-resolution mass spectroscopy.
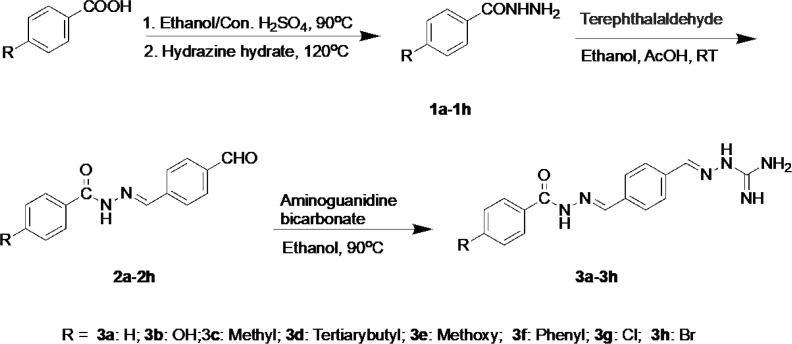
General procedures for the synthesis of 4-substituent-benzohydrazides (1a-1h)
Taking compound 1a as an example: To a solution of benzoic acid (1.22 g, 10 mmol) in dry ethanol (10 mL), concentrated sulfuric acid (2 mL) was added, and the mixture was stirred at 90 oC. After the completion of the reaction, 20% potassium carbonate solution was added into the mixture until no bubbles come out to remove the sulfuric acid and remained aromatic acid. The mixture was then extracted with dichloromethane (3 × 30 mL). The organic solution was dried over MgSO4, filtered and, concentrated under a vacuum to get the oily benzoates. 20 mL of hydrazine hydrate was added to the ethyl benzoates and refluxed at 120 oC for 8 h. The solution was removed under vacuum to get the crude residue, which was recrystallized using EtOH to afford compounds 1a. Yield 84%, White solid, m.p. 114-115 ºC, 1H-NMR (DMSO-d6, 300 MHz): δ 4.48 (s, 2H, NH2), 7.41-7.54 (m, 3H, Ph-H), 7.80-7.84 (m, 3H, Ph-H), 9.77 (s, 1H, CONH). 13C-NMR (DMSO-d6, 75 MHz): δ 127.39 , 128.76, 131.523, 133.76, 166.34. The compounds 1b-1h were obtained using the same method.
General procedures for the synthesis of N’-(4-formylbenzylidene)-4-substituted-benzohydrazides (2a-2h)
Taking compound 2a as an example: To a solution of terephthalaldehyde (1.47 g, 11 mmol) in dry ethanol (10 mL) with 3 drops of AcOH, benzohydrazides(1.36 g, 10 mmol)was added in batches with the stirring at room temperature. After the completion of the reaction, the mixture was cooled. The precipitation was filtered and recrystallized by EtOH to give compound 2a. Yield 61%, White solid, m.p. 198-200 ºC, 1H-NMR (DMSO-d6, 300 MHz): δ 7.52-7.62 (m, 3H, Ph-H), 7.92-8.01 (m, 6H, Ph-H), 8.54 (s, 1H, CH=N), 10.05 (s, 1H, CONH), 12.05 (s, 1H, CHO). 13C-NMR (DMSO-d6, 75 MHz): δ 128.05, 128.17, 128.99, 130.44, 132.43, 133.67, 137.26, 140.32, 146.78, 163.78, 193.17. The compounds 2b-2h were obtained using the same method.
General procedures for the synthesis of 2-(4-((2-(4-substituted benzoyl)hydrazineylid-ene)methyl)benzylidene)hydrazine-1-carboximidamide (3a-3h)
Taking compound 3a as an example: To a solution of aminoguanidine bicarbonate (0.67 g, 5 mmol) and compound 2a (1.26 g, 5 mmol) in dry ethanol (20 mL), 4 drops of AcOH was added. And the mixture was stirred and refluxed for 8 h. The solvent was removed, and the resulted crude residue was applied onto a silica gel column eluted with 1%-2% CH3OH in CH2Cl2 to afford compound 3a. The compounds 3b-3h were obtained using the same method. The spectral data of compounds 3a-3h were listed as below.
(2-Benzoylhydrazono)methyl)benzylidene)hydrazine-1-carboximidamide (3a)
Yield 70.6%; white solid; m.p. 234-236 ºC. 1H-NMR (DMSO-d6, 300 MHz): δ 6.55 (s, 4H, Guanidine-H), 7.51-7.61 (m, 3H, Ph-H), 7.70 (d, 2H, J = 8.2 Hz, Ph-H), 7.81 (d, 2H, J = 8.2 Hz, Ph-H), 7.92 (d, 2H, J = 7.2 Hz, Ph-H), 8.05 (s, 1H, N=C-H), 8.45 (s, 1H, N=C-H), 11.89 (s, 1H, CONH). 13C-NMR (DMSO-d6, 75 MHz): δ 127.09, 127.27, 127.67, 128.52, 131.83, 133.40, 134.51, 143.46, 147.48, 158.73, 163.24, 175.81. ESI-HRMS calcd for C16H17N6O+ ([M + H]+): 309.1464; found: 309.1459.
(2-(4-hydroxybenzoyl)hydrazono)methyl)benzylidene)hydrazine-1-carboximidamide (3b)
Yield 67.8%; white solid; m.p. 304-305 ºC,. 1H-NMR (DMSO-d6, 300 MHz): δ 6.51 (s, 4H, Guanidine-H), 6.86 (d, 2H, J = 8.4 Hz, Ph-H), 7.67 (d, 2H, J = 8.0 Hz, Ph-H), 7.76-7.82 (m, 4H, Ph-H), 8.03 (d, 1H, N=C-H), 8.41 (s, 1H, N=C-H), 11.65 (s, 1H, CONH). 13C-NMR (DMSO-d6, 75 MHz): δ 115.08, 123.75, 127.10, 127.16, 129.74, 134.90, 136.69, 143.64, 146.40, 158.38, 160.87, 175.05. ESI-HRMS calcd for C16H17N6O2+ ([M + H]+): 325.1413; found: 325.1409.
(2-(4-methylbenzoyl)hydrazono)methyl)benzylidene)hydrazine-1-carboximidamide (3c)
Yield 70.9%; white solid; m.p. 286-289 ºC. 1H-NMR (DMSO-d6, 300 MHz): δ 2.38 (s, 3H, CH3), 5.60 (s, 2H, Guanidine-H), 6.01 (s, 2H, Guanidine-H), 7.34 (d, 2H, J = 7.7 Hz, Ph-H), 7.66 (d, 2H, J = 8.1 Hz, Ph-H), 7.74 (d, 2H, J = 8.1 Hz, Ph-H), 7.83 (d, 2H, J = 7.7 Hz, Ph-H), 8.00 (s, 1H, N=C-H), 8.42 (s, 1H, N=C-H), 11.77 (s, 1H, CONH). 13C-NMR (DMSO-d6, 75 MHz): δ 20.96, 126.41, 127.04, 127.56, 128.90, 130.59, 133.40, 138.61, 141.69, 142.43, 147.35, 160.75, 162.84. ESI-HRMS calcd for C17H19N6O+ ([M + H]+): 323.1620; found: 323.1615.
(2-(4-(tert-butyl)benzoyl)hydrazono)methyl)benzylidene)hydrazine-1-carboximidamide (3d)
Yield 64.6%; white solid; m.p. 233-235 ºC. 1H-NMR (DMSO-d6, 300 MHz): δ 1.32 (s, 9H, C(CH3)3), 6.66 (s, 4H, Guanidine-H), 7.55 (d, 2H, J = 8.3 Hz, Ph-H), 7.70 (d, 2H, J = 8.2 Hz, Ph-H), 7.80 (d, 2H, J = 8.2 Hz, Ph-H), 7.87 (d, 2H, J = 8.3 Hz, Ph-H), 8.03 (d, 1H, N=C-H), 8.46 (d, 1H, N=C-H), 11.88 (s, 1H, CONH). 13C-NMR (DMSO-d6, 75 MHz): δ 30.99, 34.75, 125.26, 126.98, 127.20, 127.60, 130.75, 134.46, 137.40, 143.11, 147.33, 154.68, 159.25, 175.63. ESI-HRMS calcd for C20H25N6O+ ([M + H]+): 365.2090; found: 365.2078.
(2-(4-methoxybenzoyl)hydrazono)methyl)benzylidene)hydrazine-1-carboximidamide (3e)
Yield 68.9%; white solid; m.p. 250-252 ºC. 1H-NMR (DMSO-d6, 300 MHz): δ 3.84 (s, 3H, OCH3), 6.84 (s, 4H, Guanidine-H), 7.06 (d, 2H, J = 7.5 Hz, Ph-H), 7.69 (d, 2H, J = 7.8 Hz, Ph-H), 7.80 (d, 2H, J = 7.8 Hz, Ph-H), 7.72 (d, 2H, J = 7.5 Hz, Ph-H), 8.04 (d, 1H, N=C-H), 8.45 (d, 1H, N=C-H), 11.80 (s, 1H, CONH). 13C-NMR (DMSO-d6, 75 MHz): δ 55.47, 113.72, 125.48, 127.11, 129.63, 134.79, 136.88, 143.46, 146.90, 158.67, 162.05, 162.69, 175.40. ESI-HRMS calcd for C17H19N6O2+ ([M + H]+): 339.1569; found: 339.1558.
(2-([1,1’-biphenyl]-4-carbonyl)hydrazo-no)methyl)benzylidene)hydrazine-1-carboximidamide (3f)
Yield 63.5%; white solid; m.p. 337-340 ºC,. 1H-NMR (DMSO-d6, 300 MHz): δ 6.62 (s, 4H, Guanidine-H), 7.40-7.54 (m, 3H, Ph-H), 7.71-8.05 (m, 11H, Ph-H, N=C-H), 8.47 (s, 1H, N=C-H), 11.95 (s, 1H, CONH). 13C-NMR (DMSO-d6, 75 MHz): δ 126.72, 126.82, 126.97, 127.23, 128.21, 128.37, 129.11, 132.29, 134.02, 138.03, 139.17, 143.02, 147.61, 159.87, 162.78, 173.23. ESI-HRMS calcd for C22H21N6O+ ([M + H]+): 385.1777; found: 385.1766.
(2-(4-chlorobenzoyl)hydrazono)methyl)benzylidene)hydrazine-1-carboximidamide (3g)
Yield 66.8%; white solid; m.p. 291-293 ºC. 1H-NMR (DMSO-d6, 300 MHz): δ 6.75 (s, 4H, Guanidine-H), 7.62 (d, 2H, J = 8.4 Hz, Ph-H), 7.72 (d, 2H, J = 8.4 Hz, Ph-H), 7.82 (d, 2H, J = 8.4 Hz, Ph-H), 7.96 (d, 2H, J = 8.4 Hz, Ph-H), 8.05 (s, 1H, N=C-H), 8.45 (s, 1H, N=C-H), 11.27 (s, 1H, CONH). 13C-NMR (DMSO-d6, 75 MHz): δ 127.22, 127.34, 128.65, 129.65, 132.11, 134.63, 136.97, 143.46, 147.76, 158.38, 162.19, 175.23. ESI-HRMS calcd for C16H16ClN6O+ ([M + H]+): 343.1074; found: 343.1068.
(2-(4-bromobenzoyl)hydrazono)methyl)benzylidene)hydrazine-1-carboximidamide (3h)
Yield 71.1%; white solid; m.p. 324-325 ºC. 1H-NMR (DMSO-d6, 300 MHz): δ 5.75 (s, 2H, Guanidine-H)), 6.09 (s, 2H, Guanidine-H)), 7.68 (d, 2H, J = 8.1 Hz, Ph-H), 7.76 (d, 4H, Ph-H), 7.88 (d, 2H, J = 8.1 Hz, Ph-H), 8.01 (s, 1H, N=C-H), 8.43 (s, 1H, N=C-H), 11.95 (s, 1H, CONH). 13C-NMR (DMSO-d6, 75 MHz): δ 125.53, 126.65, 127.28, 127.60, 129.79, 131.57, 133.56, 138.59, 142.71, 148.07, 160.46, 162.21. ESI-HRMS calcd for C16H16BrN6O+ ([M + H]+): 387.0569; found: 387.0562.
Evaluation of Antibacterial Activity in-vitro
The antibacterial activity in-vitro against S. aureus (CMCC(B) 26003 and CMCC 25923, S. pyogenes CMCC 32067, E. faecalis CMCC 29212, B. subtilis CMCC 63501; E. coli CMCC 25922 and CMCC 44568, P. aeruginosa CMCC 27853 and CMCC 10104, as well as four methicillin-resistant clinical isolates (S. aureus ATCC 43300 and ATCC 33591, E. coli ATCC BAA-196, and P. aeruginosa ATCC BAA-2111), was evaluated using a two-fold serial dilution technique, and the final concentrations of compounds obtained were in the range of 0.5–128 μg/ml. Test bacteria were grown to mid-log phase in Mueller-Hinton broth (MHB) or Tryptone Soya Broth (TSB) and diluted 1000-fold in the same medium. The 105 CFU/ml bacteria were inoculated into MHB or TSB and dispensed at 0.2 ml/well in a 96-well microtiter plate. As positive controls, norfloxacin, oxacillin, and penicillin were used. Test compounds were prepared in DMSO, the final concentration of which did not exceed 0.05%. The MIC was defined as the concentration of a test compound that inhibited bacteria growth by more than 80% during 24 h incubation at 37 oC. Bacteria growth was determined by measuring the absorption at 630 nm using a microtiter enzyme-linked immunosorbent assay (ELISA) reader. All tests were triple holes.
Docking Studies
Molecular docking studies were carried out for the synthesized compounds with the E. coli FabH-CoA complex structure (PDB ID: 1HNJ) using the Discovery Studio (version 2019) (19). The structures of compounds 3a-3h were drawn using ChemBioDraw Ultra [Chemical Structure Drawing Standard; Cambridge Soft Corporation, USA (2010)], and then energetically minimized using Discovery Studio. The co-crystallized protein-ligand complex structure (pdb id: 1HNJ) was downloaded from Protein Data Bank and prepared as per the requirement of docking study, such as hydrogen atoms adding and water/impurities removing. The binding site was defined based on the volume occupied by the bound ligand in the “Define and Edit Binding site” tools of DS 2019. The input site sphere was built with a radius of 12 in x = 32.5723, y = 20.4034, and z = 29.1248. And other parameters remained at the default status. The compounds 3a-3h were docked with the receptor, and the LibDockScores were provided. Types of interactions of compound 3d with the protein were analyzed after molecular docking.
Prediction of ADME properties
A computational study of titled compounds was performed for the prediction of ADME properties. Polar surface area (TPSA), miLog P, number of rotatable bonds (n-ROTB), number of hydrogen bond donor (HBD) and acceptor (HBA) atoms and violations of Lipinski’s rule of five were calculated using Molinspiration online property calculation toolkit (21,22). Absorption (%ABS) was calculated by: % ABS = 109 - (0.345*PSA) (23).
Results and Discussion
Chemistry
The synthetic route to prepare a new class of 2-(4-((2-(4- substituted benzoyl)hydrazineylidene)methyl)benzylidene)hydrazine-1-carboximidamides from 4-substituted benzoic acid is depicted in Scheme 1. The reaction of 4-substituted benzoic acid with the alcohol in the presence of concentrated sulfuric acid produced ethyl 4-substituted-benzoates under refluxing, which transformed into the corresponding benzohydrazides immediately (1a-1h). Compounds 2a-2h were prepared by condensation of 1a-1h with terephthalaldehyde in the presence of AcOH. Finally, the target compounds 3a-3h were obtained by condensation of 2a-2h with aminoguanidine bicarbonate. The structures of the target compounds were well characterized by 1H-NMR, 13C-NMR, and high-resolution mass spectrometry.
Taking compound 3a as an example in the structure confirmation. In the 1H-NMR spectrum, a single peak due to N-H of guanidyl was observed at 6.55 ppm. And the aromatic protons of the terminal benzene ring were observed in 7.51-7.61 and 7.92. A doublet of doublets (J = 8.2 Hz) due to aromatic protons of para-substituted phenyl ring was observed at 7.70 and 7.81 ppm. Two single peaks were found to absorb C-H in imine at 8.05 ppm or 8.45 ppm, respectively. A single peak due to N-H of amide was found at 11.89 ppm. The absorption peak in the hydrogen spectrum is completely in conformity with the hydrogen signal in the structure. The 13C NMR spectra also give accurate information about the structure of the compound, which involved 12 kinds of carbon in different chemical environments. Moreover, the high-resolution mass spectrometry of 3a displayed an [M + H]+ signal at m/z 309.1459, which was corresponding to its molecular weight of 309.1464.
Antimicrobial Activity
All of the target compounds (3a-3h) were evaluated for their in vitro antibacterial activity using a serial dilution method to obtain the minimum inhibitory concentration (MIC) against five gram-positive strains (S. aureus (CMCC(B) 26003 and CMCC 25923, S. mutans BNCC 336931, E. faecalis CMCC 29212 and B. subtilis CMCC 63501), and four gram-negative strains (E. coli CMCC 25922 and CMCC 44568 and P. aeruginosa CMCC 27853 and CMCC 10104) as well as four mutidrug-resistant clinical isolates (S. aureus ATCC 43300, S. aureus ATCC 33591, E. coli ATCC BAA-196, and P. aeruginosa ATCC BAA-2111). Norfloxacin, oxacillin, and penicillin were used as positive control drugs.
The results of target compounds (3a-3h) were described in Table 1 as MIC values against the Gram-positive and Gram-negative strains. Generally compounds 3a-3h presented the antibacterial activities but didn’t achieve the expected level, which were lower than that of the lead compound and positive controls. This may be caused by introducing too many nitrogen atoms in a molecule, which may result in too much polarity, and then result in low membrane permeability and low antibacterial activity. It could be found that some of the tested compounds showed potent to moderate inhibitory effects against the strains with MICs in 4-64 μg/mL. Compounds 3a-3c hardly showed inhibitory activity at 64 μg/mL against the nine strains selected, the only compound 3b exhibited moderate inhibition against S. aureus CMCC(B) 26003. Compound 3d, with a tertiary butyl group, is effective to eight strains and showed the most potent inhibitory activity against B. subtilis CMCC 63501 with a MIC value of 4 μg/mL. Compound 3e, bearing a methoxy group,presented weaker activity only effective against S. aureus (CMCC(B) 26003, B. subtilis CMCC 63501 and P. aeruginosa CMCC 10104 with a MIC value of 64 μg/mL. Compound 3f, bearing a phenyl group, presented better activity against S. aureus (CMCC(B) 26003, B. subtilis CMCC 63501, and P. aeruginosa CMCC 10104 with a MIC value of 4 μg/mL. Compound 3g and 3h, with a chlorine and bromine atom, respectively, are effective to seven strains with MICs value of 16, 32, or 64 μg/mL.
All the compounds were also subjected to evaluate the antibacterial activity against four multidrug-resistant clinical isolates (S. aureus ATCC 43300, S. aureus ATCC 33591, E. coli ATCC BAA-196, and P. aeruginosa ATCC BAA-2111). As shown in Table 2. compound 3d also presented high activities (MIC = 8 μg/mL) against four multidrug-resistant strains, which was comparable or potent to oxacillin (MIC = 64 or 8 μg/mL) and penicillin (MIC ≥ 32 μg/mL).
Molecular docking
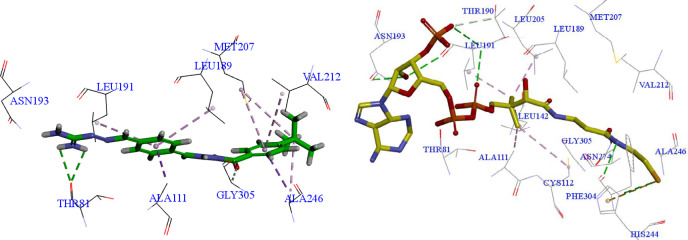
FabH receptor (also called β-ketoacyl-acyl carrier protein synthase III receptor) is a condensing enzyme that plays key role in fatty acid biosynthesis (24). It has been an important target for novel antibacterial drug design (25, 26). To illustrate the probable binding pattern, molecular docking between the aminoguanidine derivatives (3a-3h) and FabH receptor (PDB ID: 1HNJ) was performed (19, 27). Among them, tertbutyl derivative 3d showed the strongest binding affinity with a binding score of 116.62, according to the antibacterial activity results. However, the co-crystallized ligand MLC exhibited a binding score of 147.50, which was much higher than that of the synthesized compounds. It suggested that these compounds might be weak in the binding with FabH, thereby showed antibacterial activity not good enough. The docking of compound 3d and FabH receptor was analyzed to provide the detailed binding interactions (Figure 2). The C=O and NH2 groups of compound 3d function as an H-bond acceptor and donor, respectively, involved in two H-bonds formation with THR81 and GLY305. The THR81 as one of the crucial residues for the catalytic activity of FabH in various bacteria has been reported by Zhang et al. in their previous study (19). The two phenyl groups in 3d were responsible for forming Pi hydrophobic interaction and hydrophobic force interaction with LEU189, LEU191, MET207, ALA246, ALA111, VAL212. It is worth mentioning that the critical amino acid residues MET207 and ALA246 also formed alkyl hydrophobic interaction with the tertbutyl group of 3d, which explains the best antibacterial activity of 3d in the previous screening. The importance of residue ALA246 in the binding with FabH receptor was also confirmed by Muhammad’s study, in which CYS112 and ALA246 were found to be responsible for substrate binding and cleavage of the alkyl chain of CoA (28). The co-crystallized ligand MLC showed more H-bonds interactions with FabH than 3d, including the H-bonds formations withHIS 244, ASN274, PHE204, LEU191, ASN193.
Prediction of ADME properties
A computational study was conducted to predict the ADME properties and drug-likeness of all of the synthesized compounds (Table 4). It has been demonstrated experimentally that the intestinal absorption of drugs is significantly correlated with their polar surface area (PSA). The PSA of a molecule effectively represents the portion of its surface belonging to polar atoms, such as oxygen, nitrogen, and attached hydrogens, and is a descriptor related to the passive molecular transport of a molecule through membranes. With this in mind, PSA could be used to predict the transport properties of drugs in the intestines (29). Palm et al. (30) have proven, based on Caco-2 cell studies, that drugs with a PSA value below 60 were completely absorbed in the intestine. For the target compounds 3a-h, the PSA values ranged from 115.70 to 135.96 Å2 and the level of intestinal absorption (%ABS), according to the algorithm described by Zhao et al. (23), it was in the range of 62.1 to 69.1%. These findings indicated that these compounds would possess weak transport properties in the intestines.
The “Rule of 5” was established as a set of simple molecular descriptors by Lipinski based on the observation that most drugs are relatively small and lipophilic molecules (21). This rule states that most “drug-like” molecules have common parameters, including LogP ≤ 5, Mw ≤ 500, number of hydrogen bond acceptors ≤ 10, and number of hydrogen bond donors ≤ 5. Molecules violating more than one of these rules may have problems with bioavailability.
Inhibitory activity (MIC, μg/mL) of compounds 5a-5h against Gram-positive and Gram-negative bacteria
| Compd. | R | Gram-positive strains | Gram-negative strains | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 26003a | 25923b | 29212c | 63501d | 336931e | 25922f | 44568g | 27853h | 10104i | ||
| 3a | H | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 3b | OH | 32 | >64 | >64 | 64 | >64 | >64 | >64 | >64 | >64 |
| 3c | CH3 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 3d | C(CH3)3 | 8 | 8 | >64 | 4 | 32 | 8 | 16 | 16 | 8 |
| 3e | OCH3 | 64 | >64 | >64 | 64 | >64 | >64 | 64 | >64 | 64 |
| 3f | C6H5 | 4 | >64 | >64 | 4 | >64 | 32 | >64 | >64 | 4 |
| 3g | Cl | 32 | 32 | >64 | 16 | 32 | 64 | 64 | >64 | 32 |
| 3h | Br | 32 | 64 | >64 | 16 | >64 | 16 | 32 | 64 | 32 |
| Norfloxacin | 0.125 | 0.125 | 1 | 2 | 16 | 0.125 | 0.125 | 2 | 4 | |
| Oxacillin | 0.125 | 0.125 | 128 | >128 | 0.125 | 128 | >128 | >128 | 128 | |
| Penicillin | 0.125 | 0.125 | 128 | 128 | 0.125 | 128 | >128 | >128 | 32 | |
Inhibitory activity (MIC, µg/mL) of compounds 5a-5h against clinical isolates of multidrug-resistant strains
| Compd. | multidrug-resistant Gram-positive strains | multidrug-resistant Gram-negative strains | ||
|---|---|---|---|---|
| 43300a | 3359b | BAA-196c | BAA-2111d | |
| 3a | >32 | >32 | >32 | >32 |
| 3b | >32 | >32 | >32 | >32 |
| 3c | >32 | >32 | >32 | >32 |
| 3d | 8 | 8 | 8 | 8 |
| 3e | >32 | >32 | >32 | >32 |
| 3f | >32 | 32 | 32 | 16 |
| 3g | 32 | 32 | 32 | >32 |
| 3h | 16 | 16 | 32 | 32 |
| Norfloxacin | 0.5 | 0.25 | 0.5 | 1 |
| Oxacillin | 64 | 8 | ND | ND |
| Penicillin | 32 | >32 | ND | ND |
Docking scores of compounds 3a-3h and co-crystallized ligand MLC in the docking with E. coli FabH
| Compounds | LibDockScore |
|---|---|
| 3a | 107.269 |
| 3b | 109.01 |
| 3c | 107.32 |
| 3d | 116.52 |
| 3e | 111.048 |
| 3f | 113.32 |
| 3g | 107.55 |
| 3h | 109.008 |
| MLC | 147.50 |
Pharmacokinetic parameters which are important for good oral bioavailability and drug-likeness of target compounds 5a-p
| Compds | ABS (%) | TPSA (Å2) | n-ROTB | MW | miLogP | HBD | HBA | Lipinski’s violation |
|---|---|---|---|---|---|---|---|---|
| rule | - | - | ≤10 | <500 | ≤5 | ≤5 | ≤10 | ≤1 |
| 3a | 69.1 | 115.73 | 6 | 308.4 | 2.3 | 5 | 7 | 0 |
| 3b | 62.1 | 135.96 | 6 | 324.3 | 1.82 | 6 | 8 | 1 |
| 3c | 69.1 | 115.70 | 6 | 322.4 | 2.75 | 5 | 7 | 0 |
| 3d | 69.1 | 115.70 | 7 | 302.4 | 4.01 | 5 | 7 | 0 |
| 3e | 65.9 | 124.96 | 7 | 338.4 | 2.36 | 5 | 8 | 0 |
| 3f | 69.1 | 115.73 | 7 | 384.4 | 4.10 | 5 | 7 | 0 |
| 3g | 69.1 | 115.73 | 6 | 342.8 | 2.98 | 5 | 7 | 0 |
| 3h | 69.1 | 115.73 | 6 | 387.2 | 3.11 | 5 | 7 | 0 |
Conclusion
For the first time, we synthesized a series of novel aminoguanidine derivatives containing an acylhydrazone moiety and determined their antibacterial activities against Gram-positive and Gram-negative bacteria. Some compounds had potential antibacterial activities against Gram-positive bacteria (including multidrug-resistant strains of clinical isolates). In particular, compound 3d with a tertiary butyl group was found to have the broad spectrum inhibitory capacity, which is effective to eight strains and showed the most potent inhibitory activity against B. subtilis CMCC 63501 with a MIC value of 4 μg/mL. Compound 3d also presented high activities against four multidrug-resistant strains comparable to or potent than oxacillin and penicillin. Molecular docking studies revealed that H-bond interaction with amino acid residue THR81 and alkyl hydrophobic interaction with residue LA246 of FabH found to be crucial for their binding force and in vitro antimicrobial activities. What’s more, considering the performance of the antibacterial activities and the prediction of ADME properties, the acylhydrazone and aminoguanidine moieties are unsuited to coexist to avoid the low cell permeability.
Acknowledgements
References
-
1.
Bi Y, Liu XX, Zhang HY, Yang X, Liu ZY, Lu J, Lewis PJ, Wang CZ, Xu JY, Meng QG, Ma C, Yuan CS. Synthesis and Antibacterial Evaluation of Novel 3-Substituted Ocotillol-Type Derivatives as Leads. Molecules. 2017;22:590.
-
2.
Carrel M, Perencevich EN, David MZ. USA300 Methicillin-Resistant Staphylococcus aureus, United States, 2000–2013. Emerg. Infect. Dis. 2015;21:1973-80. [PubMed ID: 26484389].
-
3.
Hvistendahl M. China Takes Aim at Rampant Antibiotic Resistance. Science. 2012;336:795.
-
4.
Yezli S, Li H. Antibiotic resistance amongst healthcare-associated pathogens in China. Int. J. Antimicrob. Agents. 2012;40:389-97. [PubMed ID: 22999767].
-
5.
Azeredo da Silveira S, Perez A. Liposomes as novel anti-infectives targeting bacterial virulence factors? Expert. Rev. Anti. Infect. Ther. 2015;13:531-3.
-
6.
Simpkin VL, Renwick MJ, Kelly R, Mossialos E. Incentivising innovation in antibiotic drug discovery and development: progress, challenges and next steps. J. Antibiot. (Tokyo). 2017;70:1087-96. [PubMed ID: 29089600].
-
7.
Sidoryk K, Świtalska M, Rózga P, Wietrzyk J, Bujak I, Żerek B, Kaczmarek Ł, Cybulski M. An efficient synthesis of indolo[2,3-b]quinoline guanidine derivatives with their in-vitro and in-vivo study. Med. Chem. Res. 2017;26:3354-66. [PubMed ID: 29170613].
-
8.
Song MX, Wang SB, Wang ZT, Fu ZY, Zhou SC, Cheng HB, Liang Z, Deng XQ. Synthesis, antimicrobial and cytotoxic activities, and molecular docking studies of N-arylsulfonylindoles containing an aminoguanidine, a semicarbazide, and a thiosemicarbazide moiety. Eur. J. Med. Chem. 2019;166:108-18. [PubMed ID: 30685534].
-
9.
Wei ZY, Chi KQ, Yu ZK, Liu HY, Sun LP, Zheng CJ, Piao HR. Synthesis and biological evaluation of chalcone derivatives containing aminoguanidine or acylhydrazone moieties. Bioorg. Med. Chem. Lett. 2016;26:5920-5. [PubMed ID: 27843112].
-
10.
Li YR, Li C, Liu JC, Guo M, Zhang TY, Sun LP, Zheng CJ, Piao HR. Synthesis and Biological Evaluation of 1,3-diaryl Pyrazole Derivatives as Potential Antibacterial and Anti-Inflammatory Agents. Bioorg. Med. Chem. Lett. 2015;25:5052-7. [PubMed ID: 26490095].
-
11.
Wu J, Ma S, Zhang TY, Wei ZY, Wang HM, Guo FY, Zheng CJ, Piao HR. Synthesis and biological evaluation of ursolic acid derivatives containing an aminoguanidine moiety. Med. Chem. Res. 2019;28:959-73.
-
12.
Gao ZM, Wang TT, Li SZ, Wan HQ, Wang G, Wu YB, Deng XQ, Song MX. Synthesis and antibacterial activity evaluation of (2-Chloroquinolin-3-yl)methyleneamino guanidine derivatives. Chin. J. Org. Chem. 2016;36:2484-8.
-
13.
Yu HH, Zhou SC, Guo TT, Liang Z, Chen HB, Dai WK, Song MX. Synthesis and antimicrobial activity evaluation of aminoguanidine derivatives containing a biphenyl moiety. Chin. J. Org. Chem. 2019;39:1497-1502.
-
14.
Zhang M, Xian DM, Li HH, Zhang JC, You ZL. Synthesis and structures of halo-substituted aroylhydrazones with antimicrobial activity. Aust. J. Chem. 2012;65:343-50.
-
15.
Masunari A, Tavares LC. A new class of nifuroxazide analogues: synthesis of 5-nitrothiophene derivatives with antimicrobial activity against multidrug-resistant staphylococcus aureus. Bioorg. Med. Chem. 2007;15:4229-36. [PubMed ID: 17419064].
-
16.
Congiu C, Onnis V. Synthesis and biological evaluation of novel acylhydrazone derivatives as potential antitumor agents. Bioorg. Med. Chem. 2013;21:6592-9. [PubMed ID: 24071449].
-
17.
Main RJ. The nitrofurans - a new type of antibacterial agent. J. Am. Pharm. Assoc. 1947;36:317-20.
-
18.
Avais M, Rashid G, Ijaz M, Khan MA, Nasir A, Jahanzaib MS, Khan JA, Hameed S, Reichel MP. Evaluation of furazolidone, sulfadimidine and amprolium to treat coccidiosis in beetal goats under field conditions. Pak. J. Pharm. Sci. 2016;29:485-7. [PubMed ID: 27087093].
-
19.
Zhang HJ, Qin X, Liu K, Zhu DD, Wang XM, Zhu HL. Synthesis, antibacterial activities and molecular docking studies of schiff bases derived from N-(2/4-benzaldehyde-amino) phenyl-N’-phenyl-thiourea. Bioorg. Med. Chem. 2011;19:5708-15. [PubMed ID: 21872479].
-
20.
Zhang HJ, Li ZL, Zhu HL. Advances in the research of β-ketoacyl-ACP synthase III (FabH) inhibitors. Curr. Med. Chem. 2012;19:1225-27. [PubMed ID: 22257054].
-
21.
Lipinski CA, Lombardo L, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3-26. [PubMed ID: 11259830].
-
22.
-
23.
Zhao Y, Abraham MH, Lee J, Hersey A, Luscombe CN, Beck G, Sherborne B, Cooper I. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002;19:1446-57. [PubMed ID: 12425461].
-
24.
Heath RJ, Rock CO. Inhibition of beta-ketoacyl-acyl carrier protein synthase III (FabH) by acyl-acyl carrier protein in Escherichia coli. J. Biol. Chem. 1996;271:10996-11000. [PubMed ID: 8631920].
-
25.
Veyron-Churlet R, Guerrini O, Mourey L, Daffe M, Zerbib D. Proteinprotein interactions within the Fatty Acid Synthase-II system of Mycobacterium tuberculosis are essential for mycobacterial viability. Mol. Microbiol. 2004;54:1161-72. [PubMed ID: 15554959].
-
26.
Khandekar SS, Daines RA, Lonsdale JT. Bacterial beta-ketoacyl-acyl carrier protein synthases as targets for antibacterial agents. Curr. Protein Pept. Sci. 2003;4:21-9. [PubMed ID: 12570782].
-
27.
Chu WC, Bia PY, Yang ZQ, Cui DY, Hua YG, Yang Y, Yang QQ, Zhang E, Qin SS. Synthesis and antibacterial evaluation of novel cationic chalcone derivatives possessing broad spectrum antibacterial activity. Eur. J. Med. Chem. 2018;143:905-21. [PubMed ID: 29227931].
-
28.
Muhammad YA, Narang R, Nayak SK, Singh SK. Synthesis, antibacterial activity and molecular docking studies of N’-benzylidene/N’-(1-phenylethylidene)hexa-2,4-dienehydrazide derivatives. J. Chem. Pharm. Res. 2016;8:930-7.
-
29.
Ertl P, Rohde B, Selzer P. Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000;43:3714-7. [PubMed ID: 11020286].
-
30.
Palm K, Luthman K, Ungell AL, Strandlund G, Artursson P. Correlation of drug absorption with molecular surface properties. J. Pharm. Sci. 1996;85:32-9. [PubMed ID: 8926580].