Abstract
Background
Intravenously infused hydroxyethyl starch (HES) can be found in urine, plasma and tissues. HES remaining in plasma and tissues is thought to increase the risk of clinical complications. HES solutions of lower molecular weight and substitution have been developed to increase urinary excretion and reduce plasma persistence. However, their effect on tissue uptake of HES has not been investigated in human subjects.
Objective
Our objective was to test the hypothesis that lower molecular weight and substitution decrease tissue uptake of HES.
Data sources
Computer searches were performed of MEDLINE; EMBASE; the Cochrane Library; meeting abstract databases in surgery, anaesthesiology and intensive care; ClinicalTrials.gov; and Google. Supplementary sources were reference lists and electronic tables of journal contents. No time period or language restrictions were imposed.
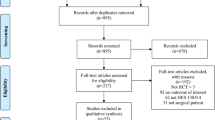
Study Selection
Clinical studies were eligible for inclusion in the meta-analysis, if data were reported both for cumulative urinary excretion of HES over 24 hours after infusion and for plasma HES concentration at 24 hours.
Data Extraction
Data were extracted on 24-hour urinary excretion of HES, 24-hour HES plasma concentration, plasma volume, HES molecular weight and substitution, study design, type and demographics of subjects, indication for fluid infusion, and HES infusion regimen. Tissue uptake of HES was computed as the difference between the infused dose and the sum of urinary excretion and residual plasma HES at 24 hours.
Data Synthesis
Twenty-five clinical studies totalling 287 subjects were included. Tissue uptake of low-molecular-weight HES (≤200 kD) was 42.3% (95% confidence interval [CI] 39.6, 45.0) compared with 24.6% (CI 17.8, 31.4) for high-molecular-weight HES (p<0.001). Similarly, tissue uptake of lower-substitution HES (≤0.5) was 42.4% (CI 39.5, 45.3) versus 26.6% (CI 19.6, 33.6) for higher-substitution HES (p<0.001). Among the three most often investigated single HES solutions, tissue uptake of 130/0.4 (42.6%; CI 35.0, 50.2) and HES 200/0.5 (43.3%; CI 39.4, 47.2) closely coincided, whereas uptake of HES 450/0.7 (22.2%; CI 14.8, 29.6) was lower (p = 0.001 and p<0.001, respectively).
Conclusions
This meta-analysis did not support the hypothesis that lower molecular weight and substitution decrease tissue uptake of HES. Further clinical studies of HES tissue uptake are needed.







Similar content being viewed by others
References
Schortgen F, Deye N, Brochard L. Preferred plasma volume expanders for critically ill patients: results of an international survey. Intensive Care Med 2004 Dec; 30(12): 2222–9
Barron ME, Wilkes MM, Navickis RJ. A systematic review of the comparative safety of colloids. Arch Surg 2004 May; 139(5): 552–63
Bork K. Pruritus precipitated by hydroxyethyl starch: a review. Br J Dermatol 2005 Jan; 152(1): 3–12
Pfeifer U, Kult J, Förster H. Ascites als Komplikation hepatischer Speicherung von Hydroxyethylstärke (HES) bei Langzeitdialyse. Klin Wochenschr 1984 Sep 17; 62(18): 862–6
Schmidt-Hieber M, Loddenkemper C, Schwartz S, et al. Hydrops lysosomalis generalisatus — an underestimated side effect of hydroxyethyl starch therapy? Eur J Haematol 2006 Jul; 77(1): 83–5
Westphal M, James MF, Kozek-Langenecker S, et al. Hydroxyethyl starches: different products — different effects. Anesthesiology 2009 Jun 8; 111(1): 187–202
Ferber HP, Nitsch E, Förster H. Studies on hydroxyethyl starch: part II. Changes of the molecular weight distribution for hydroxyethyl starch types 450/0.7, 450/0.5, 450/0.3, 300/0.4, 200/0.7, 200/0.5, 200/0.3 and 200/0.1 after infusion in serum and urine of volunteers. Arzneimittelforschung 1985; 35(3): 615–22
Treib J, Haass A, Pindur G, et al. HES 200/0.5 is not HES 200/0.5: influence of the C2/C6 hydroxyethylation ratio of hydroxyethyl starch (HES) on hemorheology, coagulation and elimination kinetics. Thromb Haemost 1995; 74(6): 1452–6
Thompson WL, Fukushima T, Rutherford RB, et al. Intravascular persistence, tissue storage, and excretion of hydroxyethyl starch. Surg Gynecol Obstet 1970 Nov; 131(5): 965–72
Lindblad G, Falk J. Konzentrationsverlauf von Hydroxyäthylstärke und Dextran in Serum und Lebergewebe von Kaninchen und die histopathologischen Folgen der Speicherung von Hydroxyäthylstärke. Infusionstherapie 1976; 3(5): 301–3
Paulini K, Sonntag W. Veränderungen des RHS der Ratte nach parenteraler Gabe von Dextran (Mw 40000) und Hydroxyäthylstärke (Mw 40000): Chemische, licht- und elektronenmikroskopische Untersuchungen. Infusionstherapie 1976; 3(5): 294–9
Jesch F, Hübner G, Zumtobel V, et al. Hydroxyäthylstärke (HÄS 450/0,7) in Plasma und Leber: Konzentrationsverlauf und histologische Veränderungen beim Menschen. Infusionsther Klin Ernahr 1979 Apr; 6(2): 112–7
Förster H, Wicarkzyk C, Dudziak R. Bestimmung der Plasmaelimination von Hydroxyaethylstärke und von Dextran mittels verbesserter analytischer Methodik. Infusionstherapie 1981 Apr; 2(2): 88–94
Jungheinrich C, Scharpf R, Wargenau M, et al. The pharmacokinetics and tolerability of an intravenous infusion of the new hydroxyethyl starch 130/0.4 (6%, 500mL) in mild-to-severe renal impairment. Anesth Analg 2002 Sep; 95(3): 544–51
Waitzinger J, Bepperling F, Pabst G, et al. Hydroxyethyl starch (HES) [130/0.4], a new HES specification: pharmacokinetics and safety after multiple infusions of 10% solution in healthy volunteers. Drugs R D 2003; 4(3): 149–57
Kalhorn TF, Yacobi A, Sum CY. Biliary excretion of hydroxyethyl starch in man. Biomed Mass Spectrom 1984 Apr; 11(4): 164–6
Lenz K, Schimetta W, Pölz W, et al. Intestinal elimination of hydroxyethyl starch? Intensive Care Med 2000 Jun; 26(6): 733–9
Sirtl C, Laubenthal H, Zumtobel V, et al. Tissue deposits of hydroxyethyl starch (HES): dose-dependent and time-related. Br J Anaesth 1999 Apr; 82(4): 510–5
Ständer S, Szépfalusi Z, Bohle B, et al. Differential storage of hydroxyethyl starch (HES) in the skin: an immunoelectron-microscopical long-term study. Cell Tissue Res 2001 May; 304(2): 261–9
Nohé B, Burchard M, Zanke C, et al. Endothelial accumulation of hydroxyethyl starch and functional consequences on leukocyte-endothelial interactions. Eur Surg Res 2002 Sep–Oct; 34(5): 364–72
Asskali F, Förster H. Zur Kumulation unterschiedlich substituierter Hydroxyethylstärke (HES) nach repetitiver Infusion bei gesunden Versuchspersonen. Anästhesiol Intensivmed Notfallmed Schmerzther 1999 Sep; 34(9): 537–41
Ballinger 2nd WF, Murray GF, Morse EE. Preliminary report on the use of hydroxyethyl starch solution in man. J Surg Res 1966 Apr; 6(4): 180–3
Lee Jr WH, Cooper N, Weidner Jr MG, et al. Clinical evaluation of a new plasma expander hydroxyethyl starch. J Trauma 1968 May; 8(3): 381–93
Solanke TF. Clinical trial of 6 per cent hydroxyethyl starch (a new plasma expander). Br Med J 1968 Sep 28; 3(621): 783–5
Metcalf W, Papadopoulos A, Tufaro R, et al. A clinical physiologic study of hydroxyethyl starch. Surg Gynecol Obstet 1970 Aug; 131(2): 255–67
Mishler JM, Borberg H, Emerson PM, et al. Hydroxyethyl starch: an agent for hypovolemic shock treatment. I: serum concentrations in normal volunteers following three consecutive daily infusions. J Surg Res 1977 Oct; 23(4): 239–45
Mishler JM, Borberg H, Emerson PM, et al. Hydroxyethyl starch: an agent for hypovolaemic schock treatment. II: urinary excretion in normal volunteers following three consecutive daily infusions. Br J Clin Pharmacol 1977 Oct; 4(5): 591–5
Mishler IV JM, Parry ES, Petrie A, et al. Plasma clearance and renal excretion of erythrocyte cryoprotectant hydroxyethylated amylopectin. Br J Haematol 1978 Oct; 40(2): 231–7
Mishler JM, Parry ES, Sutherland BA, et al. A clinical study of low molecular weight-hydroxyethyl starch, a new plasma expander. Br J Clin Pharmacol 1979 Jun; 7(6): 619–22
Mishler JM, Ricketts CR, Parkhouse EJ, et al. Catabolism of low-molecular-weight hydroxyethylated amylopectin in man: I. Changes in the circulating molecular composition. J Lab Clin Med 1979 Dec; 94(6): 841–7
Mishler IV JM. Pharmakokinetik mittelmolekularer Hydroxyäthylstärke (HÄS 200/0,5). Infusionsther Klin Ernahr 1980 Apr; 7(2): 96–102
Mishler JM, Ricketts CR, Parkhouse EJ, et al. The catabolism of low molecular weight-hydroxyethylated amylopectin in man: II. Changes in the urinary molecular profiles. Int J Clin Pharmacol Ther Toxicol 1980 Jan; 18(1): 5–9
Mishler JM, Ricketts CR, Parkhouse EJ. Urinary excretion kinetics of hydroxyethyl starch 350/0.60 in normovolaemic man. J Clin Pathol 1981 Apr; 34(4): 361–5
Köhler H, Zschiedrich H, Linfante A, et al. Die Elimination von Hydroxyäthylstärke 200/0,5, Dextran 40 und Oxypolygelatine. Klin Wochenschr 1982 Mar; 60(6): 293–301
Köhler H, Zschiedrich H, Clasen R, et al. Blutvolumen, kolloidosmotischer Druck und Nierenfunktion von Probanden nach Infusion mittelmolekularer 10% Hydroxyäthylstärke 200/0,5 und 10% Dextran 40. Anaesthesist 1982 Feb; 31(2): 61–7
Yacobi A, Stoll RG, Sum CY, et al. Pharmacokinetics of hydroxyethyl starch in normal subjects. J Clin Pharmacol 1982 Apr; 22(4): 206–12
Korttila K, Gröhn P, Gordin A, et al. Effect of hydroxyethyl starch and dextran on plasma volume and blood hemostasis and coagulation. J Clin Pharmacol 1984 Jul; 24(7): 273–82
Waitzinger J, Bepperling F, Pabst G, et al. Pharmacokinetics and tolerability of a new hydroxyethyl starch (HES) specification [HES (130/0.4)] after single-dose infusion of 6% or 10% solutions in healthy volunteers. Clin Drug Investig 1998; 16(2): 151–60
Asskali F, Warnken U, Förster H. Acetylstärke als Volumenersatz, eine mögliche Alternative zu HES. Dtsch Med Wochenschr 2001 Jan 5; 126(1–2): 1–6
Förster H, Lehmann G, Asskali F. Das in vivo mittlere Molekular-gewicht und die Nierenschwelle von Hydroxyethylstärke am Beispiel mittelsubstituierter HES (HES 70/0,5). Anästhesiol Intensivmed Notfallmed Schmerzther 2001 Jan; 36(1): 31–7
Wilkes NJ, Woolf RL, Powanda MC, et al. Hydroxyethyl starch in balanced electrolyte solution (Hextend®) — pharmacokinetic and pharmacodynamic profiles in healthy volunteers. Anesth Analg 2002 Mar; 94(3): 538–44
Lehmann G, Asskali F, Förster H. Pharmacokinetics of hydroxyethyl starch (70/0.5) following repeated infusions. Transfus Med Hemother 2003 Mar; 30(2): 72–7
Lesch A. Hydroxyethylstärke (HES) im Urin nach Mehrfachinfusion von HES (450/0,7): Physiko-chemische Veränderungen der Substanzcharakteristika einer hochsubstituierten, hochmolekularen HES [dissertation]. Frankfurt: Johann Wolfgang Goethe-Universitä, 2003
Lehmann GB, Asskali F, Boll M, et al. HES 130/0.42 shows less alteration of pharmacokinetics than HES 200/0.5 when dosed repeatedly. Br J Anaesth 2007 May; 98(5): 635–44
Lehmann G, Marx G, Förster H. Bioequivalence comparison between hydroxyethyl starch 130/0.42/6:1 and hydroxyethyl starch 130/0.4/9:1. Drugs R D 2007; 8(4): 229–40
Bogan RK, Gale GR, Walton RP. Fate of 14C-labeled hydroxyethyl starch in animals. Toxicol Appl Pharmacol 1969; 15(1): 206–11
Eisenbach C, Schönfeld AH, Vogt N, et al. Pharmacodynamics and organ storage of hydroxyethyl starch in acute hemodilution in pigs: influence of molecular weight and degree of substitution. Intensive Care Med 2007 Jun 7; 33(9): 1637–44
Lukasewitz P, Kroh U, Löwenstein O, et al. Quantitative Untersuchungen zur Gewebsspeicherung von mittelmolekularer Hydroxyethylstärke 200/0,5 bei Patienten mit Multiorganversagen. J Anaesth Intensivbeh 1998; 3(3): 42–6
Cittanova ML, Leblanc I, Legendre C, et al. Effect of hydroxyethylstarch in brain-dead kidney donors on renal function in kidney-transplant recipients. Lancet 1996 Dec 14; 348(9042): 1620–2
de Labarthe A, Jacobs F, Blot F, et al. Acute renal failure secondary to hydroxyethylstarch administration in a surgical patient. Am J Med 2001 Oct 1; 111(5): 417–8
Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis 2008 Mar; 51(3): 491–503
Kiehl P, Metze D, Kresse H, et al. Decreased activity of acid α-glucosidase in a patient with persistent periocular swelling after infusions of hydroxyethyl starch. Br J Dermatol 1998 Apr; 138(4): 672–7
Metze D, Reimann S, Szepfalusi Z, et al. Persistent pruritus after hydroxyethyl starch infusion therapy: a result of long-term storage in cutaneous nerves. Br J Dermatol 1997 Apr; 136(4): 553–9
Christidis C, Mal F, Ramos J, et al. Worsening of hepatic dysfunction as a consequence of repeated hydroxyethylstarch infusions. J Hepatol 2001 Dec; 35(6): 726–32
Wilkes MM, Navickis RJ, Sibbald WJ. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg 2001 Aug; 72(2): 527–33
Wiedermann CJ. Hydroxyethyl starch - can the safety problems be ignored? Wien Klin Wochenschr 2004 Sep 30; 116(17-18): 583–94
Davidson IJ. Renal impact of fluid management with colloids: a comparative review. Eur J Anaesthesiol 2006 Sep; 23(9): 721–38
Wiedermann CJ. Systematic review of randomized clinical trials on the use of hydroxyethyl starch for fluid management in sepsis. BMC Emerg Med 2008 Jan 24; 8(1): 1–8
Zarychanski R, Turgeon AF, Fergusson DA, et al. Renal outcomes and mortality following hydroxyethyl starch resuscitation of critically ill patients: systematic review and meta-analysis of randomized trials. Open Med 2009; 3(4): E196–209
Dart AB, Mutter TC, Ruth CA, et al. Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev 2010; (1): CD007594
Acknowledgements
This meta-analysis was conducted and the paper was prepared with no financial support from any funding organization or sponsor. Professor Wiedermann has received fees for speaking and travel reimbursements from manufacturers of plasma-derived therapies (CSL Behring, Baxter, Kedrion). Professor Bellmann has received research support from Torrex Chiesi and from Pfizer Inc. Doctor Feistritzer declares that he has no potential conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellmann, R., Feistritzer, C. & Wiedermann, C.J. Effect of Molecular Weight and Substitution on Tissue Uptake of Hydroxyethyl Starch. Clin Pharmacokinet 51, 225–236 (2012). https://doi.org/10.2165/11594700-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11594700-000000000-00000