Abstract
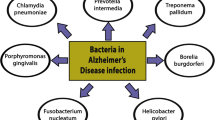
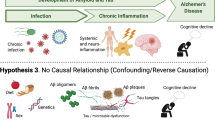
While our understanding of the neuropathology of Alzheimer’s disease continues to grow, its pathogenesis remains a subject of intense debate. Genetic mutations contribute to a minority of early-onset autosomal dominant cases, but most cases are of either late-onset familial or sporadic form. CNS infections, most notably herpes simplex virus type 1, Chlamydophila pneumoniae and several types of spirochetes, have been previously suggested as possible aetiological agents in the development of sporadic Alzheimer’s disease but with little consistent evidence. However, peripheral infections may have a role to play in accelerating neurodegeneration in Alzheimer’s disease by activating already primed microglial cells within the CNS. Potential pharmacological interventions could aim at modification of this peripheral inflammatory response through targeting various agents involved in this inflammatory pathway. However, benefit could also be gained clinically through the meticulous detection, treatment and prevention of infections in individuals either alone or in combination with anti-inflammatory therapy.
Similar content being viewed by others
References
Holmes C. The genetics and molecular pathology of dementia. In: Jacoby R, Oppenheimer C, Dening T, et al., editors. Oxford textbook of old age psychiatry. Oxford: Oxford University Press, 2008: 103–17
Bell JE. Neuropathology. In: Johnstone EC, Cunningham-Owens DG, Lawrie SM, et al., editors. Companion to psychiatric studies. London: Churchill Livingstone, 2004: 68–80
Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC FAS). Pathological correlates of late onset dementia in a multicentre, community based population in England and Wales. Lancet 2001; 357: 169–75
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992 Apr 10; 256(5054): 184–5
Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 1993 Dec 17; 75(6): 1039–42
Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease [letter]. Lancet 1976 Dec 25; 2(8000): 1403
Saunders AM. Apolipoprotein E and Alzheimer disease: an update on genetic and functional analyses. J Neuropathol Exp Neurol 2000 Sep; 59(9): 751–8
Lynch JR, Tang W, Wang H, et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem 2003; 278: 48529–33
Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer’s disease: role of spirochetes. J Alzheimers Dis 2008; 13: 381–91
McArthur JC. HIV-associated dementia. In: Davis LE, Kennedy PGE, editors. Infectious diseases of the nervous system. Oxford: Butterworth-Heinemann, 2000: 165–213
Tomlinson BE. The pathology of dementia. Contemp Neurol Ser 1977; 15: 113–53
Itzhaki RF, Wozniak MA. Herpes simplex virus type 1 in Alzheimer’s disease: the enemy within. J Alzheimers Dis 2008 May; 13(4): 393–405
Balin BJ, Little CS, Hammond CJ, et al. Chlamydophila pneumoniae and the etiology of late-onset Alzheimer’s disease. J Alzheimers Dis 2008 May; 13(4): 371–80
Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer’s disease: role of spirochetes. J Alzheimers Dis 2008 May; 13(4): 381–91
Pogo BG, Casals J, Elizan TS. A study of viral genomes and antigens in brains of patients with Alzheimer’s disease. Brain 1987 Aug; 110 (Pt 4): 907–15
Friedl RP, May C, Dahlberg J. The viral hypothesis of Alzheimer’s disease: absence of antibodies to lentiviruses. Arch Neurol 1990 Feb; 47(2): 177–8
Renvoize EB, Awad IO, Hambling MH. A sero-epidemiological study of conventional infectious agents in Alzheimer’s disease. Age Ageing 1987 Sep; 16(5): 311–4
Kountouras J, Gavalas E, Zavos C, et al. Alzheimer’s disease and Helicobacter pylori infection: defective immune regulation and apoptosis as proposed common links. Med Hypotheses 2007; 68(2): 378–88
Liedtke W, Opalka B, Zimmerman CW, et al. Age distribution of latent HSV1 and Varicella zoster virus genome in human nervous tissue. J Neurol Sci 1993; 116(1): 6–11
Ball MJ. Limbic predilection in AD: is reactivated herpes virus involved? Can J Neurol Sci 1982; 9: 303–6
Shipley SJ, Parkin ET, Itzhaki RF, et al. Herpes simplex virus interferes with amyloid precursor protein processing. BMC Microbiol 2005 Aug 18; 5: 48–55
Jamieson GA, Maitland NJ, Wilcock GK, et al. Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. J Med Virol 1991 Apr; 33(4): 224–7
Itzhaki RF, Dobson CB, Lin WR, et al. Association of HSV1 and apolipoprotein E-varepsilon4 in Alzheimer’s disease. J Neurovirol 2001 Dec; 7(6): 570–1
Itzhaki R. Herpes simplex virus type 1, apolipoprotein E and Alzheimer’ disease. Herpes 2004 Jun; 11Suppl. 2: 77A–82A
Federoff HJ. An infectious conspiracy: the case for HSV-1 and APOEepsilon4 in Alzheimer’s disease. Neurobiol Aging 1999 Jul–Aug; 20(4): 467–8
Marques AR, Straus SE, Fahle G, et al. Lack of association between HSV-1 DNA in the brain, Alzheimer’s disease and apolipoprotein E 4. J Neurovirol 2001 Feb; 7(1): 82–3
Lin WR, Wozniak MA, Esiri MM, et al. Herpes simplex encephalitis: involvement of apolipoprotein E genotype. J Neurol Neurosurg Psychiatry 2001; 70(1): 117–9
Mann DM, Tinkler AM, Yates PO. Neurological disease and herpes simplex virus: an immunohistochemical study. Acta Neuropathol (Berl) 1983; 60(1–2): 24–8
Robinson SR, Dobson C, Lyons J. Challenges and directions for the pathogen hypothesis of Alzheimer’s disease. Neurobiol Ageing 2004 May–Jun; 25(5): 629–37
Denaro FJ, Staub P, Colmer J, et al. Coexistence of Alzheimer disease neuropathology with herpes simplex encephalitis. Cell Mol Biol (Noisy-le-grand) 2003 Dec; 49(8): 1233–40
Itzhaki RF. Herpes simplex virus type 1, apolipoprotein E and Alzheimer’s disease. Herpes 2004 Jun; 11Suppl. 2: 77A–82A
Grayston JT, Campbell LA, Kuo CC, et al. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis 1990 Apr; 161(4): 618–25
Balin BJ, Gerard HC, Arking EJ, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol 1998 Jun; 187(1): 23–42
Paradowski B, Jaremko M, Dobosz T, et al. Evaluation of CSF-Chlamydia pneumoniae, CSF-tau, and CSF-Abeta42 in Alzheimer’s disease and vascular dementia. J Neurol 2007 Feb; 254(2): 154–9
Little CS, Hammond CJ, MacIntyre A, et al. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol Aging 2004 Apr; 25(4): 419–29
Appelt DM, Roupas MR, Way DS, et al. Inhibition of apoptosis in neuronal cells infected with Chlamydophila (Chlamydia) pneumoniae. BMC Neurosci 2008 Jan 24; 9: 13
Gerard HC, Wildt KL, Whittum-Hudson JA, et al. The load of Chlamydia pneumoniae in the Alzheimer’s brain varies with APOE genotype. Microb Pathog 2005 Jul–Aug; 39(1–2): 19–26
Gerard HC, Dreses-Werringloer U, Wildt KS, et al. Chlamydophila (Chlamydia) pneumoniae in the Alzheimer’s brain. FEMS Immunol Med Microbiol 2006 Dec; 48(3): 355–66
Nochlin D, Shaw CM, Campbell LA, et al. Failure to detect Chlamydophila pneumoniae in brain tissues of Alzheimer’s disease [letter]. Neurology 1999 Nov 10; 53(8): 1888
Ring RH, Lyons JM. Failure to detect Chlamydophila pneumoniae in late onset Alzheimer’s brain. J Clin Microbiol 2000 Jul; 38(7): 2591–4
Loeb MB, Molloy DW, Smieja M, et al. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer’s disease. J Am Geriatr Soc 2004 Mar; 52(3): 381–7
Choi Y, Kim HS, Shin KY. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology 2007 Nov; 32(11): 2393–404
Miklossy J. Alzheimer’s disease: a spirochetosis? Neuroreport 1993 Jul; 4(7): 841–8
Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol 2002 Apr; 17(2): 113–8
Miklossy J, Khalili K, Gem L, et al. Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease. J Alzheimers Dis 2004 Dec; 6(6): 639–49
MacDonald AB, Miranda JM. Concurrent neocortical borreliosis and Alzheimer’s disease. Hum Pathol 1987 Jul; 18(7): 759–61
Miklossy J, Kis A, Radenovic A, et al. Beta-amyloid deposition and Alzheimer’s type changes induced by Borrelia spirochetes. Neurobiol Aging 2006 Feb; 27(2): 228–36
Ohnishi S, Koide A, Koide SJ. Solution conformation and amyloid-like fibril formation of a polar peptide derived from a beta-hairpin in the OspA single-layer beta-sheet. J Mol Biol 2000 Aug 11; 301(2): 477–89
MacDonald AB. Plaques of Alzheimer’s disease originate from cysts of Borrelia burgdorferi, the Lyme disease spirochete. Med Hypotheses 2006; 67(3): 592–600
Pappolla MA, Omar R, Saran B, et al. Concurrent neuroborreliosis and Alzheimer’s disease: analysis of the evidence. Hum Pathol 1989 Aug; 20(8): 753–7
Gutacker M, Valsangiacomo C, Balmelli T, et al. Arguments against the involvement of Borrelia burgdorferi sensu lato in Alzheimer’s disease. Res Microbiol 1998 Jan; 149(1): 31–7
Marques AR, Weir SC, Fahle GA, et al. Lack of evidence of Borrelia involvement in Alzheimer’s disease [letter]. J Infect Dis 2000 Sep; 182(3): 1006–7
Galbussera A, Tremolizzo L, Isella V, et al. Lack of evidence for Borrelia burgdorferi seropositivity in Alzheimer disease. Alzheimer Dis Assoc Disord 2008 Jul–Sep; 22(3): 308
Urosevic N, Martins RN. Infection and Alzheimer’s disease: the APOE ε4 connection and lipid metabolism. J Alzheimers Dis 2008 May; 13(4): 421–35
Hart BL. Biological basis of the behaviour of sick animals. Neurosci Biobehav Rev 1988; 12: 123–37
Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in the CNS. Br JPharmacol 2006; 147Suppl. 1: S232–40
Perry VH, Cunningam C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature 2007; 7: 161–7
Tracey KJ. The inflammatory reflex. Nature 2002; 420: 853–9
LaFlamme N, Rivest S. Effects of systemic immunogenic insults and circulating proinflammatory cytokines on the transcription of the inhibitory factor kBa within specific cellular populations of the rat brain. J Neurochem 1999; 73: 309–21
Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxaemia, independent of systemic cytokines. J Neurosci 2005; 25: 1788–96
Ek M, Engblom D, Saha S, et al. Inflammatory response: pathway across the blood-brain barrier. Nature 2001 Mar 22; 410(6827): 430–1
Akiyama H, Bargar S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Ageing 2000 May–Jun; 21(3): 383–421
Ajmone-Cat MA, Nicolini A, Minghetti L. Prolonged exposure of microglia to lipopolysaccharide modifies the intracellular signalling pathways and selectively promotes prostaglandin E2 synthesis. J Neurochem 2003 Dec; 87(5): 1193–203
VonBernhardi R. Glial cell dysregulation: a new perspective on Alzheimer’s disease. Neurotox Res 2007 Dec; 12(4): 215–32
Rojo LE, Fernandez JA, Maccioni AA, et al. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch Med Res 2008 Jan; 39(1): 1–16
Sly LM, Krzesicki RF, Brashler JR, et al. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res Bull 2001 Dec; 56(6): 581–8
Herber DL, Roth LM, Wilson D. Time-dependent reduction in Abeta levels after intracranial LPS administration in APP transgenic mice. Exp Neurol 2004 Nov; 190(1): 245–53
Cunningham C, Wilcockson D, Campion S, et al. Central and systemic endotoxin challenges exacerbate the central inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci 2005; 25: 9275–84
Lee J, Chan SL, Mattson MP. Adverse effect of a presenilin-1 mutation in microglia results in enhanced nitric oxide and inflammatory cytokine responses to immune challenge in the brain. Neuromolecular Med 2002; 2(1): 29–45
Kitazawa M, Oddo S, Yamasaki TR, et al. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci 2005 Sep 28; 25(39): 8843–53
Cunningham C, Campion S, Lunnon K, et al. Systemic inflammation induces acute behavioural and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry 2008 Feb 15; 65(4): 304–12
McCusker J, Cole M, Dendukuri N, et al. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ 2001 Sep 4; 165(5): 575–83
Rahkonen T, Luukkainen-Makkula R, Paanila S, et al. Delirium episode as a sign of undetected dementia among community dwelling elderly subjects: a 2 year follow up study. J Neurol Neurosurg Psychiatry 2000 Oct; 69(4): 519–21
Holmes C, El-Okl M, Williams AL, et al. Systemic infection, interleukin 1 β and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2003; 74: 788–9
Holmes C, Perry H, Cunningham C, et al. Systemic cytokines and clinical progression in Alzheimer’s disease. Alzheimer Dementia 2008;4Suppl. 2: 159–60
Dunn N, Mullee M, Perry VH, et al. Association between dementia and infectious disease: evidence from a casecontrol study. Alzheimer Dis Assoc Disord 2005 Apr–Jun; 19(2): 91–4
Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol 2004 May; 61(5): 668–72
Kamer AR, Dasanayeke A, Craig RG, et al. Alzheimer’s disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis. J Alzheimers Dis 2008 May; 13(4): 437–49
Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin 2006 Aug; 24(3): 521–38
Barrientos RM, Higgins EA, Biedenkapp JC, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging 2006 May; 27(5): 723–32
Park KH, Hallows JL, Chakrabarty P, et al. Conditional neuronal simian virus 40 T antigen expression induces Alzheimer-like tau and amyloid pathology in mice. J Neurosci 2007 Mar 14; 27(11): 2969–78
Verreault R, Laurin D, Lindsay J, et al. Past exposure to vaccines and subsequent risk of AD. CMAJ 2001; 165: 1495–8
Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421(6291): 384–8
Tobinick E. Perispinal etanercept for treatment of Alzheimer’s disease. Curr Alzheimer Res 2007 Dec; 4(5): 550–2
Griffin WS. Perispinal etanercept: potential as an Alzheimer therapeutic [letter]. J Neuroinflammation 2008; 5: 3
Acknowledgements
We would like to acknowledge funding from the Alzheimer’s Society UK for our research on the role of infections in the progression of Alzheimer’s disease. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holmes, C., Cotterell, D. Role of Infection in the Pathogenesis of Alzheimer’s Disease. CNS Drugs 23, 993–1002 (2009). https://doi.org/10.2165/11310910-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11310910-000000000-00000