Abstract
Background and objective: Caffeine treatment is widely used in nursing care to reduce the risk of apnoea in premature neonates. To check the therapeutic efficacy of the treatment against apnoea, caffeine concentration in blood is an important indicator. The present study was aimed at building a pharmacokinetic model as a basis for a medical decision support tool.
Methods: In the proposed model, time dependence of physiological parameters is introduced to describe rapid growth of neonates. To take into account the large variability in the population, the pharmacokinetic model is embedded in a population structure. The whole model is inferred within a Bayesian framework. To update caffeine concentration predictions as data of an incoming patient are collected, we propose a fast method that can be used in a medical context. This involves the sequential updating of model parameters (at individual and population levels) via a stochastic particle algorithm.
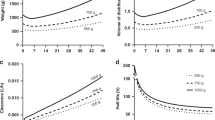
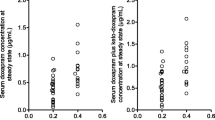
Results: Our model provides better predictions than the ones obtained with models previously published. We show, through an example, that sequential updating improves predictions of caffeine concentration in blood (reduce bias and length of credibility intervals). The update of the pharmacokinetic model using body mass and caffeine concentration data is studied. It shows how informative caffeine concentration data are in contrast to body mass data.
Conclusion: This study provides the methodological basis to predict caffeine concentration in blood, after a given treatment if data are collected on the treated neonate.











Similar content being viewed by others
References
Daily WJ, Klaus M, Meyer HB. Apnea in premature infants: monitoring, incidence, heart rate changes, and an effect of environmental temperature. Pediatrics 1969; 43(4): 510–8
Aranda JV, Turmen T, Davis J, et al. Effect of caffeine on control of breathing in infantile apnea. J Pediatr 1983; 103(6): 975–8
Aranda JV, Gorman W, Bergsteinsson H, et al. Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J Pediatr 1977; 90(3): 467–72
Aranda JV, Grondin D, Sasyniuk BI. Pharmacologic considerations in the therapy of neonatal apnea. Pediatr Clin North Am 1981; 28(1): 113–33
Zanardo V, Dani C, Trevisanuto D, et al. Methylxanthines increase renal calcium excretion in preterm infants. Biol Neonate 1995; 68(3): 169–74
Gillot I, Gouyon JB, Guignard JP. Renal effects of caffeine in preterm infants. Biol Neonate 1990; 58: 133–6
D’Urzo AD, Jhirad R, Jenne H, et al. Effect of caffeine on ventilatory responses to hypercapnia, hypoxia, and exercice in humans. J Appl Physiol 1990; 68(1): 322–8
Aranda JV, Clozel M. Pharmacologic effects of caffeine and theophylline in the premature infant. Dev Pharmacol Ther 1982; 4 Suppl.: 165–72
Karacan I, Thornby JI, Anch M, et al. Dose-related sleep disturbances induced by coffee and caffeine. Clin Pharmacol Ther 1976; 20(6): 682–9
Henderson-Smart DJ, Steer P. Methylxanthine treatment for apnea in preterm infants. Cochrane Database Syst Rev 2000; (2): CD000140
Bhatt-Mehta V, Schumacher RE. Treatment of apnea of prematurity. Paediatr Drugs 2003; 5(3): 195–210
Thomson AH, Kerr S, Wright S. Population pharmacokinetics of caffeine in neonates and young infants. Ther Drug Monit 1996; 18(3): 245–53
Lee TC, Charles B, Steer P, et al. Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clin Pharmacol Ther 1997; 61(6): 628–40
Falcao AC, Fernandez de Gatta MM, Delgado Iribarnegaray MF, et al. Population pharmacokinetics of caffeine in premature neonates. Eur J Clin Pharmacol 1997; 52(3): 211–7
Box GEP, Tiao G. Bayesian inference in statistical analysis. New York: John Wiley and Sons, 1973
Gelman A, Carlin JB, Stern HS, et al. Bayesian data analysis. London: Chapman & Hall, 1995
Bernardo JM, Smith AFM. Bayesian theory. New York: Wiley, 1994
Amzal B, Bois FY, Parent E, et al. Bayesian optimal design via interacting particle systems. J Am Stat Assoc 2006; 101(474): 773–85
Warszawski D, Gorodischer R. Tissue distribution of caffeine in premature infants and in newborn and adult dogs. Pediatr Pharmacol (New York) 1981; 1(4): 341–6
International Commission on Radiological Protection (ICRP). Basic anatomical and physiological data for use in radiological protection: reference values. Stockholm: Pergamon, 2002
Pons G, Carrier O, Richard M-O, et al. Developmental changes of caffeine elimination in infancy. Dev Pharmacol Ther 1988; 11: 258–64
Carrier O, Pons G, Rey E, et al. Maturation of caffeine metabolic pathways in infancy. Clin Pharmacol Ther 1988; 44(2): 145–51
Bourgoin H, Paintaud G, Buchler M, et al. Bayesian estimation of cyclosporin exposure for routine therapeutic drug monitoring in kidney transplant patients. Br J Clin Pharmacol 2005; 59(1): 18–27
Sandstrom M, Karlsson MO, Ljungman P, et al. Population pharmacokinetic analysis resulting in a tool for dose individualization of busulphan in bone marrow transplantation recipients. Bone Marrow Transplant 2001; 28(7): 657–64
Taright N, Mentre F, Mallet A, et al. Nonparametric estimation of population characteristics of the kinetics of lithium from observational and experimental data: individualization of chronic dosing regimen using a new Bayesian approach. Ther Drug Monit 1994; 16(3): 258–69
Arnaud M. Metabolism of caffeine and other components of coffee. In: Garattini S, editor. Caffeine, coffee and health. New York: Raven Press, 1993: 43–93
Aranda JV, Collinge JM, Zinman R, et al. Maturation of caffeine elimination in infancy. Arch Dis Child 1979; 54(12): 946–9
Le Guennec JC, Billon B, Pare C. Maturational changes of caffeine concentrations and disposition in infancy during maintenance therapy for apnea of prematurity: influence of gestational age, hepatic disease, and breast-feeding. Pediatrics 1985; 76(5): 834–40
Beal SL, Sheiner LB. Estimating population kinetics. Crit Rev Biomed Eng 1982; 8(3): 195–222
Racine-Poon A, Smith AF. Population models. In: Berry DA, editor. Statistical methodology in the pharmaceutical sciences. New York: Marcel Dekker Inc, 1990: 139–162
Garthwaite PH, Kadane JB, O’Hagan A. Statistical methods for eliciting probability distributions. J Am Stat Assoc 2005; 100(470): 680–700
Kadane JB, Wolfson LJ. Experiences in elicitation. J R Stat Soc Series D (The Statistician) 1998; 47(1): 3–19
Chen MH, Ibrahim JG, Shao QM, et al. Prior elicitation for model selection and estimation in generalized linear mixed models. J Stat Plan Inference 2003; 111(1-2): 57–76
Arnaud M, Weitzholtz H, Voegelin M, Bircher J, Presig R. Assessment of the cytochrome P-448 dependent liver enzyme system by caffeine breath test. In: Sato R, editor. Microsomes drug oxydation and drug toxicity. New York: Wiley Interscience, 1982: 443–4
Stone M. Cross-validation choice and assessment of statistical predictions. J R Stat Soc B 1974; 36: 111–47
Gelfand AE, Dey DK, Chang H. Model determination using predictive distributions with implementation via sampling-based methods. In: Bernardo JM, Berger JO, Dawid AP, et al., editors. Bayesian statistics 4. Oxford: Oxford University Press, 1992: 147–67
Robert C. Méthodes de Monte Carlo par chaînes de Markov. Paris: Economica, 1996
Gilks WR, Richardson S, Spiegelhalter DJ. Markov chain Monte Carlo in practice. London: Chapman & Hall, 1996
Bois FY, Maszle D. MCSim: a simulation program. Journal of Statistical Software 1997; 2 (9) [online]. Available from URL: http://toxi.ineris.fr/activites/toxicologie_quantitative/mcsim/mcsim.php [Accessed 2006 Nov 27]
Chopin N. A sequential particle filter method for static models. Biometrika 2002; 89: 539–52
Tierney L, Mira A. Some adaptive Monte Carlo methods for Bayesian inference. Stat Med 1999; 18: 2507–15
Doucet A, Godsill S, Andrieu C. On sequential Monte Carlo sampling methods for Bayesian filtering. Statistics and Computing 2000; 10(3): 197–208
Doucet A, De Freias J, Gordon N. Sequential Monte Carlo methods in practice. New York: Springer-Verlag, 2001
Ridgeway G, Madigan D. A sequential Monte Carlo method for Bayesian analysis of massive datasets. Data Mining and Knowledge Discovery 2003; 7(3): 301–19
Bayard DS, Jelliffe RW. A Bayesian approach to tracking patients having changing pharmacokinetic parameters. J Pharmacokinet Pharmacodyn 2004; 31(1): 75–107
Geweke J. Bayesian inference in econometric models using Monte Carlo integration. Econometrica 1989; 24: 1317–99
Rubin DB. Using the SIR algorithm to simulate posterior distributions. In: Bernardo JM, De Groot MH, Lindley DV, et al., editors. Bayesian statistics 3. Oxford: Oxford University Press, 1988: 395–402
Tierney L. Markov chains for exploring posterior distributions. Ann Stat 1994; 22(4): 1701–62
Jelliffe R, Schumitzky A, Van Guilder M. Population pharmacokinetics/pharmacodynamics modeling: parametric and nonparametric methods. Ther Drug Monit 2000; 22(3): 354–65
Wakefield J, Walker S. Bayesian nonparametric population models: formulation and comparison with likelihood approaches. J Pharmacokinet Biopharm 1997; 25(2): 235–53
Acknowledgements
The authors wish to thank Dr C. Diack for his helpful remarks and the reviewers for comments that improved the manuscript. Partial funding for this work was provided by the French Ministry of Research and Technology, project DIADEME, decision 02 C 0141. This work has also been supported by the research project BCRD-AP2001-DRC07 provided by the French Ministry of the Ecology and Sustainable Development and by the Regional Council of Picardie. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Micallef, S., Amzal, B., Bach, V. et al. Sequential Updating of a New Dynamic Pharmacokinetic Model for Caffeine in Premature Neonates. Clin Pharmacokinet 46, 59–74 (2007). https://doi.org/10.2165/00003088-200746010-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200746010-00003