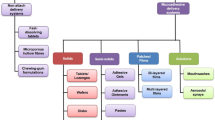
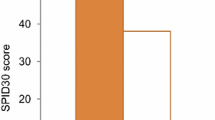Abstract
Noninvasive opioid delivery systems that provide rapid pain relief offer advantages for outpatients with high-intensity, rapid-onset pain. fentanyl effervescent buccal tablets (FEBT) employ a novel delivery system that uses an effervescence reaction to enhance the rate and efficiency of oral transmucosal fentanyl absorption. Enhanced absorption of fentanyl is considered to be caused primarily by dynamic shifts in pH within the microenvironment lying between a dissolving tablet and the buccal mucosa. The initial drop in pH is a result of the dissolution of citric acid, which combines with bicarbonate to form carbonic acid. Carbonic acid dissociates into carbon dioxide and water. As carbon dioxide dissipates, the pH increases. The initial drop in pH favors the dissolution of fentanyl citrate, the ionized form of fentanyl, whereas the subsequent increase in pH favors the formation of nonionized fentanyl. Nonionized fentanyl prefers to associate with the more lipophilic molecules in the buccal mucosa rather than stay in solution surrounded by water molecules. The dynamic changes in pH that occur on the surface of FEBT as they dissolve can be measured in vitro. Pharmacokinetic studies in healthy volunteers have demonstrated more rapid and more complete fentanyl absorption with effervescent buccal delivery compared with noneffervescent buccal delivery. Controlled clinical studies in patients with high-intensity, rapid-onset pain are the next step in defining the clinical utility of this enhanced buccal absorption of fentanyl.



Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain 1990; 41(3): 273–81
Streisand JB, Varvel JR, Stanski DR, et al. Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology 1991; 75(2): 223–9
Coluzzi PH, Schwartzberg L, Conroy JD, et al. Breakthrough cancer pain: a randomized trial comparing oral transmucosal fentanyl citrate (OTFC®) and morphine sulfate immediate release (MSIR®). Pain 2001; 91(1–2): 123–30
Resine T, Pasternak G. Opioid analgesics and antagonists. In: Hardman JG, Limbird LE, Molinoff PB, et al., editors. Goodman and Gilman’s pharmacologic basis of therapeutics. 9th rev. ed. New York: McGraw-Hill, 1996: 521–55
Prescribing information: ACTIQ® (oral transmucosal fentanyl citrate). Physicians’ desk reference. 59th ed. Montvale (NJ): Medical Economics Company, Inc., 2005: 1222–6
Prescribing information: KADIAN® (morphine sulfate sustained release capsules). Physicians’ desk reference. 59th ed. Montvale (NJ): Medical Economics Company, Inc., 2005: 569–73
Weinberg DS, Inturrisi CE, Reidenberg B, et al. Sublingual absorption of selected opioid analgesics. Clin Pharmacol Ther 1988; 44(3): 335–42
Scott JC, Ponganis KV, Stanski DR. EEG quantitation of narcotic effect: the comparative pharmacodynamics of fentanyl and alfentanil. Anesthesiology 1985; 62(3): 234–41
Kramer TH, d’Amours RH, Buettner C. Pharmacodynamic model of the effects of morphine and morphine-6-glucuronide during patient controlled analgesia [abstract]. Clin Pharmacol Ther 1996; 59: 132
Labroo RB, Paine ME, Thummel KE, et al. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos 1997; 25(9): 1072–80
Streisand JB, Zhang J, Niu S, et al. Buccal absorption of fentanyl is pH-dependent in dogs. Anesthesiology 1995; 82(3): 759–64
Stumm W, Morgan JJ. Aquatic chemistry. New York: Wiley-Interscience, 1970
Pather SI, Siebert JM, Hontz J, et al. Enhanced buccal delivery of fentanyl using the OraVescent drug delivery system. Drug Delivery Tech 2001 Oct; 1(1): 54–7 [online]. Available from URL: http://www.drugdeliverytech.com/cgi-bin/is-sues.cgi?.idlssue=1 [Accessed 2001 Jun 23]
Darwish M, Messina J, Tempero K. Relative bioavailability and dose proportionality of a novel effervescent form of fentanyl in healthy volunteers [abstract]. 2005 Annual Meeting, American Society of Anesthesiologists; 2005 Oct 22–26; Atlanta (GA)
Darwish M, Tempero K, Kirby M, et al. Pharmacokinetics and dose proportionality of fentanyl effervescent buccal tablets in healthy volunteers. Clin Pharmacokinet 2005; 44(12): 1279–86
Acknowledgments
The studies described in this manuscript were sponsored by Cephalon, Inc. (Frazer, Pennsylvania, USA) and CIMA Labs, Inc. (Eden Prairie, Minnesota, USA).
Steven Durfee, John Messina, and Raj Khankari are employed by the sponsoring companies. The authors have no other financial interests or conflicts of interest to disclose. The authors would like to acknowledge and thank Steven Shoemaker, MD, a paid consultant for Cephalon, Inc., for his dedication in preparing the article.
The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Durfee, S., Messina, J. & Khankari, R. Fentanyl effervescent buccal tablets. Am J Drug Deliv 4, 1–5 (2006). https://doi.org/10.2165/00137696-200604010-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00137696-200604010-00001