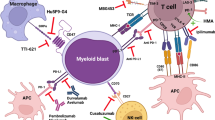
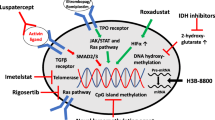
Abstract
The management of the myelodysplastic syndromes (MDS) requires insight into the complex biology of the disease. Despite this challenge, two recent developments have contributed significantly to advancements in the treatment of MDS: (i) improvements in classification systems and prognostic models; and (ii) the emergence of US FDA-approved agents such as lenalidomide, azacitidine and decitabine. Prior to these developments, supportive care measures consisting of blood and platelet transfusions, haematopoietic growth factors and antimicrobials remained standard of care for the treatment of MDS. As a result of these developments, clinicians are able to provide patient-tailored therapy for specific MDS subgroups. Clinical trials addressing combination therapies with multiple investigational agents as well as novel combination regimens are ongoing. This review focuses on supportive care modalities, the approved agents indicated for the treatment of MDS and future directions for the treatment of MDS, including agents under clinical investigation.







Similar content being viewed by others
References
Faderl S, Kantarjian H. Myelodysplastic syndromes. In: DeVita V, Hellman S, Rosenberg S, editors. Cancer: principles and practice of oncology. 7th ed. Philadelphia (PA): Lippincott Williams & Wilkins, 2005: 2144–54
Faderl S, Kantarjian H. Novel therapies for myelodysplastic syndromes. Cancer 2004 Jul 15; 101(2): 226–41
Aul C, Bowen DT, Yoshida Y. Pathogenesis, etiology and epidemiology of myelodysplastic syndromes. Haematologica 1998 Jan; 83(1): 71–86
Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol 2007 May; 25(14): 1908–15
Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 1982 Jun; 51(2): 189–99
Harris NJ, Jaffe ES, Diebold J, et al. World Health Organization lymphoid tissues: report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1977. J Clin Oncol 1999 Dec; 17(12): 3835–49
Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes [published erratum appears in Blood 1998 Feb 1; 91 (3): 1100]. Blood 1997 Mar 15; 89(6): 2079–88
Haase D, Estey E, Steidl C, et al. Multivariate evaluation of the prognostic and therapeutic relevance of cytogenetics in a merged European-American cohort of 3860 patients with MDS [abstract no. 247]. Blood 2007 Nov 16; 110(11): 247
Strom SS, Vélez-Bravo V, Estey EH. Epidemiology of myelodysplastic syndromes. Semin Hematol 2008 Jan 1; 45(1): 8–13
Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 2007 Aug 10; 25(23): 3503–10
Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer 2008 Sep 15; 113(6): 1351–61
Kantarjian H, O’Brien S, Cortes J, et al. Therapeutic advances in leukemia and myelodysplastic syndrome over the past 40 years. Cancer 2008 Oct 1; 113 (7 Suppl.): 1933–52
Andrews NC. Disorders of iron metabolism. N Engl J Med 1999 Dec 23; 341(26): 1986–95
Bennett JM. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematology 2008 Nov; 83(11): 858–61
Zurlo MG, De Stefano P, Borgna-Pignatti C, et al. Survival and causes of death in thalassaemia major. Lancet 1989 Jul 1; 2(8653): 27–30
Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med 1994 Sep 1; 331(9): 567–73
Hershko C, Link G, Cabantchik I. Pathophysiology of iron overload. Ann N Y Acad Sci 1998 Jun 30; 850: 191–201
Shalitin S, Carmi D, Weintrob N, et al. Serum ferritin level as a predictor of impaired growth and puberty in thalassemia major patients. Eur J Haematol 2005 Feb; 74(2): 93–100
Sanz G, Nomdedeu B, Such E, et al. Independent impact of iron overload and transfusion dependency on survival and leukemic evolution in patients with myelodysplastic syndrome [abstract no. 640]. Blood 2008 Nov 16; 112(11): 640
Cazzola M, Malcovati L. Myelodysplastic syndromes: coping with ineffective hematopoiesis. N Engl J Med 2005 Feb 10; 352(6): 536–8
Malcovati L, Della Porta M, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision-making. J Clin Oncol 2005 Oct; 23(30): 7594–603
Exjade (deferasirox) [prescribing information]. Novartis Pharmaceuticals Corporation, 2007 [online]. Available from URL: http://www.pharma.us.novartis.com/product/pi/pdf/exjade.pdf [Accessed 2010 Apr 10]
Porter J, Galanello R, Saglio G, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol 2008 Feb; 80(2): 168–76
Gattermann N, Schmid M, Della Porta M, et al. Efficacy and safety of deferasirox (Exjade®) during 1 year of treatment in transfusion-dependent patients with myelodysplastic syndromes: results from EPIC trial [abstract no. 633]. Blood 2008 Nov 16; 112(11): 633
Cappellini MD, Vichinsky E, Galanello R, et al. Long-term treatment with deferasirox (Exjade®, ICL670), a once-daily oral iron chelator, is effective in patients with transfusion-dependent anemias [abstract no. 2777]. Blood 2007 Nov 16; 110(11): 2777
Alessandrino EP, Amadori S, Barosi G, et al. Evidence-and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes: a statement from the Italian Society of Hematology. Haematologica 2002 Dec; 87(12): 1286–306
Bowen D, Culligan D, Jowitt S, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol 2003 Jan; 120(2): 187–200
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Myelodysplastic syndromes. V.1.2009 [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/mds.pdf [Accessed 2009 May 23]
The Nordic MDS Group Care Program. Guidelines for the diagnosis and treatment of the myelodysplastic syndromes [online]. Available from URL: http://www.nmds.org/ez4/index.php?/nmds/Nordic-Care-Programme [Accessed 2010 Jun 14]
Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of Epoetin and Darbepoetin in Patients With Cancer: 2007 American Society of Clinical Oncology/American Society of Hematology Clinical Practice Guideline Update. J Clin Oncol 2008 Jan; 26(1): 132–49
Stein RS, Abels RI, Krantz SB. Pharmacologic doses of recombinant human erythropoietin in the treatment of myelodysplastic syndromes. Blood 1991 Oct 1; 78(7): 1658–63
Italian Cooperative Study Group for rHuEpo in Myelodysplastic Syndromes. A randomized double-blind placebocontrolled study with subcutaneous recombinant human erythropoietin in patients with low-risk myelodysplastic syndromes: Italian Cooperative Study Group for rHuEpo in Myelodysplastic Syndromes. Br J Haematol 1998 Dec; 103(4): 1070–4
Hellström-Lindberg E. Efficacy of erythropoietin in the myelodysplastic syndromes: a meta-analysis of 205 patients from 17 studies. Br J Haematol 1995 Jan; 89(1): 67–71
Mannone L, Gardin C, Quarre MC, et al. High-dose darbepoetin alpha in the treatment of anaemia of lower risk myelodysplastic syndrome: results of a phase II study. Br J Haematol 2006 Jun; 133(5): 513–9
Musto P, Lanza F, Balleari E, et al. Darbepoetin alpha for the treatment of anaemia in low-intermediate risk myelodysplastic syndromes. Br J Haematol 2005 Jan; 128(2): 204–9
Stasi R, Abruzzese E, Lanzetta G, et al. Darbepoetin alfa for the treatment of anemic patients with low-and intermediate-1-risk myelodysplastic syndromes. Ann Oncol 2005 Dec; 16(12): 1921–7
Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 2000 Dec 1; 96(12): 3671–4
Moyo V, Lefebvre P, Duh MS, et al. Erythropoiesis-stimulating agents in the treatment of anemia in myelodysplastic syndromes: a meta-analysis. Ann Hematol 2008 Jul; 87(7): 527–36
Balleari E, Rossi E, Clavio M, et al. Erythropoietin plus granulocyte colony-stimulating factor is better than erythropoietin alone to treat anemia in low-risk myelodysplastic syndromes: results from a randomized single-centre study. Ann Hematol 2006 Mar; 85(3): 174–80
Casadevall N, Durieux P, Dubois S, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood 2004 Jul 15; 104(2): 321–7
Jadersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol 2008 Jul 20; 26(21): 3607–13
Hellström-Lindberg E, Negrin R, Stein R, et al. Erythroid response to treatment with G-CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: proposal for a predictive model. Br J Haematol 1997 Nov; 99(2): 344–51
Rose EH, Abels RI, Nelson RA, et al. The use of r-HuEpo in the treatment of anaemia related to myelodysplasia (MDS). Br J Haematol 1995 Apr; 89(4): 831–7
Hellström-Lindberg E, Gulbrandsen N, Lindberg G, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol 2003 Mar; 120(6): 1037–46
Greenberg PL, Sun Z, Miller KB, et al. Treatment of myelodysplastic syndrome patients with erythropoietin with or without granulocyte colony-stimulating factor: results of a prospective randomized phase 3 trial by the Eastern Cooperative Oncology Group (E1996). Blood 2009 Jun 29; 114(12): 2393–400
Saunthararajah Y, Nakamura R, Wesley R, et al. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood 2003 Oct 15; 102(8): 3025–7
Saunthararajah Y, Nakamura R, Nam JM, et al. HLADR15 (DR2) is overrepresented in myelodysplastic syndrome and aplastic anemia and predicts a response to immunosuppression in myelodysplastic syndrome. Blood 2002 Sep 1; 100(5): 1570–4
Wang H, Chuhjo T, Yasue S, et al. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood 2002 Dec 1; 100(12): 3897–902
Sloand EM, Rezvani K. The role of the immune system in myelodysplasia: implications for therapy. Semin Hematol 2008 Jan; 45(1): 39–48
Molldrem JJ, Leifer E, Bahceci E, et al. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med 2002 Aug 6; 137(3): 156–63
Stadler M, Germing U, Kliche K-O, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymoycte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia 2004 Mar; 18(3): 460–5
Apperley JF, Gardembas M, Melo JV, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med 2002 Aug 15; 347(7): 481–7
Magnusson MK, Meade KE, Nakamura R, et al. Activity of STI571 in chronic myelomonocytic leukemia with a platelet-derived growth factor β receptor fusion oncogene. Blood 2002 Aug 1; 100(3): 1088–91
Raza A, Meyer P, Dutt D, et al. Thalidomide produces transfusion independence in long-standing refractory anemias of patients with myelodysplastic syndromes. Blood 2001 Aug 15; 98(4): 958–65
List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 2005 Feb 10; 352(6): 549–57
List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006 Oct 5; 355(14): 1456–65
Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1-risk myelodysplastic syndromes with karyo-types other than deletion 5q. Blood 2008 Jan 1; 111(1): 86–93
Garcia-Manero G. Modifying the epigenome as a therapeutic strategy in myelodysplasia. Hematology Am Soc Hematol Educ Program 2007: 405–11
Silverman LR, Holland JF, Weinberg RS, et al. Effects of treatment with 5-azacytidine on the in vivo and in vitro hematopoiesis in patients with myelodysplastic syndromes. Leukemia 1993 May; 7 Suppl. 1: 21–9
Silverman LR, Holland JF, Demakos EP, et al. Azacitidine (Aza C) in myelodysplastic syndromes (MDS), CALGB studies 8421 and 8921 [abstract no. 46]. Ann Hematol 1994; 68: A12
Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the Cancer and Leukemia Group B. J Clin Oncol 2002 May 15; 20(10): 2429–40
Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol 2006 Aug 20; 24(24): 3895–903
Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009 Mar; 10(3): 223–32
Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006 Apr 15; 106(8): 1794–803
WijerMans P, Suciu S, Baila L, et al. Low dose decitabine versus best supportive care in elderly patients with intermediate or high risk MDS not eligible for intensive chemotherapy: final results of the randomized phase III study (06011) of the EORTC Leukemia and German MDS Study Groups [abstract no. 226]. Blood 2008 Nov 16; 112(11): 226
Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2007 Jan 1; 109(1): 52–7
Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006 Jul 15; 108(2): 419–25
Steensma DP, Baer MR, Slack JL, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the Alternative Dosing for OutPatient Treatment (ADOPT) trial. J Clin Oncol 2009 Aug 10; 27(23): 3842–8
Kantarjian HM, O’Brien S, Huang X, et al. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience. Cancer 2007 Mar 15; 109: 1133–7
Atallah E, Kantarjian H, Garcia-Manero G. The role of decitabine in the treatment of myelodysplastic syndromes. Expert Opin Pharmacother 2007 Jan; 8(1): 65–73
Silverman LR, Fenaux P, Mufti GJ, et al. The effects of continued azacitidine (AZA) treatment cycles on response in higher-risk patients (pts) with myelodysplastic syndromes (MDS) [abstract no. 227]. Blood 2008 Nov 16; 112(11): 227
Garcia-Manero G, Couriel DR, Tambaro PF, et al. A phase II randomized bayesian study of very low dose subcutaneous decitabine administered daily or weekly times three in patients with lower risk myelodysplastic syndrome (MDS) [abstract no. 119]. Blood 2009 Nov 20; 114(22): 119
Escalation study to determine bioavailability of a single oral dose of decitabine in patients with myelodysplastic syndrome (MDS) [ClinicalTrials.gov identifier NCT00941109]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2010 Jun 14]
Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol 2009 Apr 10; 27(11): 1850–6
Martin MG, Walgren RA, Procknow E, et al. A phase II study of 5-day intravenous azacitidine in patients with myelodysplastic syndromes. Am J Hematol 2009 Sep; 84(9): 560–4
Kuendgen A, Strupp C, Aivado M, et al. Treatment of myelodysplastic syndromes with valproic acid alone or in combination with all-trans retinoic acid. Blood 2004 Sep 1; 104(5): 1266–9
Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplstic syndrome. Blood 2007 Oct 1; 110(7): 2302–8
Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood 2006 Nov 15; 108(10): 3271–9
Faderl S, Garcia-Manero G, Ravandi F, et al. Oral (po) and intravenous (iv) clofarabine for patients (pts) with myelodysplastic syndrome (MDS) [abstract no. 222]. Blood 2008 Nov 16; 112(11): 222
A dose confirmation study of oral clofarabine for previously treated adult patients with intermediate-2 or high risk myelodysplastic syndromes (MDS) [ClinicalTrials.gov identifier NCT00531232]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2010 Jun 14]
Safety and tolerability of oral clofarabine in intermediate to high risk myelodysplastic patients [ClinicalTrials.gov identifier NCT01003678]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2010 Jun 14]
Oral clofarabine study in patients with myelodysplastic syndrome [ClinicalTrials.gov identifier NCT00299156]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2010 Jun 14]
Clofarabine in high risk myelodysplastic syndrome (MDS) [ClinicalTrials.gov identifier NCT01063257]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2010 Jun 14]
Kurzrock R, Kantarjian HM, Cortes JE, et al. Farnesyl-transferase inhibitor R115777 in myelodysplastic syndrome: clinical and biologic activities in the phase 1 setting. Blood 2003 Dec 15; 102(13): 4527–34
Kurzrock R, Albitar M, Cortes JE, et al. Phase II study of R115777, a farnesyl transferase inhibitor, in myelodysplastic syndrome. J Clin Oncol 2004 Apr 1; 22(7): 1287–92
Fenaux P, Raza A, Mufti GJ, et al. A multicenter phase 2 study of the farnesyltransferase inhibitor tipifarnib in intermediate-to high-risk myelodysplastic syndrome. Blood 2007 May 15; 109(10): 4158–63
Feldman EJ, Cortes J, DeAngelo DJ, et al. On the use of lonafarnib in myelodysplastic syndrome and chronic myelomonocytic leukemia. Leukemia 2008 Sep; 22(9): 1707–11
Kantarjian H, Fenaux P, Sekeres MA, et al. Phase 1/2 study of AMG 531 in thrombocytopenic patients (pts) with low-risk myelodysplastic syndrome (MDS): update including extended treatment [abstract no. 250]. Blood 2007 Nov 16; 110(11): 250
Kantarjian H, Giles F, Greenberg P, et al. Effect of romiplostim in patients (pts) with low or intermediate risk myelodysplastic syndrome (MDS) receiving azacytidine [abstract no. 224]. Blood 2008 Nov 16; 112(11): 224
Fenaux P, Kantarjian H, Lyons R, et al. An open-label extension study evaluating the long-term safety and efficacy of romiplostim in thrombocytopenic patients (pts) with myelodysplastic syndromes (MDS) [abstract no. 2765]. Blood 2009 Nov 20; 114(22): 2765
Greenberg PL, Garcia-Manero G, Moore MR, et al. Efficacy and safety of romiplostim in patients with low or intermediate-risk myelodysplastic syndrome (MDS) receiving decitabine [abstract no. 1769]. Blood 2009 Nov 20; 114(22): 1769
Lyons RM, Larson RA, Kosmo MA, et al. Randomized phase II study evaluating the efficacy and safety of romiplostim treatment of patients with low or intermediate risk myelodysplastic syndrome (MDS) receiving lenalidomide [abstract no. 1770]. Blood 2009 Nov 20; 114(22): 1770
Acknowledgements
No sources of funding were used to assist in the preparation of this review. Dr Jabbour reports receiving honoraria from Bristol-Myers Squibb and Novartis, Dr Issa reports receiving grants from Eisai, Celgene and Merck, and Dr Kantarjian reports receiving grants from Bristol-Myers Squibb and Novartis. The other authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bryan, J., Jabbour, E., Prescott, H. et al. Current and Future Management Options for Myelodysplastic Syndromes. Drugs 70, 1381–1394 (2010). https://doi.org/10.2165/11537920-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11537920-000000000-00000