Abstract
Objectives: To assess the cost effectiveness and cost utility of preventing postexposure influenza infection using the neuraminidase inhibitor oseltamivir from a healthcare payer’s perspective in the UK.
Methods: A simulation model was developed, based on clinical trial results and published data, to predict morbidity and mortality due to influenza and to compare oseltamivir post-exposure prophylaxis (PEP) with no prophylaxis within families with members aged ≥13 years. Two scenarios were tested
-
1.
Comparison of patients receiving PEP versus patients not receiving PEP and not being treated with oseltamivir should they become infected.
-
2.
Comparison of patients receiving PEP versus patients not receiving PEP but being treated with oseltamivir should they become infected.
The model was run with an attack rate in household contacts of 8% for the base case, with higher rates (up to 30%, representing pandemic conditions) tested in sensitivity analyses. A societal perspective and other key parameters were tested in sensitivity analysis. The year of costing was 2002. The time span for the model was up to 1 year (including one influenza season), but loss of life was included in the QALY calculation and based on expected life expectancy.
Results: PEP with oseltamivir results in reduced morbidity (i.e. fewer influenza cases) and associated reductions in complications, hospitalisations and mortality due to influenza. When comparing oseltamivir PEP with no prophylaxis for contact attack rates of 8%, 12% and 30%, the mean costs per QALY gained for scenario one were estimated at £29 938, £18 697 and £5403, respectively; the mean costs per case avoided were £467, £293 and £84, respectively. The corresponding results for scenario two were £52 202, £31 610 and £9688 per QALY gained.
Conclusions: PEP with oseltamivir is likely to be a cost-effective strategy for family contacts in the UK from a healthcare payer perspective when influenza-like illness contact attack rates are 8% or higher and the only treatment given is ‘usual care’.
Similar content being viewed by others
Influenza virus infections remain a major cause of morbidity and mortality worldwide. In the UK, clinical attack rates for influenza in recent years have been around 4% of the population in each year.[1] Influenza is also associated with a substantial number of complications such as pneumonia and bronchitis. Depending on the circulating strain and population considered, clinical complication rates of about 10% are typical.[1] Vaccination is the mainstay option for the prevention of influenza.[2] However, the effectiveness can vary widely because of low uptake rates,[3] inefficient immune responses in some recipients and mismatching of the virus with the circulating strain.[4] Thus, other treatments for the prevention of influenza are necessary.
The neuraminidase inhibitor oseltamivir effectively prevents influenza A and B virus infection in people aged ≥13 years (including the elderly [≥65 years])[5–7] and is therapeutically effective in treating both influenza A and B virus in patients aged ≥1 year.[8–10] When used for post-exposure prophylaxis (PEP) in household contacts of individuals with influenza-like illness (ILI), once-daily administration of oseltamivir for 10 days reduced the risk of proven influenza illness by 89%, compared with placebo, in contacts aged ≥13 years.[7] A recent household-based study also documented the efficacy of PEP with oseltamivir in households including children aged 1–12 years.[11] Although the study presented in this paper focused on the use of oseltamivir in those aged >13 years, the efficacy of PEP with oseltamivir in children is important because children seem to be at particular risk from influenza, with high rates of influenza complications and hospitalisations in those aged <3 years.[12] Children also play a major role in the spread of influenza within families and in the community.[10,13]
Oseltamivir is currently recommended for the treatment of influenza in at-risk adults and children (i.e. those with chronic comorbidities, immunocompromised individuals and the elderly) and for the PEP of influenza in at-risk people (aged ≥13 years) who have been exposed to influenza and who are not effectively protected by vaccination.[14] Oseltamivir is under review for PEP in children aged 1–12 years. The UK National Institute for Health and Clinical Excellence (NICE) evaluated PEP with oseltamivir for influenza for several subpopulations and estimated average cost-effectiveness ratios to be £28 000/QALY gained for otherwise healthy adults and adolescents, £7000/QALY gained for at-risk unvaccinated individuals, and £29 000/QALY gained for at-risk vaccinated individuals (year of costing 2002).[14] Considerable uncertainty exists around these estimates given the variations in clinical attack rates and the exposure of the individual to infection.
In this paper we evaluate the cost effectiveness and cost utility, from a healthcare payer’s perspective, of PEP with oseltamivir within families during an average influenza season in a UK setting. We also consider other scenarios, such as high contact attack rates that might be observed in a major influenza epidemic or pandemic event.
Methods
The SAVE (Simulating Anti-influenza Value and Effectiveness) model, which was developed initially to compare other influenza treatments with oseltamivir (Tamiflu®, F. Hoffmann-La Roche Ltd.)Footnote 1 for several populations,[15] has been extended to cover PEP within families with family members aged ≥13 years. The model simulates virtual patients and gives estimates of both the overall health outcome (morbidity and mortality) and the associated costs.
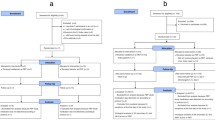
Figure 1 shows the decision tree for the model population. The population has been exposed to a person with ILI (exhibiting symptoms of influenza infection without virological confirmation) within its close environment. Initially, contacts will have a general practitioner (GP) visit to discuss receiving PEP. GP visits to treat ill index cases are not included in the prophylaxis model. The outcomes are calculated through the pathways consequent to this decision, allowing a comparison of what the total costs and health effects would be with or without prescription of oseltamivir at this point. In published studies of PEP oseltamivir use within the family setting the dose administered was 75mg once daily orally for 7–10 days.[7,11] In either branch, with or without prophylaxis, the person may or may not develop ILI. If the person develops ILI, he or she will then enter the treatment part of the model.
In the treatment part of the model, two options have been examined. In scenario one, no treatment other than usual care is given. In scenario two, patients who didn’t receive PEP with oseltamivir, and who develop an ILI, receive treatment with oseltamivir. Usual care is defined as symptomatic treatment only (i.e. bed rest and over-the-counter [OTC] medication such as antipyretics and cough suppressants). Treatment with oseltamivir is only proven to be effective in influenza-virus positive patients (rather than ILI) and if started within 48 hours of onset of symptoms.[16] If the patient receives oseltamivir treatment within 48 hours, then the treatment will have an effect. If the patient receives oseltamivir too late, they will not get any benefit of treatment. It is assumed in the base case that a GP will only consider oseltamivir treatment when they believe that the duration of symptoms is <48 hours. If it is known to the GP that duration of symptoms is >48 hours then they should not consider treatment with oseltamivir; the possible pathway for starting treatment with oseltamivir after 48 hours is included for the small fraction of patients who state the wrong duration of symptoms or don’t remember correctly.
A proportion of patients will not be true influenza positive and will therefore not benefit from treatment with oseltamivir. Generally, influenza infection tests are not used so, upon presentation, the infectious state of the patient is unknown and treatment is based on the GP’s assessment of the symptoms. Thus, the proportion of influenza-positive patients depends on the diagnostic accuracy of the GP. However, the patient may develop complications such as pneumonia or bronchitis. As the model was based on ILI rather than proven influenza, those classified as influenza negative could still develop complications as a result of the ILI but oseltamivir is assumed to not have any effect in this situation. Patients are assumed to develop no more than one complication. All patients (with or without complications) will experience one of the three disease states: outpatient (GP, specialist visits only), inpatient (hospitalisation due to ILI or the complications) or death (due to ILI or the complications). Patients who recovered and required no further care (other than the initial GP visit regarding whether PEP should be administered) were included in the outpatient arm.
As described earlier, the model compares PEP with oseltamivir with the current standard of no PEP within families. For both prophylactic intervention and the current standard approach, the model has two possibilities for treatment: oseltamivir and usual care. Two major scenarios, shown in table I, are studied in this paper: (i) oseltamivir PEP versus no PEP (no oseltamivir treatment after infection for either group), and (ii) oseltamivir PEP (with no post-infection oseltamivir treatment) versus no PEP with post-infection oseltamivir treatment.
Oseltamivir is licensed for PEP for persons aged ≥13 years, and this is considered as the study population for this model and analysis. The complications selected for inclusion in the model are pneumonia and bronchitis because they not only have a high incidence within the chosen population but also have a definite association with influenza.[17] Early oseltamivir treatment has been found to significantly reduce the risk of these complications and of hospitalisation for any reason in the month following influenza diagnosis.[18]
First- and second-order Monte Carlo simulations were performed to take into account the uncertainty around input variables and the variability between patients. While second-order Monte Carlo simulation uses distributions rather than point values, first-order Monte Carlo simulation models a chosen number of single virtual patients (100 000), randomly walking through the model, rather than a cohort. For each possible action (i.e. no PEP or PEP with oseltamivir) in the decision tree, 100 000 patients were individually sent through the pathway up to the next decision point (treatment model). From here, either no treatment or treatment with oseltamivir for the no PEP patients in scenario two was then assigned and the patients continued the walk through the model. The model was then re-run 1000 times. The analyses provide as the output incremental cost-effectiveness and cost-utility ratios for the costs and health outcomes associated with the scenarios compared.
The analyses were performed from the perspective of the healthcare payer — the UK NHS. Hence, all direct healthcare costs are included and no indirect costs, such as lost productivity or patient time, are considered. Costs are year 2002 values.
Most of the events occur within 1 year and are therefore not discounted. Only part of the QALYs, life years gained due to avoided premature mortality, occur in the future and are discounted at a rate of 1.5% per annum, a rate that was recommended for economic evaluations in the healthcare field in the UK at the time of this analysis.[19]
Data
The underlying clinical part of the model mainly draws from epidemiological data[1,20–23] and clinical trials conducted by Roche (M76001, WV15670, WV15671, WV15730).[15,18,24]
A systematic literature search (MEDLINE) was performed to retrieve relevant epidemiological data using various clinical, epidemiological and health economic keywords. Epidemiological data were used for the usual care population. Relative changes, as observed in the oseltamivir clinical trials, were applied to derive estimates for the oseltamivir population. Clinical trial data and literature data were complemented with assumptions, supported by expert opinion.Footnote 2
Country-specific resource use and cost data were assigned to events in the model and collected separately.[25–28]
Distributions for second-order Monte Carlo simulation have been used for probabilities of complications, hospitalisations and mortality and days to return to normal activity (see table II).
The likelihood of the index case being infected is included in the model via the attack rate. In the case where the index case has ILI but is not actually infected with influenza, the likelihood of contacts developing ILI is low, whereas the risk would be high in the case where the index ILI case truly has influenza. It is therefore the attack rate for the contacts that drives the cost effectiveness of PEP with oseltamivir. A low attack rate indicates a low likelihood of the index case being influenza positive, while a high attack rate indicates the opposite. However, even if the index ILI case is influenza negative, it was shown in the clinical trials that oseltamivir still offers protection because an individual can also be infected outside the family. The model is run using different assumptions on attack rate.
Diagnostic certainty (i.e. the likelihood that the patient is truly influenza positive) is influenced by a number of factors. Since virological testing for influenza is generally not performed by the GP, diagnostic accuracy depends on the GP’s experience with influenza symptoms, the clinical manifestation of the disease in the individual, and whether influenza is indeed circulating in the community. Diagnostic accuracy was used in the model to adjust for the number of times oseltamivir did offer an effect for treatment (true influenza positive) and the number of times where the drug was wasted as the patient was not influenza positive.
The attack rate in contacts for all base-case scenarios was 8%, and the diagnostic certainty rate (i.e. the proportion of contacts infected with influenza who were correctly diagnosed) was 70%. The rate of true influenza infection in index cases was taken from two studies[7,11] that reported rates of laboratory-confirmed influenza amongst index cases to be between 43% and 65%.
The Likelihood of Infection at Base Case
Table II shows key input parameters (and their sources) both for the PEP intervention and no prophylaxis and for the two treatment possibilities, usual care and oseltamivir for ILI cases.
Clinical trials have shown an effectiveness of 89% for oseltamivir PEP for the unvaccinated older individuals (aged ≥13 years) within families of an ILI case.[7] To evaluate uncertainty around this parameter, a beta-distribution was assigned to this value for probabilistic sensitivity analysis. This means that in the simulations, values for protective efficacy of oseltamivir are picked randomly between 0% and 100% but with a distribution mean of 89%.
Oseltamivir treatment has an impact on duration of illness and the incidence but not the course of complications.[18] This means that treatment of ILI with oseltamivir will reduce the probability of a complication such as pneumonia or bronchitis, but when pneumonia or bronchitis occurs, the probabilities of hospitalisation and mortality following on from these events are the same as for a patient not treated with oseltamivir. Because of limited data availability for bronchitis, no additional hospitalisation is assumed, and the probability of dying from bronchitis as a complication of ILI is assumed to be the same as the probability of dying from an uncomplicated ILI. The duration of illness is not relevant in terms of costs but has an impact on the QALYs saved and work loss (from the societal perspective).
Probabilistic sensitivity analysis (second-order Monte Carlo simulation) has been used to determine the impact of uncertainty around these input parameters (probability of complications, hospitalisations and mortality).
Adverse effects due to oseltamivir PEP and treatment have not been included because the adverse effects observed in clinical trials were generally mild, self-limiting and did not result in healthcare service utilisation.[30] However, the impact of adverse effects on quality of life has been reported in the clinical trials of oseltamivir and is therefore included in the QALY estimate.
Costs and Resource Utilisation
Direct cost and resource use have been estimated for prevention, as well as for the treatment of persons developing influenza (table III). Data on resource utilisation related to treatment of influenza have been drawn from a comprehensive US database (National Ambulatory Medical Care Survey [NAMCS]),[26] because such detailed data are not available for the UK. The database gives estimates on drug prescriptions (particularly antibacterials), tests and investigations performed, as well as primary and secondary care for patients with influenza and selected complications. The overall rate of antibacterial prescriptions is very similar to rates reported in the UK. According to the NAMCS, 83% of complicated influenza cases receive antibacterials, while a UK study has estimated a rate of 80%.[1] The rates for uncomplicated influenza are 36% and 40%, respectively.[1] UK unit costs were attached to resource utilisation.[25,27]
Health Outcome
Health outcome (the denominator of the cost-effectiveness or cost-utility ratio) was measured in two ways: ILI cases avoided and QALYs saved. ILI cases avoided is an important disease-specific outcome for cost-effectiveness analysis. However, it does not cover avoided morbidity and premature mortality. QALYs saved were therefore used for cost-utility analysis, giving an estimate of the quality of life gain due to avoided ILI infections. This also enables a comparison with interventions other than influenza PEP.
Calculation of Utility
The patient’s health state was evaluated as part of the clinical trials used within this analysis (Bettis et al., unpublished data). The patients were asked to rate their wellbeing on three eleven-point Likert visual analogue scales (VAS; health, sleep and usual activities) daily for 21 days. The scales used had been pilot tested in 1997 in 52 patients and were found to have acceptable psychometric properties, showing a very high correlation between influenza symptom scores (ISS) and wellbeing indicators.[31] In order to measure impact on quality of life (QALYs), the health VAS scale was assumed to represent the broadest aspect of health and therefore used in the cost-utility analysis.
In order to transform this health valuation into a utility measure, the VAS Likert health scale, which spans 0–10 was recalibrated to a VAS scale with 1 as uppermost value and allowing negative values. This was necessary because of the Likert scale’s top end definition of ‘normal health for someone at your age’ and because the patients in the Roche trials were only allowed to circle whole numbers between 0 and 10. Then the VAS scales were transformed into time trade-off (TTO) scores using the following algorithm: TTO = −0.445 + (2.112 × VAS) + (-0.580 × VAS2). The algorithm is based on econometric work done by York University.[32] This is the same approach as adopted by NICE in their technology report.[14] The utility of experiencing pneumonia is calculated as the utility weight for pneumonia multiplied by the time (days) spent with pneumonia. This process is then repeated for influenza. The utility weights calculated are shown in table II. Average time to development of a ILI complication was derived from the clinical trials (Bettis et al., unpublished data). In order to calculate QALYs for years of life lost due to premature death, an average age of death was assumed and from UK life tables it was estimated that 34.24 years of life were lost.[33] A lost life year was assumed equal to 1 QALY.
Sensitivity Analyses
A number of sensitivity analyses were performed in addition to the underlying probabilistic analysis. These explored the impact of varying influenza seasons (change in ILI attack rate for contacts of the index case), healthcare resource utilisation related to PEP and uncertainty of accurate diagnosis in influenza patients treated with oseltamivir. Additionally, an analysis from the societal perspective was performed.
Overall prophylactic effectiveness depends on whether the index case is infected and on the overall attack rate in the community. This analysis uses an 89% reduction in number of ILI cases in the base case, even though <50% of the index cases were infected (laboratory confirmed influenza A or B).[7] This is lower than the percentages observed in oseltamivir treatment trials (about 70%) or in the other household-based trials of oseltamivir PEP (62%).[11] The underlying study’s inclusion criteria allowed enrolment of index cases without fever, decreasing the probability of true influenza in the index case, and hence the potential influenza transmission to family members.[7] A low prophylactic effectiveness of 60% was assumed for sensitivity analysis. The underlying assumption is that a rate of <50% of infected index cases results in lower overall prophylactic effectiveness.
Several clinical attack rates in household contacts observed in various influenza seasons were used: 8% for base case and 12% in sensitivity analyses.[7,10,34] An additional sensitivity analysis with an assumed high attack rate of 30% (i.e. a ‘pandemic scenario’) to simulate the potential impact when a novel virus is circulating and affects a high proportion of a naïve population was performed. One study of PEP with amantadine conducted during the 1968 pandemic found that 41% of 86 family contacts of an influenza-positive index case had serological evidence of influenza A virus infection.[35] During a pandemic outbreak, attack rates are higher in the family than in the wider community as a result of closer contact circumstances.
The base-case analysis includes a GP visit for each contact to obtain a prescription for PEP. However, it may be unrealistic to expect healthy persons to make a GP visit and to be able to gain an appointment quickly. Rather, PEP prescriptions for contacts may be given to the index case if visiting the GP, or may be dispensed by the office nurse or pharmacy. Therefore, a sensitivity analysis with no initial GP visit for contacts is performed as a lower bound for healthcare utilisation costs related to PEP.
Diagnostic certainty describes the proportion of persons infected with influenza among the population having ILI. This rate is much higher when influenza is circulating and has been assumed to be 70% for the base case based on observations from clinical trials with oseltamivir. This rate was increased to 85% for sensitivity analyses to indicate the diagnostic certainty in a pandemic situation.
A sensitivity analysis from the societal perspective was also performed. Productivity loss was valued by applying the human capital approach, thus using the time to return to normal activity (days) as evaluated in epidemiological studies and clinical trials, and valuing the time with the average gross UK income according to the Office for National Statistics (£10.53 per hour).[28] Time to return to normal activities after an ILI episode was calculated to be 12.34 days (gamma distributionFootnote 3 [9.26, 15.30]) for usual care and 9.29 days (gamma distribution3 [6.97, 11.61]) with oseltamivir PEP, in line with the NICE approach.[14] Time loss (including work and leisure time lost) was measured by using the time to return to normal activities. For uncomplicated influenza cases, this is the time to return to normal activities as measured in the clinical trials. For patients with complications but no hospitalisation this is the average duration until the complication develops and the average duration of the complication (Bettis et al., unpublished trial data). For hospitalised influenza patients, the time loss is equal to half the time of an outpatient plus the length of stay in hospital.[24] For hospitalised patients with a complication, the time loss is the average duration until the complication develops plus the time of an outpatient with that complication plus the length of stay in hospital with that complication.
The loss of working days appears relatively long, but this is because the population includes high-risk individuals who have a longer time to return to normal activity than otherwise healthy adults. In addition, the number of lost work days is taking into account a proportion of patients that experience complications. Based on the clinical trial data, time to return to normal health was 9.29 days for oseltamivir-treated patients, 12.34 for usual care-treated patients, 16.23 days for bronchitis outpatients, 19.67 days for pneumonia outpatients, and 25.82 days in total for pneumonia inpatients (Bettis et al., unpublished data). This is allowing for an average time elapsing before the complication occurred. We did not subtract weekend days as we are explicitly modelling time loss that includes work loss and leisure time lost.
The scenarios analysed are outlined in table IV.
Results
Base-Case Analyses
Base-case analyses compared no prophylaxis with oseltamivir PEP in two scenarios defined by subsequent treatment of infected cases (table I).
Table V shows the simulation results (100 000 contacts per iteration, 1000 iterations) for both base-case scenarios in terms of overall costs, number of ILI cases and QALYs gained, as well as the incremental cost-effectiveness and cost-utility ratios. For both scenarios there are fewer cases of influenza with oseltamivir PEP than with no prophylaxis: approximately 880 versus 8000 cases, respectively. However, the overall costs including prophylaxis and treatment of infected persons are higher with oseltamivir PEP (£38 vs £4–5 per person with no prophylaxis). This results in costs per case avoided of £467 and £451 for the two scenarios.
QALYs not only include a measure for cases avoided but also the differences in quality of life due because of different treatment strategies (usual care or oseltamivir) for infected cases. QALYs gained are 0.0011 for scenario one and 0.0006 for scenario two. Consequently, costs per QALY gained are lower for scenario one (£29 938) than for scenario two (£52 202). The choice of treatment for infected cases obviously has a larger impact if no PEP has been taken, as the number of infected cases is higher. The cost acceptability curve for scenario one is shown in figure 2. It can be seen that 50% of all simulations in the probabilistic sensitivity analysis fall below a willingness to pay of £30 000 per QALY.
Sensitivity Analyses
Sensitivity analyses were performed for both base-case scenarios for a lower prophylactic effectiveness rate (60%), higher attack rates (12% and 30%), diagnostic certainty rates for infected contacts of 85% and the societal perspective. Generally, a similar pattern was observed as for base-case analyses: similar cost-effectiveness ratios across the two scenarios but lower (i.e. better) cost-utility ratios for scenario one than for scenario two (table VI).
A decrease of the prophylactic effectiveness to 60% (analysis B) increases cost-effectiveness ratios to £709 and £686 per case averted and cost-utility ratios to £45 323 and £128 560 per QALY for scenario one and two, respectively.
Higher attack rates (analyses D and E) show more favourable cost-effectiveness and cost-utility ratios than lower ones (table VI; e.g. for scenario one: £5403 per QALY for 30% attack rate vs £18 697 per QALY for 12% attack rate). The cost-acceptability curve for an attack rate of 30% (with a diagnostic certainty rate of 85%) representing a pandemic situation for scenario one is shown in figure 3. Under these pandemic conditions, there was a close to 100% likelihood of oseltamivir PEP being cost effective at a threshold of £10 000–15 000 per QALY.
Cost-acceptability curve for sensitivity analysis of scenario one of oseltamivir for post-exposure prophylaxis against influenza vs no prophylaxis (cost per QALY from a healthcare payer perspective). Analysis of a contact attack rate of 30% (with a diagnostic certainty rate of 85%), representing a pandemic situation. Ceiling ratio represents the maximum willingness to pay.
Excluding the initial GP visit for contacts to obtain a prescription for PEP (analysis C), cost-effectiveness and cost-utility ratios are much lower than in the base-case analysis (table VI).
PEP is the dominant strategy (i.e. more effective and less costly than no PEP) from the societal perspective. However, work loss related to the initial GP visit for contacts to get a prescription for PEP with oseltamivir was not included.
Discussion
Although annual vaccination is the cornerstone of influenza management, neuraminidase inhibitors such as oseltamivir are an important adjunctive option in both prophylaxis and treatment. Our analysis found that PEP with oseltamivir is likely to be a cost-effective strategy for adolescent and adult family members in the UK from a healthcare payer perspective when ILI contact attack rates in the family are assumed to be 8% or higher and in the absence of a specific treatment option. The incremental cost-utility ratios range from £5403 to £128 560 per QALY gained depending on prophylactic effectiveness, ILI attack rate and treatment strategies used for infected cases. Probabilistic modelling also shows that the probability of oseltamivir PEP being cost effective from the healthcare payer perspective is highest in the case of a pandemic.
In addition, our sensitivity analyses illustrate the large economic impact of the mechanism by which contacts access the drug. Although the base case incorporated a GP visit to obtain a prescription for each contact, other scenarios could reduce or eliminate the need for such visits (e.g. multiple scripts written at one visit, prescriptions given at time of illness visit for index case, telephone prescribing or non-prescription availability of oseltamivir through pharmacy) and markedly reduce the cost of preventing ILI.
The results of the current analysis suggest that prophylaxis with oseltamivir is cost effective compared with no prophylaxis when usual care is the only treatment option. However, considering data recently published on healthy adults,[15] it appears that treatment of influenza with oseltamivir is a more cost-effective use of resources than prophylaxis. Thus, superficially, it seems that there is no need for prophylaxis and that all patients can be treated once symptoms develop. However, this viewpoint misses two factors. First, the scenario that we adopted for determining the cost effectiveness of prophylactic oseltamivir was very conservative with regards to the ILI contact attack rate within families (very low at 8%), and the number of GP visits per family (one per person, when one visit may cover the whole family). Second, treatment may be more cost effective in terms of resources, but this does not take into account the impact of influenza on the patient, family and workplace. Indeed, from a societal point of view both prophylaxis and treatment are cost-saving interventions in the healthy adult population.[15] The main impact of influenza on the otherwise healthy adult population is on work participation, and the price of the drug is well below the value of a day’s work. The main driver in cost effectiveness of both treatment and prophylaxis of influenza from a society’s point of view would therefore be to make sure that the drug is given to influenza-positive patients and that prophylaxis is offered when the contact attack rate can be assumed to be medium or high, or when the value or opportunity cost of lost work participation is high.
Despite numerous publications on influenza and its control, specific data on attack rates and influenza-related hospitalisations, complications and mortality are limited and depend on viral strain and population characteristics.[36] Further, data are limited with respect to the effects of antiviral treatment and prophylaxis, so that the assumptions for the treatment part of our model are based on only a few studies (reviewed in Cooper et al.[37]). Also, we have used ILI cases rather than laboratory-defined cases for both the index cases potentially exposing family members to influenza and the number of symptomatic cases who receive treatment. To use only actual influenza cases would improve the effect of oseltamivir PEP and treatment, since its antiviral and clinical effects are virus specific. The model incorporates probabilistic sensitivity analysis to examine the impact of these limitations. As aggregated resource use data were unavailable for the UK, we used a detailed US database (DHHS/NAMCS 1997),[26] as the aggregated data have been shown to be comparable to that from UK sources.[1] The resource use data were then combined with UK unit costs for use in the analyses.
For PEP, the number of cases prevented is an important endpoint. The preventive efficacy of PEP with oseltamivir within families[7,11] and of seasonal prophylaxis in open and institutionalised populations of adults[5,6] has been estimated in four controlled clinical trials. The point estimates for protection of individuals ranged from 58% to 92% in these studies. In the current analysis, a probability of 0.89 with a beta distribution (0.89; 0.11) was used for prophylactic effectiveness.
Various contact attack rates were tested in the sensitivity analysis, allowing for the increased virulence of influenza, and the possible vaccination of a proportion of the contacts. Prior studies of PEP with oseltamivir and other anti-influenza agents found secondary attack rates in contacts not receiving prophylaxis of 5–29% in households with a proven influenza virus introduction.[7,34,38] If the household consists of unvaccinated individuals, then the ILI attack rate is high, while with vaccinated household members the attack rate is naturally low. The decision to provide PEP therefore also depends on the vaccination status of the individuals in the household. Our 8% scenario represents a relatively low ILI contact attack rate following introduction of influenza in the household, but obviously also reflect the fact that the model assumes that a number of index cases are not true influenza cases.
To illustrate a pandemic influenza season, an attack rate of 30% was chosen for analysis. However, this approach has to be treated with caution. New strains of influenza emerging in a pandemic may be more virulent (i.e. cause greater morbidity [complications, hospitalisations] and mortality). It is not known how effective neuraminidase inhibitors such as oseltamivir would be in such situations, although oseltamivir has in vitro antiviral activity against all nine neuraminidase subtypes recognised in nature and has in vivo activity against pandemic threat viruses such as H5N1 and H9N2 viruses.[39] In addition, the M2 inhibitors (amantadine and rimantadine) have proven prophylactic activity in pandemic influenza, although with somewhat lower efficacy compared with the inter-pandemic period (reviewed in Hayden[40]). In view of the fact that most people would be expected to have no specific immunity against such a new strain and that the availability of an effective vaccine is uncertain, oseltamivir could be a valuable intervention for prophylaxis in pandemics. Nevertheless, pandemic situations are unpredictable in terms of occurrence and impact and the ability to produce large quantities of antiviral drugs during a pandemic response is negligible at present. Consequently, pre-event stockpiling of neuraminidase inhibitors as part of a pandemic preparedness plan will be required to guarantee supplies.[40,41]
One of the potential problems with the use of any antiviral is the development of resistance. However, current rates of resistance to oseltamivir in the community remain low despite extensive use. In a recent screening of community-derived influenza isolates in Japan during the 2003–4 influenza season, 0.4% of isolates were oseltamivir-resistant,[42] even though approximately 6 million treatment courses were used, and 5% of the population received oseltamivir. Furthermore, cumulative clinical trial data reveal that, during treatment with oseltamivir, 0.33% of adults and 4% of children developed resistance.[43] A higher rate of resistance (18%) has been reported in Japanese children.[44] However, this may be as a result of underexposure to oseltamivir in young children as Japan is the only country that does not use a unit-administration approach approved globally and uses 2 mg/kg across all child age groups.
Conclusion
In conclusion, under a set of very conservative assumptions where the effects of herd immunity were not assessed, the current cost-effectiveness and cost-utility analysis suggests that, from a healthcare payer perspective, post-exposure influenza prophylaxis with oseltamivir is a cost-effective strategy for family contacts in the UK, when contact attack rates are high (>8%) and no specific treatment option is available. Under real-life conditions when attack rates within families will be much higher, and when contacts are able to obtain oseltamivir without a physician visit/prescription, the cost effectiveness of oseltamivir will increase.
While targeting the healthy adult/adolescent population, as in the current analysis, will minimise social disruption and economic loss, it should be noted that if minimisation of morbidity and mortality is the goal, then children and the elderly may in fact be the most important target populations.
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
Expert opinion was sought by formal consensus meeting with two top medical experts in the field of influenza.
Gamma (alpha, beta): the two parameters are fitted using the mean \(({\bar \mu})\) and variance (s2) from the observed sample:
$$\matrix{{\alpha = {{{{\bar \mu}^2}} \over {{{\rm{s}}^2}}}} \;\;\;\; {\beta = {{{{\rm{s}}^2}} \over {\bar \mu}}} \cr}$$
References
Meier CR, Napalkov PN, Wegmuller Y, et al. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis 2000; 19: 834–842
Hak E, Hoes AW, Verheij TJM. Influenza vaccinations: who needs them and when? Drugs 2002; 62: 2413–2420
Tacken M, Braspenning J, Spreeuwenberg P, et al. Patient characteristics determine differences in the influenza vaccination rate more so than practice features. Prev Med 2002; 35: 401–406
de Jong JC, Beyer WE, Palache AM, et al. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A (H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol 2000; 61: 94–99
Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med 1999; 341: 1336–1343
Peters PH, Gravenstein S, Norwood P, et al. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001; 49: 1025–1031
Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA 2001; 285: 748–754
Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283: 1016–1024
Nicholson KG, Aoki FY, Ostethaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355: 1845–1850
Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001; 20: 127–133
Hayden FG, Belshe R, Villanueva C, et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis 2004; 189: 440–449
Neuzil KM, Mellen BG, Wright PF, et al. The effect of influenza on hospitalisations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342: 225–231
Fox JP, Cooney MK, Hall CE, et al. Influenza virus infections in Seattle families, 1975–1979: II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am J Epidemiol 1982; 116: 228–242
National Institute for Clinical Excellence. Guidance on the use of oseltamivir and amantadine for the prophylaxis of influenza. NICE Technology Appraisal Guidance. No 67. Ref: N0292. London: NICE, 2003 Sep [online]. Available from URL: http:/www.nice.org.uk [Accessed 2002 Feb]
Vindt Holm M, Gyldmark M, Holme Hansen E. Pharmacoeconomic assessment of oseltamivir in treating influenza: the case of otherwise healthy Danish adults and adolescents. Pharm World Sci 2004 Dec; 26 (6): 339–345
Aoki FY, Macleod MD, Paggiaro P, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother 2003; 51 (1): 123–129
Nicholson KG, Webster RG, Hay AI. Textbook of influenza. London: Blackwell Science Ltd, 1998
Kaiser L, Wat C, Mills T, et al. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalisations. Arch Intern Med 2003; 163: 1667–1672
National Institute for Clinical Excellence. Guidance for manufacturers and sponsors: technological appraisals process series 5. London: NICE, 2001
Simonsen L, Fukuda K, Schonberger LB, et al. The impact of influenza epidemics on hospitalisations. J Infect Dis 2000; 181: 831–837
Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289: 179–186
Bochud PY, Moser F, Erard P, et al. Community-acquired pneumonia: a prospective outpatient study. Medicine (Baltimore) 2001; 80: 75–87
Die Spitaler der Schweiz. H+ Spitalstatistiken, Medizinische Gesarrtstatistik der Schweizer Spitalër 1996. H+ Verlag CH-5001 Aarau, 1997
Data on file, Hoffmann-La Roche
Netten A, Curtis L. Unit costs of health and social care. Personal Social Services Research Unit, University of Kent at Canterbury [online]. Available from URL: http://www.pssru.ac.uk/pdf/uc2003/uc2003.pdf [Accessed 2003 Sep 9]
Department of Health and Human Services. National ambulatory medical care survey (NAMCS). Ann Arbor (MI): National Centre for Health Statistics, 1997
British National Formulary No 42. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2001
Office for National Statistics. New earnings survey 2000. Rev. ed. London: 2001 [online]. Available from URL: http://www.statistics.gov.uk [Accessed 2003 Sep]
Stouthard M, Essink-Bot M, Bonsel G, et al. Disability weights for diseases in the Netherlands. Rotterdam, Netherlands: Department of Public Health, Erasmus University Rotterdam, 1997
Enger C, Nordstrom BL, Thakrar B, et al. Health outcomes among patients receiving oseltamivir. Pharmacoepidemiol Drug Saf 2004; 13: 227–237
Osborne R, Hawthorne G, Papanicolaou M, et al. Measurement of rapid changes in health outcomes in people with influenza symptoms. J Outcomes Res 2000; 4: 15–30
MVH Group. The measurement and valuation of health: final report on the modelling of valuation tariffs. York University: Centre for Health Economics, 1995
Pearce D, Goldblatt P, editors. United Kingdom health statistics life expectancy: the government actuary’s department: interim life tables. London: The Stationery Office Health Statistics Quarterly (12), Winter 2001
Hayden FG, Belshe RB, Clover RD, et al. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N Engl J Med 1989; 321: 1696–1702
Galbraith AW, Oxford JS, Schild GC, et al. Study of 1-adamantanamine hydrochloride used prophylactically during the Hong Kong influenza epidemic in the family environment. Bull World Health Organ 1969; 41: 677–682
Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalisations in the United States. JAMA 2004; 292: 1333–1340
Cooper NJ, Sutton AJ, Abrams KR, et al. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326: 1235–1241
Hayden FG, Gubareva LV, Monto AS, et al. Inhaled zanamivir for the prevention of influenza in families: Zanamivir Family Study Group. N Engl J Med 2000; 343: 1282–1289
Leneva IA, Roberts N, Govorkova EA, et al. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res 2000; 48: 101–115
Hayden FG. Perspectives on antiviral use during pandemic influenza. Philos Trans R Soc Lond B Biol Sci 2001; 356: 1877–1884
Monto AS. The threat of an avian influenza pandemic. N Engl J Med 2005; 352: 323–325
Neuraminidase Inhibitor Susceptibility Network. Use of influenza antivirals during 2003–2004 and monitoring of neuraminidase inhibitor resistance. Wkly Epidemiol Rec 2005; 17: 156
Ward P, Small I, Smith J, et al. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother 2005; 55 Suppl. 1: 15–21
Kiso M, Mitamura K, Sakai-Tagwaw Y, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 2004; 364: 759–765
Acknowledgements
Financial support for this study was provided entirely by a contract with F. Hoffmann-La Roche Ltd, Pharmaceuticals Division. The funding agreement ensured the non-Roche authors’ independence in designing the study, interpreting the data and writing and publishing the report. We thank Dr Iain Bartlett, Wolters Kluwer Health, for editorial assistance. Marlene Gyldmark and Louis Garrison are employed by the sponsor. Both Beate Sander and Frederick Hayden have presented at meetings for F. Hoffmann-La Roche Ltd. All authors were involved in the design of the study and in the critical review of the manuscript; Beate Sander, Frederick Hayden and Marlene Gyldmark were involved in data analysis.#
Parts of a previous version of the paper were presented at the 2003 Conference of the International Society for Pharmacoeconomics and Outcomes Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sander, B., Hayden, F.G., Gyldmark, M. et al. Post-Exposure Influenza Prophylaxis with Oseltamivir. Pharmacoeconomics 24, 373–386 (2006). https://doi.org/10.2165/00019053-200624040-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200624040-00007