Abstract
Objective: The pharmacokinetics of mycophenolic acid (MPA) were compared in renal transplant patients receiving either mycophenolate mofetil (MMF) or enteric-coated mycophenolate sodium (EC-MPS).
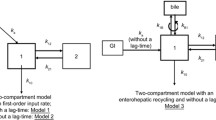
Methods: MPA concentration-time profiles were included from EC-MPS- (n = 208) and MMF-treated (n = 184) patients 4–257 months after renal transplantation. Population pharmacokinetic analysis was performed using nonlinear mixed-effects modelling (NONMEM®). A two-compartment model with first-order absorption and elimination was used to describe the data.
Results: No differences were detected in MPA clearance, intercompartmental clearance, or the central or peripheral volume of distribution. Respective values and interindividual variability (IIV) were 16 L/h (39%), 22 L/h (78%), 40 L (100%) and 518 L (490%). EC-MPS was absorbed more slowly than MMF with respective absorption rate constant values of 3.0 h−1 and 4.1 h−1 (p < 0.001) [IIV 187%]. A mixture model was used for the change-point parameter lag-time (tlag) in order to describe IIV in this parameter adequately for EC-MPS. Following the morning dose of EC-MPS, the tlag values were 0.95, 1.88 and 4.83 h for 51%, 32% and 17% of the population (IIV 8%), respectively. The morning tlag following EC-MPS administration was significantly different from both the tlag following MMF administration (0.30 h; p < 0.001 [IIV 11%]) and the tlag following the evening dose of EC-MPS (9.04 h; p < 0.001 [IIV 40%]). Post hoc analysis showed that the tlag was longer and more variable following EC-MPS administration (morning median 2.0 h [0.9–5.5 h], evening median 8.9 h [5.4–12.3 h]) than following MMF administration (median 0.30 h [0.26–0.34 h]; p < 0.001). The morning MPA predose concentrations were higher and more variable following EC-MPS administration than following MMF administration, with respective values of 2.6 mg/L (0.4–24.4 mg/L) and 1.6 mg/L (0.2–7.6 mg/L). The correlation between predose concentrations and the area under the plasma concentration-time curve (AUC) was lower in EC-MPS-treated patients (r2 = 0.02) than in MMF-treated patients (r2 = 0.48).
Conclusion: Absorption of MPA was delayed and also slower following EC-MPS administration than following MMF administration. Furthermore, the tlag varied more in EC-MPS-treated patients. MPA predose concentrations were poorly correlated with the MPA AUC in both MMF- and EC-MPS-treated patients.










Similar content being viewed by others
References:
Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection: European Mycophenolate Mofetil Cooperative Study Group. Lancet 1995; 345(8961): 1321–5
Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients: US Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 1995; 60(3): 225–32
A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation: the Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation 1996; 61(7): 1029–37
Allison AC, Eugui EM. Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF). Clin Transplant 1996; 10 (1 Pt 2): 77–84
Budde K, Curtis J, Knoll G, et al. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant 2003; 4(2): 237–43
Salvadori M, Holzer H, de Mattos A, et al. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 2003; 4(2): 231–6
Arns W, Breuer S, Choudhury S, et al. Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant 2005; 19(2): 199–206
Budde K, Bauer S, Hambach P, et al. Pharmacokinetic and pharmacodynamic comparison of enteric-coated mycophenolate sodium and mycophenolate mofetil in maintenance renal transplant patients. Am J Transplant 2007; 7(4): 888–98
Budde K, Glander P, Kramer BK, et al. Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in maintenance renal transplant recipients receiving tacrolimus: clinical, pharmacokinetic, and pharmacodynamic outcomes. Transplantation 2007; 83(4): 417–24
Hale MD, Nicholls AJ, Bullingham RE, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther 1998; 64(6): 672–83
Shaw LM, Holt DW, Oellerich M, et al. Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit 2001; 23(4): 305–15
Le Meur Y, Buchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant 2007; 7(11): 2496–503
Tedesco-Silva H, Bastien MC, Choi L, et al. Mycophenolic acid metabolite profile in renal transplant patients receiving enteric-coated mycophenolate sodium or mycophenolate mofetil. Transplant Proc 2005; 37(2): 852–5
Pescovitz MD, Guasch A, Gaston R, et al. Equivalent pharmacokinetics of mycophenolate mofetil in African-American and Caucasian male and female stable renal allograft recipients. Am J Transplant 2003; 3(12): 1581–6
Cattaneo D, Cortinovis M, Baldelli S, et al. Pharmacokinetics of mycophenolate sodium and comparison with the mofetil formulation in stable kidney transplant recipients. Clin J Am Soc Nephrol 2007; 2(6): 1147–55
van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation 1999; 68(2): 261–6
Budde K, Glander P, Schuhmann R, et al. Conversion from cyclosporine to everolimus leads to better renal function and profound changes in everolimus pharmacokinetics [abstract]. Am J Transplant 2006; 6(S2): 999
Arns W, Glander P, Schuhmann R, et al. Conversion from tacrolimus to everolimus does not influence the pharmacokinetics but increases pharmacodynamic response of mycophenolate sodium in renal transplant patients [abstract]. Am J Transplant 2006; 6(S2): 488
Frame B, Miller R, Lalonde RL. Evaluation of mixture modeling with count data using NONMEM. J Pharmacokinet Pharmacodyn 2003; 30(3): 167–83
Lesaffre E, Rizopoulos D, Tsonaka R. The logistic transform for bounded outcome scores. Biostatistics (Oxford) 2007; 8(1): 72–85
Jonsson EN, Karlsson MO. Xpose: an S-PLUS based population pharmacokinetic/ pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 1999; 58(1): 51–64
Ette EI, Williams PJ, Kim YH, et al. Model appropriateness and population pharmacokinetic modeling. J Clin Pharmacol 2003; 43(6): 610–23
Jadhav PR, Gobburu JV. A new equivalence based metric for predictive check to qualify mixed-effects models. AAPS J 2005; 7(3): E523–31
Piotrovskii VK. The use of Weibull distribution to describe the in vivo absorption kinetics. J Pharmacokinet Biopharm 1987; 15(6): 681–6
Rietbrock S, Merz PG, Fuhr U, et al. Absorption behavior of sulpiride described using Weibull functions. Int J Clin Pharmacol Ther 1995; 33(5): 299–303
Savic RM, Jonker DM, Kerbusch T, et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 2007; 34(5): 711–26
Osterberg O, Savic RM, Karlsson MO, et al. Pharmacokinetics of desmopressin administrated as an oral lyophilisate dosage form in children with primary nocturnal enuresis and healthy adults. J Clin Pharmacol 2006; 46(10): 1204–11
Cremers S, Schoemaker R, Scholten E, et al. Characterizing the role of enterohepatic recycling in the interactions between mycophenolate mofetil and calcineurin inhibitors in renal transplant patients by pharmacokinetic modelling. Br J Clin Pharmacol 2005; 60(3): 249–56
Shum B, Duffull SB, Taylor PJ, et al. Population pharmacokinetic analysis of mycophenolic acid in renal transplant recipients following oral administration of mycophenolate mofetil. Br J Clin Pharmacol 2003; 56(2): 188–97
van Hest RM, Mathot RA, Pescovitz MD, et al. Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: a population pharmacokinetic meta-analysis of mycophenolic acid in renal transplant recipients. J Am Soc Nephrol 2006; 17(3): 871–80
van Hest RM, van Gelder T, Vulto AG, et al. Population pharmacokinetics of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet 2005; 44(10): 1083–96
Premaud A, Debord J, Rousseau A, et al. A double absorption-phase model adequately describes mycophenolic acid plasma profiles in de novo renal transplant recipients given oral mycophenolate mofetil. Clin Pharmacokinet 2005; 44(8): 837–47
Le Guellec C, Bourgoin H, Buchler M, et al. Population pharmacokinetics and Bayesian estimation of mycophenolic acid concentrations in stable renal transplant patients. Clin Pharmacokinet 2004; 43(4): 253–66
Goo RH, Moore JG, Greenberg E, et al. Circadian variation in gastric emptying of meals in humans. Gastroenterology 1987; 93(3): 515–8
Satoh S, Tada H, Murakami M, et al. Circadian pharmacokinetics of mycophenolic acid and implication of genetic polymorphisms for early clinical events in renal transplant recipients. Transplantation 2006; 82(4): 486–93
Kagaya H, Inoue K, Miura M, et al. Influence of UGT1A8 and UGT2B7 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol 2007; 63(3): 279–88
Hesselink DA, van Hest RM, Mathot RA, et al. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am J Transplant 2005; 5(5): 987–94
Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet 1998; 34(6): 429–55
Nowak I, Shaw LM. Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin Chem 1995; 41(7): 1011–7
Budde K, Tedesco-Silva H, Pestana JM, et al. Enteric-coated mycophenolate sodium provides higher mycophenolic acid predose levels compared with mycophenolate mofetil: implications for therapeutic drug monitoring. Ther Drug Monit 2007; 29(3): 381–4
van Gelder T, Meur YL, Shaw LM, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit 2006; 28(2): 145–54
de Winter BC, Mathot RA, van Hest RM, et al. Therapeutic drug monitoring of mycophenolic acid: does it improve patient outcome? Expert Opin Drug Metab Toxicol 2007; 3(2): 251–61
Premaud A, Le Meur Y, Debord J, et al. Maximum a posteriori Bayesian estimation of mycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods. Ther Drug Monit 2005; 27(3): 354–61
Acknowledgements
No sources of funding were used to assist in the preparation of this study. Dr Teun van Gelder has received financial support for research, and lecture and consultancy fees from Roche Pharma and Novartis. Dr Dario Cattaneo has received lecture fees from Roche Pharma and a travel grant for attending a conference. Dr Helio Tedesco-Silva has received consultancy fees and grants from Roche Pharma and Novartis to design, conduct, analyse and review data obtained from clinical trials. Dr Luuk Hilbrands has received a grant from Roche Pharma for performing a clinical trial. Drs Mark Pescovitz and Klemens Budde have received honoraria, consultancy fees and research grants from Roche Pharma and Novartis. The other authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Winter, B.C.M., van Gelder, T., Glander, P. et al. Population Pharmacokinetics of Mycophenolic Acid. Clin Pharmacokinet 47, 827–838 (2008). https://doi.org/10.2165/0003088-200847120-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/0003088-200847120-00007