Abstract
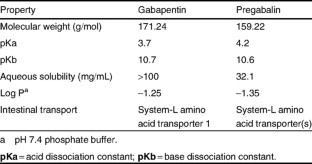
Pregabalin and gabapentin share a similar mechanism of action, inhibiting calcium influx and subsequent release of excitatory neurotransmitters; however, the compounds differ in their pharmacokinetic and pharmacodynamic characteristics. Gabapentin is absorbed slowly after oral administration, with maximum plasma concentrations attained within 3–4 hours. Orally administered gabapentin exhibits saturable absorption — a nonlinear (zero-order) process — making its pharmacokinetics less predictable. Plasma concentrations of gabapentin do not increase proportionally with increasing dose. In contrast, orally administered pregabalin is absorbed more rapidly, with maximum plasma concentrations attained within 1 hour. Absorption is linear (first order), with plasma concentrations increasing proportionately with increasing dose. The absolute bioavailability of gabapentin drops from 60% to 33% as the dosage increases from 900 to 3600 mg/day, while the absolute bioavailability of pregabalin remains at <-90% irrespective ofthe dosage. Both drugs can be given without regard to meals. Neither drug binds to plasma proteins. Neither drug is metabolized by nor inhibits hepatic enzymes that are responsible for the metabolism of other drugs. Both drugs are excreted renally, with elimination half-lives of approximately 6 hours.
Pregabalin and gabapentin both show dose-response relationships in the treatment of postherpetic neuralgia and partial seizures. For neuropathic pain, a pregabalin dosage of 450 mg/day appears to reduce pain comparably to the predicted maximum effect of gabapentin. As an antiepileptic, pregabalin may be more effective than gabapentin, on the basis of the magnitude of the reduction in the seizure frequency. In conclusion, pregabalin appearsto have some distinct pharmacokinetic advantages over gabapentin that may translate into an improved pharmacodynamic effect.







Similar content being viewed by others
References
Gee NS, Brown JP, Dissanayake VUK, et al. The novel anticonvulsant drug, gabapentin (Neurontin®), binds to the a2d subunit of a calcium channel. J Biol Chem 1996 Mar; 271(10): 5768–76
Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel a2d ligands: novel modulators of neurotransmission. Trends Pharmacol Sci 2007 Feb; 28(2): 75–82
Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel a2-d (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 2007 Feb; 73(2): 137–50
Field MJ, Cox PJ, Stott E, et al. Identification of the α2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A 2006 Nov; 103(46): 17537–42
Belliotti TR, Capiris T, Ekhato IV, et al. Structure-activity relationships of pregabalin and analogues that target the α2-δ protein. J Med Chem 2005 Apr; 48(7): 2294–307
Timmerman W, Bouma M, De Vries JB, et al. A microdialysis study on the mechanism of action of gabapentin. Eur J Pharmacol 2000 Jun; 398(1): 53–7
Stringer JL, Aribi AM. Modulation of the in vivo effects of gabapentin by vigabatrin and SKF89976A. Epilepsy Res 2002 Dec; 52(2): 129–37
Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol 2006 Feb; 6(1): 108–13
Thurlow RJ, Brown JP, Gee NS, et al. [3H]Gabapentin may label a systemL-like neutral amino acid carrier in brain. Eur J Pharmacol 1993 Nov; 247(3): 341–5
Wesche D, Bockbrader HN. A pharmacokinetic comparison of pregabalin and gabapentin [abstract]. J Pain 2005; 6 Suppl. 3: S29
Stewart BH, Kugler AR, Thompson PR, et al. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res 1993 Feb; 10(2): 276–81
Uchino H, Kanai Y, Kim DK, et al. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol 2002 Apr; 61(4): 729–37
Berry DJ, Beran RG, Plunkeft MJ, et al. The absorption of gabapentin following high dose escalation. Seizure 2003 Jan; 12(1): 28–36
Bockbrader HN. Clinical pharmacokinetics of gabapentin. Drugs of Today 1995; 318: 613–9
Piyapolrungroj N, Li C, Bockbrader H, et al. Mucosal uptake of gabapentin (neurontin) vs pregagablin in the small intestine. Pharm Res 2001 Aug; 18(8): 1126–30
Su TZ, Feng MR, Weber ML. Mediation of highly concentrative uptake of pregabalin by L-type amino acid transport in Chinese hamster ovary and Caco-2 cells. J Pharmacol Exp Ther 2005 Jun; 313(3): 1406–15
Busch J, Strand J, Posvar E, et al. Pregabalin (CI-1008) multiple-dose pharmacokinetics and safety/tolerence in healthy volunteers [abstract]. Pharm Sci 1999; 1: 2033
Radulovic LL, Turck D, von Hodenberg A, et al. Disposition of gabapentin (Neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos 1995 Apr; 23(4): 441–8
Bockbrader HN, Radulovic LL, Strand JC, et al. Regional differences in the colonic absorption of pregabalin [abstract no. CI-1008]. Pharm Sci 2000; 2: 2080
Bockbrader HN, Radulovic LL, Posvar EL, et al. Clinical pharmacokinetics of pregabalin in healthy volunteers. J Clin Pharmacol 2010 Aug; 50(8): 941–50
Bockbrader HN, Breslin EM, Uderwood BA, et al. Multiple-dose, dose-proportionality study of Neurontin® (gabapentin) in healthy volunteers [abstract]. Epilepsia 1996; 37(S5): 159
Corrigan BW, Pool WF, Posvar EL, et al. Metabolic disposition of pregabalin in healthy volunteers [abstract no. PI-68]. Clin Pharmacol Ther 2001; 69(2): P18 [online]. Available from URL: http://www.nature.com/clpt/journal/v69/n2/pdf/clpt200111a.pdf [Accessed 2010 Jun 30]
Gidal BE, Radulovic LL, Kruger S, et al. Inter- and intra-subject variability in gabapentin absorption and absolute bioavailability. Epilepsy Res 2000 Jul; 40(2–3): 123–7
Alvey C, Bockbrader H, Gonyea-Polski S, et al. An oral, rising, single- and multiple-dose, tolerance and pharmacokinetic study of pregabalin (CI-1008) capsules in healthy volunteers. New York: Pfizer Inc., 2000 Mar 13. (Data on file)
Busch J, Gonyea-Polski S, Posvar EL, et al. An oral, rising single-dose tolerance and pharmacokinetic study of CI-1008 solution and capsule doses in health volunteers. New York: Pfizer Inc., 2002 Jan 8. (Data on file)
Gidal BE, Maly MM, Budde J, et al. Effect of a high-protein meal on gabapentin pharmacokinetics. Epilepsy Res 1996 Feb; 23(1): 71–6
Vollmer KO, von Hodenberg A, Kolle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung 1986 May; 36(5): 830–9
Ben-Menachem E, Persson LI, Hedner T. Selected CSF biochemistry and gabapentin concentrations in the CSF and plasma in patients with partial seizures after a single oral dose of gabapentin. Epilepsy Res 1992 Mar; 11(1): 45–9
Buvanendran A, Kroin JS, Reuban SS, et al. Cerebrospinal fluid bioavailability of oral pregabalin [abstract no. S-206]. Anesth Analg 2008; 106(3S): S–206 [online]. Available from URL: http://www.anesthesia-analgesia.org/content/106/3S_Suppl/S1.full.pdf+html [Accessed 2010 Jun 30]
Tomson T, Battino D. Pharmacokinetics and therapeutic drug monitoring of newer antiepileptic drugs during pregnancy and the puerperium. Clin Phar-macokinet 2007; 46(3): 209–19
Ohman I, Vitols S, Tomson T. Pharmacokinetics of gabapentin during delivery, in the neonatal period, and lactation: does a fetal accumulation occur during pregnancy? Epilepsia 2005 Oct; 46(10): 1621–4
Randinitis EJ, Posvar EL, Alvey CW, et al. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J Clin Pharmacol 2003 Mar; 43(3): 277–83
Blum RA, Comstock TJ, Sica DA, et al. Pharmacokinetics of gabapentin in subjects with various degrees of renal function. Clin Pharmacol Ther 1994 Aug; 56(2): 154–9
Wilson EA, Sills GJ, Forrest G, et al. High dose gabapentin inrefractory partial epilepsy: clinical observations in 50 patients. Epilepsy Res 1998 Jan; 29(2): 161–6
Eckhardt K, Ammon S, Hofmann U, et al. Gabapentin enhances the analgesic effectofmorphine inhealthy volunteers. Anesth Analg 2000 Jul; 91(1): 185–91
Data on file, Pfizer Inc., 2005
Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 2006 Mar; 61(3): 246–55
Aills M, Allen R, Bockbrader HN, et al. A 5-week, double-blind, placebo-controlled, parallel-group study of pregabalin (75 and 150 mg/day) in patients with postherpetic neuralgia. New York: Pfizer Inc., 2001 Oct 15. (Dataonfile)
Dworkin RH, Corbin AE, Young JP, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2003 Apr; 60(8): 1274–83
Rice AS, Maton S, Postherpetic Neuralgia Study Group. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain 2001 Nov; 94(2): 215–24
Rowbotham M, Harden N, Stacey B, et al. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA 1998 Dec; 280(21): 1837–42
Sabatowski R, Galvez R, Cherry DA, et al., 1008-045 Study Group. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 2004 May; 109(1-2): 26–35
van Seventer R, Feister HA, Young JP, et al. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin 2006 Feb; 22(2): 375 neuralgia:-84
Kowalski K, Burger P, Miller R, et al. Exposure response relationship of pregabalin: a novel therapy for the treatment of neuropathic pain [abstract]. Eur J Neurol 2003; 10 Suppl. 1: 36–7
Chapel S, Kowalski K, Hutmacher M, et al. Pregabalin exposure-response analysis in patients with postherpetic neuralgia [abstract no. PII-143]. Clin Pharmacol Ther 2005; 77(2): P88 [online]. Available from URL: http://www.nature.com/clpt/journal/v77/n2/pdf/clpt2005340a.pdf [Accessed 2010 Jun 30]
Gabapentin in partial epilepsy. UK Gabapentin Study Group. Lancet 1990 May; 335(8698): 1114–7
Gabapentin as add-on therapy in refractory partial epilepsy: a double-blind, placebo-controlled, parallel-group study. The US Gabapentin Study Group No. 5. Neurology 1993 Nov; 43 (11): 2292-8
Anhut H, Ashman P, Feuerstein TJ, et al. Gabapentin (Neurontin) as add-on therapy in patients with partial seizures: a double-blind, placebo-controlled study. The International Gabapentin Study Group. Epilepsia 1994 Jul-Aug; 35(4): 795–801
Beydoun A, Uthman BM, Kugler AR, et al., Pregabalin 1008-009 Study Group. Safety and efficacy of two pregabalin regimens for add-on treatment of partial epilepsy. Neurology 2005 Feb; 64(3): 475–80
Arroyo S, Anhut H, Kugler AR, et al., Pregabalin 1008-011 International Study Group. Pregabalin add-on treatment: a randomized, double-blind, placebo-controlled, dose-response study in adults with partial seizures. Epi-lepsia 2004 Jan; 45(1): 20–7
French JA, Kugler AR, Robbins JL, et al. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology 2003 May; 60(10): 1631–7
Eisenberg E, River Y, Shifrin A, et al. Antiepileptic drugs in the treatment of neuropathic pain. Drugs 2007; 67(9): 1265–89
Acknowledgements
Several of the studies discussed in this paper were funded by Pfizer Inc. Howard N. Bockbrader, PhD, David Wesche, MD, PhD, Raymond Miller, PhD, Sunny Chapel, PhD, Nancy Janiczek, PhD and Paula Burger, BS, were employees of Pfizer Inc. and owned stock in Pfizer Inc. during the development of this paper. Editorial and administrative assistance was provided by Thomas G. Hedberg, PhD, of UBC Scientific Solutions, and was funded by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bockbrader, H.N., Wesche, D., Miller, R. et al. A Comparison of the Pharmacokinetics and Pharmacodynamics of Pregabalin and Gabapentin. Clin Pharmacokinet 49, 661–669 (2010). https://doi.org/10.2165/11536200-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11536200-000000000-00000




