Summary
Abstract
The two-compound product containing calcipotriol 50 µg/g plus betamethasone dipropionate 0.5 mg/g (Dovobet®, Daivobet®) [referred to here as calcipotriol/betamethasone dipropionate], is a topical treatment for psoriasis vulgaris, combining a vitamin D analog and a corticosteroid.
For most adult patients with psoriasis vulgaris on the trunk and limbs, up to 4 weeks of therapy with calcipotriol/betamethasone dipropionate provides an effective and well tolerated treatment. In clinical trials, patients with a mean baseline psoriasis area and severity index (PASI) of 9.5–10.9 experienced a mean 65.0–74.4% PASI improvement within 4 weeks, significantly better than improvements with calcipotriol 50 µg/g monotherapy, betamethasone dipropionate 0.5 mg/g monotherapy, or placebo. In addition, in 6.4%–20.1% of patients, lesions cleared. In patients who were subsequently treated with calcipotriol maintenance therapy, benefits were retained for at least 4 weeks. The safety of calcipotriol/betamethasone dipropionate in patients treated for up to 1 year was generally good; fewer than 5% of patients experienced adverse events possibly associated with long-term corticosteroid use.
Pharmacologic Properties
The different modes of action of the active constituents enables the combination of calcipotriol/betamethasone dipropionate to provide a response to the processes that occur in psoriasis vulgaris. Calcipotriol, a vitamin D analog, binds to the vitamin D3 receptor, regulating cell proliferation and promoting differentiation. Betamethasone dipropionate, a group III corticosteroid, acts via a glucocorticoid receptor and suppresses inflammation and hyperproliferation. Most pharmacologic information is available from studies of calcipotriol and betamethasone dipropionate as monotherapy.
In patients with psoriasis vulgaris treated for 4 weeks with calcipotriol/betamethasone dipropionate, keratinocyte differentiation improved to 51.6%, similar to differentiation after betamethasone dipropionate 0.5 mg/g (48.2%), but significantly better than with placebo (unmedicated vehicle) [29.3%]. Calcipotriol 50 µg/g monotherapy (≊32%) was similar to placebo. Proliferation decreased with all active treatments from 11.7–14.1% to 6.8–8.6% and increased (from 10.2% to 13.6%) with placebo. Inflammation (vimentin-positive cells) decreased from 3.2–4.3% to 1.6% with calcipotriol/betamethasone dipropionate, but (unexpectedly) increased with both monotherapies and placebo. Statistically significant skin thinning occurred in 30 healthy adults who applied calcipotriol/betamethasone dipropionate twice daily for 4 weeks (maximum 12.9%, similar to that with betamethasone monotherapy [14.0%]).
In patients with psoriasis vulgaris, calcipotriol 50 µg/g monotherapy generally improved cell differentiation and hyperproliferation within 4 weeks, and dermal inflammation after 8 weeks. At high doses in some studies, calcipotriol had dose-dependent effects on calcium metabolism in patients with extensive psoriasis vulgaris. The activity of betamethasone dipropionate 0.05% (0.5 mg/g) as measured by skin blanching, was similar in monotherapy and two-compound product formulations.
Less than 1% of calcipotriol/betamethasone dipropionate (2.5g 12-hourly dosage) is absorbed through normal skin. After topical application, elimination from the depot created by dermal application occurs over several days. Although 5–6% of radiolabeled 3H-calcipotriol 2.5g (monotherapy) was absorbed, no calcipotriol or active metabolites were detected over 21 days, suggesting fast transformation to inactive metabolites. There was a linear increase in the stratum corneum concentration of betamethasone dipropionate at treatment durations of up to 2 hours; concentrations also increased significantly as drug concentration increased from 0.02% to 0.05%.
Therapeutic Efficacy
Six large, randomized trials (n = 501–1603) have assessed the efficacy of calcipotriol/betamethasone dipropionate in adults with psoriasis vulgaris. Five were double-blind trials; calcipotriol/betamethasone dipropionate once or twice daily for up to 4 weeks improved the PASI by 65.0–74.4% from mean baseline scores of 9.5–10.9. This was significantly better than seen with placebo (22.7–28.8%) or once- or twice-daily monotherapy with calcipotriol 50 µg/g (46.1–58.8%), betamethasone dipropionate 0.5 mg/g (57.2–63.1%), and tacalcitol 4 µg/g (once daily only, 33.3%) [comparators varied between trials, but all differences were significant]. There was no significant difference between once- and twice-daily calcipotriol/betamethasone dipropionate.
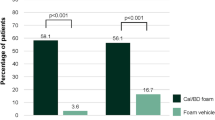
Over 4 weeks or less, there were significantly more investigator-assessed responders (with a marked improvement or clearance of, or absent/very mild, disease) among recipients of two-compound product therapy (56.3–76.1%) than placebo (7.5–10.2%), or calcipotriol 50 µg/g (22.3–50.7%), betamethasone dipropionate 0.5 mg/g (37.0–55.8%) or tacalcitol 4 µg/g monotherapy (17.0%). The percentage of patients achieving complete clearance of lesions within 4 weeks with calcipotriol/betamethasone dipropionate was 6.4% and 14.0% (once daily) and 20.1% (twice daily), versus 9.7% with twice-daily calcipotriol, 1.2% with once-daily tacalcitol and 0% with placebo; no statistical analysis was reported. One double-blind trial continued for 8 weeks: PASI improvements were significantly better with up to 4 weeks of calcipotriol/betamethasone dipropionate therapy followed by 4 weeks of calcipotriol monotherapy than with 8 weeks of tacalcitol (all once daily). A 4-week double-blind trial of two-compound product versus monotherapy continued with a 4-week open-label extension phase of treatment with twice-daily calcipotriol; at the end of this phase, PASI improvements were similar irrespective of treatment received in weeks 1–4.
In the sixth, partly blinded trial, treatment with the two-compound product formulation once daily for 8 weeks was also better than either 8 weeks’ treatment with twice-daily calcipotriol monotherapy or once-daily two-compound product therapy (up to 4 weeks) followed by two-compound product formulation at weekends and calcipotriol monotherapy on weekdays (once daily for 4 weeks).
Tolerability
In six large clinical trials, calcipotriol/betamethasone dipropionate was generally well tolerated, with only one serious possibly drug-related adverse event (a patient with extensive psoriasis vulgaris developed facial edema, which resolved when treatment was discontinued). Lesional/perilesional drug reactions (most commonly pruritus) occurred in up to 10.6% of recipients of calcipotriol/betamethasone dipropionate, with no significant difference between once- or twice-daily administration. This was significantly better than with calcipotriol 50 µg/g (11.4–19.8%), similar to betamethasone dipropionate 0.5 mg/g (4.7–8.6%), and, in one trial, significantly better than placebo (12.5–15.7%). Pruritus affected 2.6–5.1% of calcipotriol/betamethasone dipropionate recipients, and 2.6–14.3% of patients overall. Mild or moderate skin atrophy was reported for seven patients in three trials; in recipients of two-compound product therapy (three patients), calcipotriol 50 µg/g monotherapy (one patient), betamethasone dipropionate 0.5 mg/g monotherapy (two patients) and placebo (one patient).
Fewer than 1% of calcipotriol/betamethasone dipropionate recipients and ≊1% of betamethasone recipients withdrew from short-term clinical trials because of adverse events, versus 1.0–7.6% of recipients of other monotherapies and placebo. Over 8 weeks, all-cause adverse events affected 24.3–38.3% of active treatment recipients and 31.5–34.4% of placebo recipients; the incidence was no higher with the two-compound product than with monotherapy.
In a large 1-year safety trial, significantly fewer recipients of calcipotriol/betamethasone dipropionate were affected by adverse drug reactions than recipients of 4 weeks of two-compound product therapy followed by 48 weeks of calcipotriol monotherapy (21.7% vs 37.9%); 29.6% of recipients of alternating 4-weekly cycles of two-compound product and calcipotriol monotherapy experienced adverse reactions. Skin atrophy affected seven patients in the trial, including four who received the two-compound product daily, as required, throughout the study period. No recipients of calcipotriol/betamethasone dipropionate for ≤1 year experienced adrenal suppression and fewer than 5% experienced adverse events possibly associated with long-term corticosteroid use.







Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement
References
Gottlieb AB. Psoriasis. Dis Manage Clin Outcomes 1998 Nov-Dec; 1 (6): 195–202
Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med 1995; 332 (9): 381–8
Feldman SR. A quantitative definition of severe psoriasis for use in clinical trials. J Dermatol Treat 2004; 15: 27–9
Gawkrodger DJ. On behalf of the Therapy Guidelines and Audit Subcommittee of the British Association of Dermatologists. Current management of psoriasis. J Dermatol Treat 1997 Mar; 8: 27–55
Gudjonsson JE, Johnston A, Sigmundsdottir H, et al. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol 2004 Jan; 135 (1): 1–8
Lebwohl M, Ali S. Treatment of psoriasis. Part 1: topical therapy and phototherapy. J Am Acad Dermatol 2001; 45 (4): 487–98
Scott LJ, Dunn CJ, Goa KL. Calcipotriol ointment: a review of its use in the management of psoriasis. Am J Clin Dermatol 2001; 2 (2): 95–120
Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol 2002 Jan; 46: 1–23
Guilhou JJ. The therapeutic effects of vitamin D3 and its analogues in psoriasis. Expert Opin Invest Drugs 1998 Jan; 7 (1): 77–84
Bos JD, Krieg SR. Psoriasis infiltrating cell immunophenotype: changes induced by PUVA or corticosteroid treatment in T-cell subsets, Langerhans!!!2019; cells and interdigitating cells. Acta Derm Venereol (Stockh) 1985; 65: 390–7
de Jong EMGJ, Ferrier CM, de Zwart A, et al. Effects of topical treatment with budesonide on parameters for epidermal proliferation, keratinization and inflammation in psoriasis. J Dermatol Sci 1995; 9 (1995): 185–94
Reichrath J, Muller SM, Kerber A, et al. Biologic effects of topical calcipotriol (MC 903) treatment in psoriatic skin. J Am Acad Dermatol 1997 Jan; 36 (1): 19–28
Jensen AM, Llado MB, Skov L, et al. Calcipotriol inhibits the proliferation of hyperproliferative CD29 positive keratinocytes in psoriatic epidermis in the absence of an effect on the function and number of antigen-presenting cells. Br J Dermatol 1998 Dec; 139 (6): 984–91
Cagnoni ML, Ghersetich I, Lotti T, et al. Treatment of psoriasis vulgaris with topical calcipotriol: is the clinical improvement of lesional skin related to a down-regulation of some cell adhesion molecules? Acta Derm Venereol Suppl (Stockh) 1994; 186: 55–7
Jacob SE, Nassiri M, Kerdel FA, et al. Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity. Mediators Inflamm 2003 Oct; 12 (5): 309–13
Michel G, Gailis A, Jarzebska-Deussen B, et al. 1,25-(OH)2-vitamin D3 and calcipotriol induce IL-10 receptor gene expression in human epidermal cells. Inflamm Res 1997 Jan; 46 (1): 32–4
van der Vleuten CJM, de Jong EMGJ, van de Kerkhof PCM. Epidermal differentiation characteristics of the psoriatic plaque during treatment with calcipotriol. Arch Dermatol Res 1996; 288: 366–72
van Rossum MM, van Erp PEJ, van de Kerkhof PCM. Treatment of psoriasis with a new combination of calcipotriol and betamethasone dipropionate: a flow cytometric study. Dermatology 2001; 203 (2): 148–52
Bernerd F, Magnaldo T, Darmon M. Delayed onset of epidermal differentiation in psoriasis. J Invest Dermatol 1992; 98: 902–10
van de Kerkhof PCM. Reduction of epidermal abnormalities and inflammatory changes in psoriatic plaques during treatment with vitamin D3 analogs. J Investig Dermatol Symp Proc 1996 Apr; 1: 78–81
Berth-Jones J. The emergence of vitamin D as a first-line treatment for psoriasis. J Dermatol Treat 1998; 9 Suppl. 3: 13–8
Candi E, Oddi S, Terrinoni A, et al. Transglutaminase 5 cross-links loricrin, involucrin, and small proline-rich proteins in vitro. J Biol Chem 2001 Sep; 276 (37): 35014–23
Vissers WH, Berends M, Muys L, et al. The effect of the combination of calcipotriol and betamethasone dipropionate versus both monotherapies on epidermal proliferation, keratinization and T-cell subsets in chronic plaque psoriasis. Exp Dermatol 2004 Feb; 13 (2): 106–12
Traulsen J. Bioavailability of betamethasone dipropionate when combined with calcipotriol. Int J Dermatol 2004; 43 (8): 611–7
Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol 1985 Jan; 121: 63–7
Thaci D, Boehncke W, Diehl S, et al. Individual variations of treatment response with calcipotriol or/and betamethasone [abstract no. P2032]. Ann Dermatol Venereol 2002 Jul; 129 Suppl.: 769
Traulsen J, Hughes-Formella BJ. The atrophogenic potential and dermal tolerance of calcipotriol/betamethasone dipropionate ointment compared with betamethasone dipropionate ointment. Dermatology 2003; 207 (2): 166–72
Kragballe K, Noerrelund KL, Lui H, et al. Efficacy of once daily treatment regimens with calcipotriol/betamethasone dipropionate ointment and calcipotriol ointment in psoriasis vulgaris. Br J Dermatol 2004 Jun; 150 (6): 1167–73
Kragballe K, Bibby AJ, Jolliffe D. A 52-week safety study of a calcipotriol/betamethasone dipropionate two-compound product (Daivobet!!!00AE;) in the treatment of psoriasis vulgaris [plus poster]. 13th Annual Congress of the European Academy of Dermatology and Venereology; 2004 Nov 17–21; Florence
Nissen JB, Avrach WW, Hansen ES, et al. Decrease in enkephalin levels in psoriatic lesions after calcipotriol and mometasone furoate treatment. Dermatology 1999; 198 (1): 11–7
Cavicchini S, Brezzi A, Gasparini G, et al. Skin ultrastructure after calcipotriol treatment: a transmission electron microscopic and freeze-fracture study on psoriatic patients. Acta Derm Venereol (Stockh) 1996 May; 76: 186–9
Palleschi GM, Gentili A, Caproni M, et al. Structural alterations of basal keratinocytes and capillary loop in psoriasis during treatment with topical calcipotriol. Acta Derm Venereol (Stockh) 1994; (Suppl. 186): 49–51
Guzzo C, Lazarus G, Goffe BS, et al. Topical calcipotriene has no short-term effect on calcium and bone metabolism of patients with psoriasis. J Am Acad Dermatol 1996 Mar; 34: 429–33
Gumowski-Sunek D, Rizzoli R, Saurat JH. Oral calcium tolerance test in extensive psoriasis treated with topical calcipotriol. Dermatology 1995; 190 (1): 43–7
Bourke JF, Berth-Jones J, Mumford R, et al. High dose topical calcipotriol consistently reduces serum parathyroid hormone levels. Clin Endocrinol (Oxf) 1994 Sep; 41 (3): 295–7
Bourke JF, Mumford R, Whittaker P, et al. The effects of topical calcipotriol on systemic calcium homeostasis in patients with chronic plaque psoriasis. J Am Acad Dermatol 1997 Dec; 37 (6): 929–34
Pershing LK, Lambert L, Wright ED, et al. Topical 0.050% betamethasone dipropionate: pharmacokinetic and pharmacodynamic dose-response studies in humans. Arch Dermatol 1994 Jun; 130: 740–7
Pershing LK, Corlett JL, Lambert LD, et al. Cirdacian activity of topical 0.05% betamethasone dipropionate in human skin in vivo. J Invest Dermatol 1994 May; 102 (5): 734–9
Pershing LK, Silver BS, Krueger GG, et al. Feasibility of measuring the bioavailability of topical betamethasone dipropionate in commercial formulations using drug content in skin and a skin blanching bioassay. Pharm Res 1992 Jan; 9 (1): 45–51
Leo Laboratories Limited. Dovobet!!!00AE; ointment: product information 2004 Oct [online]. Available from URL: http://emc.medicines.org.uk [Accessed 2004 Feb 16]
Delaney C, Liao W, Cohen M, et al. Percutaneous absorption of calcipotriene (calcipotriol) in normal volunteers and psoriatic patients after a single dose of 3H-calcipotriene and in psoriatic patients after a single dose of 3H-calcipotriene following multiple dose treatment with non-radiolabeled calcipotriene 0.005% ointment [abstract no. 46]. J Investig Dermatol Symp Proc 1996 Apr; 1 (1): 112
Yoshikawa K, Nomura M, Higashiyama M, et al. Clinical pharmacological study on MC903 ointment: pharmacokinetic investigation in patients with psoriasis vulgaris (in Japanese). Rinsho Iyaku 1995; 11 (11): 2379–89
Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology 2002; 205 (4): 389–93
Douglas WS, Poulin Y, Decroix J, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol 2002; 82 (2): 131–5
Papp KA, Guenther L, Boyden B, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J Am Acad Dermatol 2003 Jan; 48 (1): 48–54
Ortonne JP, Kaufmann R, Lecha M, et al. Efficacy of treatment with calcipotriol/betamethasone dipropionate followed by calcipotriol alone compared with tacalcitol for the treatment of psoriasis vulgaris: a randomised, double-blind trial. Dermatology 2004; 209: 308–13
Guenther L, Cambazard F, Van de Kerkhof PC, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double-blind, vehicle-controlled clinical trial. Br J Dermatol 2002 Aug; 147 (2): 316–23
van De Kerkhof PCM. The impact of a two-compound product containing calcipotriol and betamethasone dipropionate (Daivobet/ Dovobet) on the quality of life in patients with psoriasis vulgaris: a randomized controlled trial. Br J Dermatol 2004 Sep; 151 (3): 663–8
LEO Pharma. Data on file. 2004 Dec 3
Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol 2002; 146 (3): 351–64
Lea AP, Goa KL. Calcipotriol: a review of its pharmacological properties and therapeutic efficacy in the management of psoriasis. Clin Immunother 1996 Mar; 5 (3): 230–48
Kragballe K, Olsen P. Efficacy of once daily application of a new two-compound calcipotriene/betamethasone dipropionate ointment in extensive psoriasis [abstract no. 558]. J Am Acad Dermatol 2004 Mar; 50 (3 Suppl.): 144
Wasel R, Kragballe K, van de Kerkhof PCM, et al. A new anti-psoriatic ointment yields high efficacy irrespective of initial disease levels [abstract no. P555]. J Am Acad Dermatol 2004; 50 (3 Suppl.): P143 Plus poster presented at the 62nd Annual Meeting of the American Academy of Dermatology, 2004 Feb 6–11, Washington DC
Noerregaard J, Lowson D. The cost-utility of calcipotriol/betamethasone (Dovobet) ointment in the treatment of psoriasis vulgaris in the United Kingdom [abstract no. PSN5]. Value Health 2003; 6 (6): 785
Sorensen M, Norregaard J. Pharmacoeconomic evaluation of a new two compound ointment (Daivobet!!!00AE;) and calcipotriol (Daivonex!!!00AE;) in the treatment of psoriasis vulgaris in Sweden [abstract no. PES10]. Value Health 2002; 5: 554–5
Freeman AK, Linowski GJ, Brady C, et al. Tacrolimus ointment for the treatment of psoriasis on the face and intertriginous areas. J Am Acad Dermatol 2003 Apr; 48 (4): 564–8
Lebwohl M. Topical application of calcipotriene and corticosteroids: combination regimes. J Am Acad Dermatol 1997; 37 (3 Pt 2): S55–8
Poulin Y. Calcipotriol and betamethasone dipropionate (Dovobet, Daivobet): a new formulation for the treatment of psoriasis. Skin Therapy Lett 2002 Jun; 7 (6): 1–3
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: E.C.D. de Jong, Department of Dermatology, University Hospital Nijmegen, Nijmegen, The Netherlands; M. Delfino, Clinica Dermatologica, Universita deglo Studi di Napoli Federico II, Naples, Italy; L. Fry, Dermatology Research, Imperial College of Science, Technology and Medicine, London, UK; R.W. Groves, Chelsea & Westminster Hospital, London, UK; J.E. Gudjonsson, Department of Dermatology, University of Michigan, Ann Arbor, Michigan, USA; P.I. Spuls, Department of Dermatology, Mt Sinai School of Medicine, New York, New York, USA; W.D. Tutrone, Department of Dermatology, St Luke’s-Roosevelt Hospital Center, New York, New York, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on calcipotriol/betamethasone-dipropionate, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographic information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘calcipotriol/betamethasone dipropionate’. EMBASE search terms were ‘calcipotriol/betamethasone dipropionate’. AdisBase search terms were ‘calcipotriol betamethasone-dipropionate’. Searches were last updated 7 December 2004.
Selection: Studies in patients with psoriasis vulgaris who received calcipotriol/betamethasone dipropionate. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Calcipotriol/betamethasone dipropionate, pharmacodynamics, pharmacokinetics, psoriasis vulgaris, therapeutic use, vitamin D analog.
Rights and permissions
About this article
Cite this article
Fenton, C., Plosker, G.L. Calcipotriol/Betamethasone Dipropionate. Am J Clin Dermatol 5, 463–478 (2004). https://doi.org/10.2165/00128071-200405060-00012
Published:
Issue Date:
DOI: https://doi.org/10.2165/00128071-200405060-00012