Summary
Synopsis
Acamprosate (calcium acetylhomotaurinate), a synthetic compound with a similar chemical structure to that of γ-aminobutyric acid, is thought to act via several mechanisms affecting multiple neurotransmitter systems; inhibition of neuronal hyperexcitability by antagonism of excitatory amino acid activity and reduction of calcium ion fluxes has been suggested as its predominant mechanism of action. The drug is the first agent specifically designed to maintain abstinence in alcohol (ethanol)-dependent patients after detoxification.
Voluntary oral ethanol consumption in ethanol-preferring or ethanol-dependent rats is dose-dependently reduced by acamprosate; total fluid intake and food consumption are not affected. The drug does not potentiate the acute or chronic toxic effects of ethanol and has no hypnotic, antidepressant, anxiolytic or muscle-relaxant effects in animals. There is no evidence of abuse potential with acamprosate.
Oral acamprosate 1.3 or 2 g/day in 3 divided doses administered for 3 to 12 months to alcohol-dependent patients after detoxification was more effective than placebo in preventing alcohol relapse according to abstinence rates, duration of abstinence, γ-glutamyl transferase levels and/or a variety of other clinical or biological end-points. Concomitant psychosocial/behavioural therapies were used in some trials. Compared with those with placebo, the superior abstinence rates and durations of abstinence with acamprosate were maintained during 6-to 12-month post-treatment follow-up periods, and greater abstinence rates with acamprosate were confirmed in a pooled analysis of data from 11 randomised placebo-controlled trials involving a total of 3338 patients with alcohol dependence. The efficacy of acamprosate appears to be dose dependent and enhanced by the addition of disulfiram.
Acamprosate was generally well tolerated in placebo-controlled trials. The most common adverse events were gastrointestinal (especially diarrhoea) or dermatological and were mostly mild and transient. The percentage of patient withdrawals because of adverse events was similar in acamprosate and placebo groups.
No trials have compared the efficacy or tolerability of acamprosate with those of other treatment approaches (including opiate antagonists or selective serotonin reuptake inhibitors) aimed at maintaining abstinence in detoxified alcohol-dependent patients.
Thus, acamprosate, as an adjunct to psychosocial/behavioural therapies, represents a novel advance for the management of alcohol-dependent patients in the postdetoxification period. Longer term and comparative trials with other active therapies are required to confirm these promising results.
Pharmacodynamic Properties
Acamprosate (calcium acetylhomotaurinate) is a synthetic compound with a chemical structure similar to that of γ-aminobutyric acid (GABA). The exact cellular target of acamprosate is not clear; it has been suggested that inhibition of neuronal hyperexcitability by antagonism of excitatory amino acid activity and reduction of calcium ion fluxes is its predominant mechanism of action. Several other possible mechanisms of action have been suggested, including suppression of conditioned alcohol withdrawal-induced craving.
The drug binds preferentially to GABAB receptors and enhances synaptosomal [3H]GABA uptake. Serotonergic properties of acamprosate have been demonstrated by acamprosate-induced increases in blood and cerebral serotonin (5-hydroxytryptamine; 5-HT) levels in rats exposed to ethanol vapour, intensification of convulsions caused by tryptamine, potentiation of ‘heat twitches’ induced by 5-methoxy-NN-dimethyl-tryptamine and increases in the binding capacity of 5-HT1D and 5-HT2 receptors. Noradrenergic antagonist activity is supported by acamprosate-induced increases in the number of adrenergic receptors, antagonism of the effect of ethanol on β-adrenoceptors, antagonism of reserpine-induced hypothermia and potentiation of yohimbine-induced toxicity.
Acamprosate dose-dependently reduces or suppresses voluntary oral ethanol consumption in ethanol-preferring or ethanol-dependent rats without affecting total fluid intake or food consumption. This effect is inhibited by the GABAA-receptor antagonist bicuculline. Long term acamprosate administration (for 15 days) did not potentiate the acute or chronic toxic effects of ethanol in rats.
Acamprosate was not self-administered by animals (rhesus monkeys) that freely used cocaine or pentobarbital, and the drug did not induce pentobarbital-or d-amphetamine-appropriate responding in animals (rhesus monkeys or pigeons) trained to discriminate between these agents. The drug also had no hypnotic, antidepressant, anxiolytic or muscle-relaxant effects in animals.
Pharmacokinetic Properties
Based on pharmacokinetic data from healthy volunteers, the mean maximum plasma concentration (Cmax) of acamprosate was 180 μg/L after a single oral dose of 666mg and the area under the plasma concentration-time curve was 3700 μg/L · h. Absorption of acamprosate via the gastrointestinal tract is slow and there is high interindividual variation; however, most of the drug appears to be absorbed within 4 hours. Steady-state acamprosate concentrations are achieved after 7 days’ administration. Concomitant food decreases the bioavailability of acamprosate by approximately 20%; the pharmacokinetics of the drug are unaffected by concomitant alcohol (ethanol) intake.
The apparent half-life of acamprosate is 13 hours after oral administration; the drug is not metabolised and is excreted unchanged in the urine. Acamprosate crosses the blood-brain barrier.
The pharmacokinetics of acamprosate in alcohol-dependent patients weaned from alcohol are similar to those in healthy volunteers.
After single dose administration of acamprosate 666mg, Cmax values were significantly higher, time to Cmax was significantly longer and plasma elimination half-life was significantly longer in patients with severe renal impairment (creatinine clearance 0.3 to 1.7 L/h/1.73m3) than in healthy volunteers; total apparent plasma clearance and renal clearance values were also significantly lower in patients with moderate (creatinine clearance 1.8 to 3.6 L/h/1.73m2) or severe renal impairment. These results suggest that accumulation of acamprosate would occur with prolonged administration of therapeutic dosages of the drug to patients with renal impairment.
The pharmacokinetic profile of acamprosate is not modified by hepatic impairment; there are no data from the elderly. Studies assessing the pharmacokinetics of acamprosate specifically in patients with alcohol dependence have not been assessed.
Clinical Potential
The efficacy of oral acamprosate 1.3 or 2 g/day, given in 3 divided doses to alcohol-dependent patients after detoxification, has been assessed in several well designed double-blind placebo-controlled trials. Treatment was initiated approximately 1 to 4 weeks after the start of detoxification and was continued for 3 to 12 months; various concomitant therapies including psychotherapy/behavioural therapies and treatments for coexisting conditions were used in some studies. Data were generally assessed according to intention-to-treat principles.
In these trials, acamprosate was more effective than placebo in preventing alcohol relapse as assessed by absolute, cumulative and/or continuous abstinence rates and durations of abstinence. When assessed, between-treatment differences in γ-glutamyl transferase levels and/or a variety of other clinical or biological end-points (including patient and/or physician impressions of efficacy, clinic attendance and physical signs of alcohol intake) support the greater efficacy of acamprosate when compared with placebo. Superior abstinence rates and durations of abstinence with acamprosate, compared with placebo, were sustained during 6-to 12-month post-treatment follow-up periods. The efficacy of acamprosate appears to be dose dependent and enhanced by the addition of di-sulfiram.
In a pooled analysis of data from 11 randomised placebo-controlled trials involving a total of 3338 patients with alcohol dependence, abstinence rates after 6 and 12 months were significantly greater with acamprosate than with placebo (35 vs 25% and 33 vs 21%, respectively).
No trials have compared the efficacy of acamprosate with that of other active treatments aimed at maintaining abstinence in alcohol-dependent patients after detoxification.
Tolerability
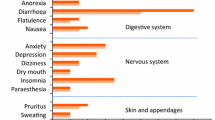
In short (3 to 6 months) and longer term (1 to 2 years) placebo-controlled trials of alcohol-dependent patients in the postdetoxification period, acamprosate was generally well tolerated, with most adverse events being mild and transient. The most common adverse effect was dose-related diarrhoea, which occurred in a similar or significantly greater number of acamprosate than placebo recipients (7 to 20% vs 3 to 12%). Other adverse events which may have been related to acamprosate therapy were mainly gastrointestinal (abdominal pain, nausea and vomiting) or dermatological (pruritus, maculopapular rash and bullous skin reactions). Dizziness, confusion, drowsiness, headache and fluctuations in libido have also been reported. The percentage of patients withdrawing from these trials because of adverse events was similar in acamprosate and placebo groups.
Transient but significant reductions in diastolic, but not systolic, blood pressure were observed in patients with alcohol-induced hepatic cirrhosis and low or moderate hepatic impairment (Pugh grade A or B) who received acamprosate 666mg (2 tablets). No clinically significant effects on heart rate, renal or hepatic function or haematological or biochemical parameters have been reported with this agent to date.
There is no clinical evidence of abuse potential with acamprosate, and over-dosage (≤43g) in 5 patients was uneventful.
Tolerability data from longer term trials, postmarketing surveillance and comparative trials of acamprosate and other active therapies are required.
Dosage and Administration
The currently recommended daily dosage of acamprosate for alcohol-dependent patients after detoxification is 1.3 g/day in patients with a body weight of <60kg and 2 g/day in those with a body weight of >60kg. The drug should be administered orally in 3 divided doses; the recommended duration of treatment is 1 year. Acamprosate should be initiated in conjunction with counselling as soon as possible after the acute alcohol withdrawal period.
Acamprosate is contraindicated in patients with a known hypersensitivity to the drug, in pregnant or lactating women, in patients with renal impairment (serum creatinine >0.12 mmol/L), in patients with severe hepatic failure (Pugh grade C), in children and in the elderly. Specific dosage recommendations for patients with coexisting diseases have not been made.
Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association, 1994: 176–81
Liskow BI, Goodwin DW. Pharmacological treatment of alcohol intoxication, withdrawal and dependence: a critical review. J Stud Alcohol 1987; 48: 356–70
Peters DH, Faulds D. Tiapride: a review of its pharmacology and therapeutic potential in the management of alcohol dependence syndrome. Drugs 1994 Jun; 47: 1010–32
Deitrich RA, Dunwiddie TV, Harris RA, et al. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev 1989 Dec; 41: 489–537
Chick J. Acamprosate as an aid in the treatment of alcoholism. Alcohol Alcohol 1995 Nov; 30: 785–7
Rassnick S, D’Amico E, Riley E, et al. GABA and nucleus accumbens glutamate neurotransmission modulate ethanol self-administration in rats. Ann NY Acad Sci 1992 Jun 28; 654: 502–5
Durlach J, Rinjard P, Sprince H, et al. Similar antagonistic effects of Ca N-acetylhomotaurinate on depression of motor activity and lethality induced by acetaldehyde or ethanol. Methods Find Exp Clin Pharmacol 1988 Jul; 10: 437–47
Littleton J. Acamprosate in alcohol dependence: how does it work? Addiction 1995; 90: 1179–88
Durbin P, Hulot T, Chabac S. Pharmacodynamics and pharmacokinetics of acamprosate: an overview. In: Soyka M, editor. Acamprosate in relapse prevention of alcoholism. Proceedings of the 1st Campral-symposium ESBRA Stuttgart. Germany, Springer, 1995: 47–64
Soyka M. Relapse prevention in alcoholism: recent advances and future possibilities. CNS Drugs 1997; 7(4): 313–27
Zeise ML, Kasparov S, Capogna M, et al. Acamprosate (calciumacetylhomotaurinate) decreases postsynaptic potentials in the rat neocortex: possible involvement of excitory amino acid receptors. Eur J Pharmacol 1993 Jan 26; 231: 47–52
Zieglgänsberger W, Hauser C, Putzke J, et al. Acamprosate reduces the enhanced excitability of central neurons following chronic alcohol intake. Pharmacopsychiatry 1995 Sep; 28: 231
Nalpas B, Dabadie H, Parot P, et al. Acamprosate: from pharmacology to therapeutics [in French]. Encephale 1990 May–Jun; 16: 175–9
Bouchenafa O, El-Qatari M, Khan S, et al. Potential mechanisms of action of the anti-craving drug, acamprosate, in alcohol dependence [abstract]. Behav Pharmacol 1995 Aug; 6: 614
Spanagel R, Zieglgänsberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci 1997; 18: 54–9
Madamba SG, Schweitzer P, Zieglgänsberger W, et al. Acamprosate (calcium acetylhomotaurinate) enhances the N-methyl-d-Aspartate component of excitatory neurotransmission in rat hippocampal CA1 neurons in vitro. Alcohol Clin Exp Res 1996; 20(4): 651–8
Daoust M, Lhuintre JP, Saligaut C, et al. Calcium bis acetyl homotaurine: un novel agoniste Gabaergique? J Pharmacol 1985; 16: 521
Daoust M, Legrand E, Gewiss M, et al. Acamprosate modulates synaptosomal GABA transmission in chronically alcoholised rats. Pharmacol Biochem Behav 1992 Apr; 41: 669–74
Boismare F, Daoust M, Moore N, et al. A homotaurine derivative reduces the voluntary intake of ethanol by rats: are cerebral GABA receptors involved? Pharmacol Biochem Behav 1984 Nov; 21: 787–9
Le Magnen J, Tran G, Durlach J, et al. Dose-dependent suppression of the high alcohol intake of chronically intoxicated rats by Ca-acetyl homotaurinate. Alcohol 1987 Mar–Apr; 4: 97–102
Boismare F, Daoust M, Lhuintre JP, et al. Which GABA receptors are involved in the voluntary ethanol intake by rats [abstract]? Alcohol Alcohol 1986; 21: A30
Gewiss M, Heidbreder C, Opsomer L, et al. Acamprosate and diazepam differentially modulate alcohol-induced behavioural and cortical alterations in rats following chronic inhalation of ethanol vapour. Alcohol Alcohol 1991; 26(2): 129–37
Spanagel R, Höher SM, Allingham K, et al. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol 1996; 305: 39–44
Le Magnen J, Tran G, Durlach J. Lack of effects of Ca-acetyl homotaurinate on chronic and acute toxicities of ethanol in rats. Alcohol 1987 Mar–Apr; 4: 103–8
Grant KA, Woolverton WL. Reinforcing and discriminative stimulus effects of Ca-acetyl homotaurine in animals. Pharmacol Biochem Behav 1989 Mar; 32: 607–11
Zieglgänsberger W, Hauser C, Wetzel C, et al. Actions of acamprosate on neurons of the central nervous system. In: Soyka M, editor. Acamprosate in relapse prevention of alcoholism. Proceedings of the lst Campral-symposium. ESBRA, Stuttgart. Germany, Springer, 1995; 65–70
Gerra G, Caccavari R, Delsignore R, et al. Pituitary responses to Ca-acetyl homotaurinate in normal subjects and alcoholics. Neuroendocrinol Lett 1992; 14(2): 119–26
Guiet-Bara A, Bara M, Durlach J, et al. Ethanol effect on the ionic transfer through isolated human amnion. I. Preventive and antagonistic actions of some nutrients and of their synthetic congeners. Alcohol 1988 Jan-Feb; 5: 63–71
Lipha. Campral, prescribing information. European summary of product characteristics. Lyon, France, 1995
Girault J, Gobin P, Fourtillan JB. Determination of calcium acetylhomotaurinate in human plasma and urine by combined gas chromatography-negative-ion chemical ionization mass spectrometry. J Chromatogr 1990 Sep 14; 530: 295–305
Durbin PH, Belleville M, Chabac S. Evidence of acamprosate penetration into the rat brain [abstract]. Behav Pharmacol 1995 Aug; 6: 620
Chabenat C, Chretien P, Daoust M, et al. Physicochemical, pharmacological and pharmacokinetic study of a new GABA-ergic compound, calcium acetylhomotaurinate. Methods Find Exp Clin Pharmacol 1988 May; 10: 311–7
Aubin H-J, Lehert P, Beaupere B, et al. Tolerability of the combination of acamprosate with drug used to prevent alcohol withdrawal syndrome. Alcoholism 1995; 31(1–2): 25–38
Sennesael J. Acamprosate pharmacokinetic study after single oral administration of two acamprosate tablets (2 × 333 mg) to subjects with normal or impaired renal function. Lipha, France, 1994. Reference: AOTA-CIN IR1-AD 1003 H. (Data on file)
Besson J, Aeby F, Kasas A, et al. Combined efficacy of acamprosate and disulfiram for enhancing abstinence of chronic alcoholic patients during a one year post detoxication period [abstract]. Neuropsychopharmacology 1994 May; 10 Suppl. Pt 2: 74
Ladewig D, Knecht T, Leher P, et al. Acamprosate — a stabilising factor in the long-term treatment of alcoholics [in German]. Ther Umsch 1993 Mar; 50: 182–8
Lhuintre JP, Moore N, Tran G, et al. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol Alcohol 1990; 25: 613–22
Paille FM, Guelfi JD, Perkins AC, et al. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol 1995 Mar; 30: 239–47
Pelc I, Le Bon O, Lehert P, et al. Acamprosate in the treatment of alcohol dependence: a 6-month postdetoxifcation study. In: Soyka M, editor. Acamprosate in relapse prevention of alcoholism. Proceedings of the 1st Campral-symposium ESBRA Stuttgart. Germany, Springer, 1995; 133–142
Sass H, Soyka M, Mann K, et al. Relapse prevention by acamprosate: results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry 1996 Aug; 53: 673–80
Tempesta E, Janiri L, Bignamini AA. The effectiveness and safety of calcium-acetylhomotaurinate (acamprosate) on the maintenance of abstinence in weaned alcoholics [abstract]. Eur Psychiatry 1994; 9 Suppl. 1: 203
Whitworth AB, Fischer F, Lesch OM, et al. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet 1996 May 25; 347: 1438–42
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, DSM-III, 3rd ed. Washington, DC: American Psychiatric Association, 1980: 163–70
Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA 1984; 252: 1905–7
Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry 1971; 127: 1653–8
Feuerlein W, Ringer C, Kiifner H, et al. Diagnosis of alcoholism: the Munich Alcoholisms Test (MALT). In: Galanter M, editor. Currents of alcoholism. New York, New York: Grune &Sratton, 1980: 137–47
Sass H. Results from a pooled analysis of 11 European trials comparing acamprosate and placebo in the treatment of alcohol dependence [abstract]. Alcohol Alcohol 1995; 30(4): 551
Delgrange T, Khater J, Capron D, et al. Effect of acute administration of acamprosate on the risk of hepatic encephalopathy and on blood pressure in alcoholic cirrhotic patients [in French]. Gastroenterol Clin Biol 1992; 16: 687–91
Robins LN, Heizer JE, Weissman MM, et al. Lifetime prevalence of specific psychiatric disorders in three sties. Arch Gen Psychiatry 1984; 41: 949–58
Howard MO, McGuffin RW, Saxon AJ, et al. Clinical issues related to the costs of alcoholism. PharmacoEconomics 1996 Feb; 9: 134–45
Dongier M. Brain, alcohol and alcoholism treatment. Ann R Coll Phys Surg Can 1993; 26(1): 26–8
Soyka M. Pharmacotherapy and psychotherapy for the treatment of alcoholism in Germany. Drug Alcohol Depend 1995 Sep; 39 Suppl. 1: S9–13
Volpicelli JR, Volpicelli LA, O’Brien CP. Medical management of alcohol dependence: clinical use and limitations of naltrexone treatment. Alcohol Alcohol 1995 Nov; 30: 789–98
Naranjo CA, Ozdemir V, Bremner KE. Diagnosis and pharmacological treatment of alcoholic patients. CNS Drugs 1994 May; 1: 330–40
Gorelick DA. Medications for the treatment of substance abuse. Curr Opin Psychiatry 1992; 5(3): 430–5
Sellers EM, Higgins GA, Tomkins DM, et al. Opportunities for treatment of psychoactive substance use disorders with serotonergic medications. J Clin Psychiatry 1991 Dec; 52 Suppl.: 49–54
O’Malley SS. Current strategies for the treatment of alcohol dependence in the United States. Drug Alcohol Depend 1995 Sep; 39 Suppl. 1: S3–7
Batel P. The treatment of alcoholism in France. Drug Alcohol Depend 1995 Sep; 39 Suppl. 1: S15–21
Litten RZ, Allen J, Fertig J. Pharmacotherapies for alcohol problems: a review of research with focus on developments since 1991. Alcohol Clin Exp Res 1996 Aug; 20: 859–76
Perlmutter SJ. Pharmacologic treatment of substance abuse. Child Adolesc Psychiatr Clin North Am 1995; 4(2): 435–52
Chick J. Emergent treatment concepts. Annu Rev Addcit Res Treat 1992; 2(−): 297–312
Gallimberti L, Ferri M, Ferrara SD, et al. Gamma-hydroxybutyric acid in the treatment of alcohol dependence: a double-blind study. Alcohol Clin Exp Res 1992; 16(4): 673–6
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: J. Chick, Royal Edinburgh Hospital, Morningside Park, Edinburgh, Scotland; G. Gerra, Centro Studi Farmacotossicodipendenze, Azienda Unità Sanitaria Locale, Parma, Italy; J. Littleton, Tobacco and Health Research Institute, University of Kentucky, Lexington, Kentucky, USA; M.G. Monteiro, World Health Organization, Programme on Substance Abuse, Geneva, Switzerland; B. Nalpas, Unité d’Hépatologie, Hôpital Necker, Paris, France; F. Paille, Centre d’Alcoologie, Hôpital Fournier, Nancy, France; E.M. Sellers, Psychopharmacology and Dependence Research Unit, Women’s College Hospital, Toronto, Ontario, Canada.
Rights and permissions
About this article
Cite this article
Wilde, M.I., Wagstaff, A.J. Acamprosate. Drugs 53, 1038–1053 (1997). https://doi.org/10.2165/00003495-199753060-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199753060-00008