Abstract
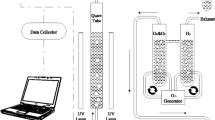
Chemical oxygen demand (COD) is important for water quality assessment as it represents the level of reductive organic pollution from eutrophication in aquatic systems. For surface water quality monitoring, permanganate is usually applied as an oxidizing reagent, and the routine CODMn determination is mostly achieved by titration method. However, this titration method is tedious and time consuming, and the results suffer from environmental temperature fluctuations and complicated operation techniques. In this study, a novel CODMn determination method was developed using gas-phase molecular absorption spectrometry equipped with an online automated digestion device for the first time. The effects of digestion temperature, digestion time and sulfuric acid content were thoroughly studied. This method exhibited good linearity (0.35 to 12 mg/L), a low detection limit (0.12 mg/L), and good RSD from various water samples (0.71 - 2.37%). When used for CODMn determination in routine water quality monitoring, this automated GPMAS can considerably improve analysis speed, efficiency, accuracy and stability compared to the traditional titration method.
Similar content being viewed by others
References
Q. Zheng, B. Zhou, J. Bai, L. Li, Z. Jin, J. Zhang, J. Li, Y. Liu, W. Cai, and X. Zhu, Adv. Mater, 2008, 20, 1044.
W. Liu, Q. Zhang, and G. Liu, Hydrobiologia, 2010, 644, 289.
W. Liu, Q. Zhang, and G. Liu, Hydrological Processes, 2012, 26, 570.
W. A. Moore, F. J. Ludzack, and C. C. Ruchhoft, Anal. Chem., 1951, 23, 1297.
R. A. Dobbs and R. T. Williams, Anal. Chem., 1963, 35, 1064.
F. J. Baumann, Anal. Chem., 1974, 46, 1336
J. Wang, C. Wu, K. Wu, Q. Cheng, and Y. Zhou, Anal. Chim. Acta, 2012, 736, 55
I. H. A. Badr, H. H. Hassan, E. Hamed, and A. M. Abdel-Aziz, Electroanalysis, 2017, 29, 2401.
G. Le, H. Yang and X. Yu, Water Sci. Technol., 2018, 77, 1271.
G. Cazaudehore, B. Schraauwers, C. Peyrelasse, C. Lagnet, and F. Monlau, J. Environ. Manage., 2019, 250, 109464.
D. T. K. Hue, S. Hashimoto, H. Nishikawa, Y. Maeda, and N. Takenaka, Anal. Sci., 2017, 33, 931.
J. Chen, S. Liu, X. Qi, S. Yan, and Q. Guo, Sens. Actuators, B, 2018, 254, 778.
J. Li, L. Li, L. Zheng, Y. Xian, S. Ai, and L. Jin, Anal. Chim. Acta, 2005, 548, 199.
H. Yu, C. Ma, X. Quan, S. Chen, and H. Zhao, Environ. Sci. Technol., 2009, 43, 1935.
C. A. Almeida, P. González, M. Mallea, L. D. Martinez, and R. A. Gil, Talanta, 2012, 97, 273.
H. Zhang, J. Zhu, Q. Chen, S. Jiang, Y. Zhang, and T. Fu, Microchem. J., 2018, 143, 292.
H. Matsuura, T. Takahashi, S. Sakamoto, T. Kitamura, and S. Uchiyama, Anal. Sci. 2017, 33, 703.
M. Ahmed, M. Asghar, M. Yaqoob, N. Munawar, and A. Nabi, Anal. Sci., 2017, 33, 1259.
J. Li, G. Luo, L. He, J. Xu, and J. Lyu, Crit. Rev. Anal. Chem., 2017, 48, 47.
L. Ebdon, S. J. Hill, M. Jameel, W. T. Corns, and P. B. Stockwell, Analyst, 1997, 122, 689.
K. Zhou, H. Jing, Y. Liu, and H. Zhou, Environ. Sci. Technol., 2012, 35, 147.
N. Ozbek and S. Akman, J. Anal. At. Spectrom., 2018, 33, 111.
D. Chen, S. Li, H. Liu, T. Chen, C. Chen, and C. Yu, Anal. Methods, 2014, 6, 9085.
B. Haghighi and S. F. Kurd, Talanta, 2004, 64, 688.
J. Sanz-Asensio, M. T. Martínez-Soria, M. Plaza-Medina, and M. P. Clavijo, Talanta, 2001, 54, 953.
S. Priyanka, B. Y. Raza, and N. G. Ram, Talanta, 2019, 191, 364.
A. Safavi, B. Haghighi, and F. Peiravian, Anal. Lett., 2003, 36, 479.
S. Cabredo, J. Galbán, and J. Sanz, Talanta, 1998, 46, 631
K. J. Chae, I. Am, S. K. Yim, and I. S. Kim, Bioresource Technol., 2008, 99, 1.
K. Takashi, Anal. Lett., 1980, 73, 1001.
D. Dan, R. C. Sandford, and P. J. Worsfold, Analyst, 2005, 130, 227.
M. Zenki, S. Fujiwara, and T. Yokoyama, Anal. Sci., 2006, 22, 77.
Y. Su, X. Li, H. Chen, Y. Lv, and X. Hou, Microchem. J., 2007, 87, 56.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51909012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Peng, L., Wang, J. et al. Determination of Chemical Oxygen Demand in Water Samples Using Gas-phase Molecular Absorption Spectrometry. ANAL. SCI. 36, 841–846 (2020). https://doi.org/10.2116/analsci.19P444
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.19P444