Abstract
Darunavir is a nonpeptidic HIV type 1 (HIV-1) protease inhibitor (PI) that binds with high affinity to the HIV-1 protease, including multi-drug resistant proteases.
This drug is highly potent against a range of laboratory strains and clinical isolates of wild-type and multidrug-resistant HIV and has limited potential to cause cytotoxicity. Darunavir did not display cross-resistance with other PIs in vitro.
The coadministration of a low boosting dose of ritonavir with darunavir (boosted darunavir) increases the bioavailablity of darunavir. The drug is also administered together with other highly active antiretroviral agents.
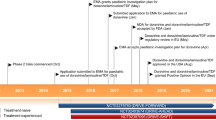
The efficacy of twice-daily boosted darunavir (11–19 mg/kg plus ritonavir 1.5–2.5 mg/kg) in treatment-experienced pediatric patients (aged 6–17 years and weighing ≥20 kg; n = 80) was demonstrated in the phase II DELPHI trial, where a virologic response (HIV-1 RNA reduction from baseline of ≥1 log10 copies/mL) at week 24 (primary endpoint) was achieved in 74% of patients, and 88% of these patients sustained this level of response at week 48.
Boosted darunavir was generally well tolerated in the DELPHI trial, with a similar profile to that observed in adults. The mean triglyceride level at week 48 was lower than that at baseline, and cholesterol levels increased slightly but remained within the normal range.



Similar content being viewed by others
References
Gupta RK, Gibb DM, Pillay D. Management of paediatric HIV-1 resistance. Curr Opin Infect Dis 2009 Jun; 22(3): 256–63
Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection [online]. Available from URL: http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf [Accessed 2009 Nov 24]
McKeage K, Perry CM, Keam SJ. Darunavir: a review of its use in the management of HIV infection in adults. Drugs 2009; 69(4): 477–503
Koh Y, Nakata H, Maeda K, et al. Novel bis-tetrahydrofuranylurethane-containing nonpeptide protease inhibitor (PI) IUC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob Agents Chemother 2003 Oct; 47(10): 3123–9
De Meyer S, Azijn H, Surleraux D, et al. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother 2005 Jun; 49(6): 2314–21
Blanche S, Bologna R, Cahn P, et al. Pharmacokinetics, safety and efficacy of darunavir/ritonavir in treatment-experienced children and adolescents. AIDS 2009 Aug 30; 23(15): 2005–13
Koh Y, Matsumi S, Das D, et al. Potent inhibition of HIV-1 replication by novel non-peptidyl small molecule inhibitors of protease dimerization. J Biol Chem 2007 Sep 28; 282(39): 28709–20
King NM, Prabu-Jeyabalan M, Nalivaika EA, et al. Structural and thermodynamic basis for the binding of TMC114, a next-generation human immunodeficiency virus type 1 protease inhibitor. J Virol 2004 Nov; 78(21): 12012–21
European Medicines Agency. Prezista® film coated tablets: summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/Humans/EPAR [Accessed 2009 Nov 17]
Tibotec Therapeutics. Prezista® (darunavir) tablet. Full prescribing information [online]. Available from URL: http://www.prezista.com/prezista/documents/us_package_insert.pdf [Accessed 2009 Nov 3]
Dierynck I, De Wit M, Gustin E, et al. Binding kinetics of darunavir to human immunodeficiency virus type 1 protease explain the potent antiviral activity and high genetic barrier. J Virol 2007 Dec; 81(24): 13845–51
De Meyer S, Lathouwers E, Dierynck I, et al. Characterization of virologic failure patients on darunavir/ritonavir in treatment-experienced patients. AIDS 2009; 23(14): 1829–40
Lathouwers E, De Meyer S, Dierynck I, et al. Update on the prevalence of the 2007 darunavir resistance-associated mutations in samples received for routine clinical resistance testing [abstract plus poster]. 6th European HIV Drug Resistance Workshop; 2008 Mar 26–28; Budapest
Thuret I, Chaix M-L, Tamalet C. Raltegravir, etravirine and r-darunavir combination in adolescents with multidrug-resistant virus. AIDS 2009; 23(17): 2364–6
Back D, Sekar V, Hoetelmans RM. Darunavir: pharmacokinetics and drug interactions. Antivir Ther 2008; 13(1): 1–13
Sekar VJ, De Pauw M, Marien K, et al. Pharmacokinetic interaction between TMC114/r and efavirenz in healthy volunteers. Antivir Ther 2007; 12(4): 509–14
Sekar VJ, Lefebvre E, De Marez T, et al. Pharmacokinetic interaction between indinavir and darunavir with low-dose ritonavir in healthy volunteers. Intervirol. In press
Sekar VJ, Lefebvre E, Boogaerts G, et al. Pharmacokinetic interaction between the protease inhibitors TMC114 and lopinavir/ritonavir [abstract no. A-367]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Sekar VJ, Lefebvre E, Marien K, et al. Pharmacokinetic interaction between darunavir and saquinavir in HIV-negative volunteers. Ther Drug Monit 2007 Dec; 29(6): 795–801
Sekar VJ, Spinosa-Guzman S, De Paepe E, et al. Darunavir/ritonavir pharmacokinetics following coadministration with clarithromycin in healthy volunteers. J Clin Pharmacol 2008 Jan; 48(1): 60–5
Sekar VJ, Lefebvre E, De Pauw M, et al. Pharmacokinetics of darunavir/ritonavir and ketoconazole following co-administration in HIV-healthy volunteers. Br J Clin Pharmacol 2008 May 5; 66(2): 215–21
Sekar V, De Paepe E, De Marez T, et al. Pharmacokinetic interaction between darunavir (TMC114), a new protease inhibitor, and the selective serotonin reuptake inhibitors (SSRIs), paroxetine and sertraline [abstract no. P295 plus poster]. 8th International Congress on Drug Therapy in HIV Infection; 2006 Nov 12–16; Glasgow
Sekar VJ, Lavreys L, De Paepe E, et al. Pharmacokinetic interaction between darunavir in combination with low-dose ritonavir and rifabutin [abstract no. H-4053]. Joint Annual Meeting of 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Meeting of the Infectious Diseases Society of America; 2008 Oct 25–28; Washingon, DC
Sekar VJ, Spinosa-Guzman S, Marien K, et al. Pharmacokinetic drug-drug interaction between the new HIV protease inhibitor darunavir (TMC114) and the lipid-lowering agent pravastatin [abstract no. 54]. 8th International Workshop on Clinical Pharmacology of HIV Therapy; 2007 Apr 16–18; Budapest
Sekar VJ, Lefebvre E, De Marez T, et al. Pharmacokinetics of darunavir (TMC114) and atazanavir during coadministration in HIV-negative, healthy volunteers. Drugs R D 2007; 8(4): 241–8
Sekar VJ, Spinosa-Guzman S, De Paepe E, et al. Pharmacokinetic interaction trial between darunavir in combination with low-dose ritonavir and didanosine [abstract]. 4th International AIDS Society Conference; 2007 Jul 22–25; Sydney
Mathias AA, Hinkle J, Shen G, et al. Effect of ritonavir boosted tipranavir or darunavir on the steady-state pharmacokinetics of elvitegravir. J Acquir Immune Defic Syndr 2008; 49(2): 156–62
Scholler-Gyure M, Kakuda TN, Sekar V, et al. Pharmacokinetics of darunavir/ritonavir and TMC125 alone and coadministered in HIV-negative volunteers. Antivir Ther 2007; 12(5): 789–96
Sekar V, Lefebvre E, Marien K, et al. Pharmacokinetic interaction between nevirapine and darunavir with low-dose ritonavir in HIV-1-infected patients. Br J Clin Pharmacol 2009; 68(1): 116–9
Hoetelmans RMW, Mariën K, De Pauw M, et al. Pharmacokinetic interaction between TMC114/ritonavir and tenofovir disoproxil fumarate in healthy volunteers. Br J Clin Pharmacol 2007 Nov; 64(5): 655–61
Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet 2007 Jul 7; 370(9581): 49–58
Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet 2007 Apr 7; 369(9568): 1169–78
Molina JM, Cohen C, Katlama C, et al. Safety and efficacy of darunavir (TMC114) with low-dose ritonavir in treatment-experienced patients: 24-week results of POWER 3. J Acquir Immune Defic Syndr 2007 Sep; 46(1): 24–31
Arasteh K, Yeni P, Pozniak A, et al. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antiviral Ther 2009; 14(6): 859–64
Pozniak A, Arasteh K, Molina JM, et al. POWER 3 analysis: 144-week efficacy and safety results for darunavir/ritonavir 600/100 mg bid in treatment-experienced HIV patients [abstract no. P24 plus poster]. 9th International Congress on Drug Therapy in HIV Infection; 2008 Nov 9–13; Glasgow
Katlama C, Bellos N, Grinsztejn B, et al. POWER 1 and 2: combined final 144-week efficacy and safety results for darunavir/ritonavir (DRV/r) 600/100 mg bid in treatment-experienced HIV patients [abstract no. P21 plus poster]. 9th International Congress on Drug Therapy in HIV Infection; 2008 Nov 9–13; Glasgow
Sekar VJ, Lavreys L, De Paepe E, et al. Bioavailability and food effect of darunavir (DRV) following administration of an oral suspension [abstract no. H233]. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009 Sep 12–15; San Francisco (CA)
Tibotec Pharmaceuticals, Ireland. TMC114-TiDP29-C228: a safety study to evaluate the antiviral activity of darunavir (DRV) in combination with ritonavir (RTV) in HIV-1 infected children from 3 years to below 6 years of age [ClinicalTrials.gov identifier NCT00919854]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2010 Jan 12]
Tibotec Pharmaceuticals, Ireland. TMC114-TiDP29-C230: a safety study to evaluate the antiviral activity of darunavir in combination with ritonavir in HIV-1 infected adolescents between 12 and 18 years of age who have not received previous treatment with antiretroviral drugs [ClinicalTrials.gov identifier NCT00915655]. US National Institutes of Health, ClinicalTrials. gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2010 Jan 12]
Acknowledgments and Disclosures
This manuscript was reviewed by: S. Esposito, Department of Maternal and Pediatric Sciences, University of Milan Fondazione IRCCS, Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena, Milan, Italy. M. Neely, Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McKeage, K., Scott, L.J. Darunavir. Pediatr-Drugs 12, 123–131 (2010). https://doi.org/10.2165/11204530-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11204530-000000000-00000