
A pilot study of anlotinib with third-generation epidermal growth factor receptor tyrosine kinase inhibitors in untreated EGFR-mutant patients with advanced non-small cell lung cancer
Highlight box
Key findings
• Combining third-generation EGFR-TKIs with anlotinib as the initial treatment might significantly increase toxicity in EGFR-mutant patients with advanced NSCLC.
What is known, and what is new?
• Third-generation EGFR-TKIs have been recommended as a first-line treatment for EGFR-mutant advanced NSCLC.
• Anlotinib was combined with third-generation EGFR-TKIs to explore better combination treatment strategies for postponing drug resistance and prolonging survival benefits.
What is the implication, and what should change now?
• Combining anlotinib and third-generation EGFR-TKIs in untreated EGFR-mutant patients with advanced NSCLC is not an appropriate therapeutic choice. Oncologists intending to use such combined therapy should evaluate comprehensively and choose carefully before treatment.
Introduction
Epidermal growth factor receptor (EGFR) mutations are the most common driver genes in non-small cell lung cancer (NSCLC) which constitutes approximately 75–80% of all lung cancers (1). EGFR mutations are present in approximately 10% of cases in North America and Western Europe and about 30–50% of cases in East Asian countries (2). In selected NSCLC groups, the mutation rate could be over 50% (3). With the further understanding of molecular mechanisms in the etiology and drug resistance of EGFR mutations in lung cancers, tyrosine kinase inhibitors [TKIs; small molecular inhibitors targeting EGFR-mutations (EGFR-TKIs)] have been rapidly developed in recent years (4-7). EGFR-TKIs have dramatically improved outcomes and are the first-line treatment choice for patients with locally advanced or metastatic NSCLC harboring EGFR mutations (8). For these patients, osimertinib, a third-generation EGFR-TKI, showed superior efficacy, similar safety, and lower rates of serious adverse events (AEs) when compared to first- or second-generation EGFR-TKIs, such as gefitinib, erlotinib, or afatinib (9,10). Consequently, osimertinib has been recommended as a first-line treatment of EGFR-mutant advanced NSCLC. Positive results have also been reported from the AENEAS study, a randomized phase III clinical trial designed to assess the efficacy and safety of aumolertinib, another third-generation EGFR-TKI, vs. gefitinib as a first-line treatment in patients with locally advanced or metastatic NSCLC harboring EGFR mutations (11). The primary end point, progression-free survival (PFS), was significantly longer with aumolertinib than with gefitinib. The secondary end point, overall survival (OS), has not been reached yet. Despite their improvements in PFS and OS over first- and second-generation EGFR-TKIs, the benefits from third-generation EGFR-TKIs are still transient, and disease progression inevitably occurs. Acquired resistance remains the major limitation of monotherapy with EGFR-TKIs (12). Thus, combination treatment strategies to postpone drug resistance and prolong survival benefits remain to be explored.
Vascular endothelial growth factor (VEGF) is another therapeutic target in NSCLC. The binding of VEGF to the VEGF receptor (VEGFR) promotes tumor angiogenesis and indirectly encourages tumor growth. It has been reported that the activation of EGFR could induce VEGF expression (13-16). Preclinical studies have suggested that re-induction of VEGF and subsequent direct or indirect VEGF-dependent tumor growth was a major mechanism of EGFR-TKI resistance, and simultaneous blockade of VEGF/VEGFR and EGFR pathways could eliminate both primary resistance to EGFR-TKIs and acquired resistance due to the EGFR T790M mutation (17,18). Consequently, many clinical studies have investigated the dual inhibition of EGFR-TKIs combined with VEGF or VEGFR inhibitors to treat EGFR-mutant advanced NSCLC. Bevacizumab is a VEGF monoclonal antibody. A phase II trial suggested gefitinib combined with bevacizumab was a favorable and well-tolerated first-line therapy for patients with advanced NSCLC with activating EGFR mutations (19). The ORR of the combined treatment was 73.8% and the median OS was not reached at the time of survival analysis. In the J025567 and NEJ026 studies, combined erlotinib and bevacizumab therapy improved PFS compared with erlotinib alone in patients with EGFR-positive advanced non-squamous NSCLC (20,21). However, in the J025567 study, the combined therapy did not show significant OS benefit (47.0 vs. 47.4 months erlotinib alone, hazard ratio 0.81; P=0.3267). Similarly, erlotinib plus ramucirumab, a VEGFR2 monoclonal antibody, demonstrated superior PFS compared with placebo plus erlotinib in patients with untreated EGFR-mutated metastatic NSCLC (22). EGFR-TKIs plus bevacizumab showed better survival benefits for patients with multiple brain metastases than EGFR-TKIs alone (23). Addition of bevacizumab had a significantly higher intracranial ORR (66.1% vs. 41.6%, P=0.001), systematic ORR (74.6% vs. 57.1%, P=0.019) and longer OS (29.6 vs. 21.7 months, P<0.001). Furthermore, a phase 1/2 study combining osimertinib and bevacizumab as a first-line treatment for EGFR-mutant lung cancers achieved the primary effectiveness end point of 12-month PFS and showed promising activity against central nerve system (CNS) metastases (24). The ORR was 80% (95% confidence interval, 67–91%), the median OS was not reached at the time of analysis.
However, bevacizumab and ramucirumab need to be administrated intravenously and may cause allergies, which limit their clinical application to some extent. Anlotinib is an oral multitarget TKI for inhibiting tumor angiogenesis and proliferative signaling (25,26). The ALTER-0303 trial showed that patients with advanced NSCLC who received anlotinib as third-line or further therapy showed greater survival benefits, and anlotinib was well tolerated among these patients (27). Based on these facts, China’s National Medical Products Administration (NMPA) approved anlotinib as a third-line treatment for NSCLC in 2018. Currently, clinical trials using anlotinib combined with chemotherapy as a first- or second-line treatment for NSCLC are in progress, with great expectations of positive results. It has been reported that anlotinib combined with gefitinib significantly inhibited the proliferation of EGFR-TKI-resistant NSCLC cells in vitro and in vivo (28,29). Concurrent use of anlotinib overcomes acquired resistance to EGFR-TKIs (gefitinib, erlotinib, icotinib, afatinib) in advanced EGFR-mutant NSCLC patients (29). A case report suggested that a combination of osimertinib and anlotinib might overcome the resistance mediated by in cis EGFR T790M-C797S in NSCLC (30). It has also been reported that anlotinib overcame acquired resistance to EGFR-TKIs via fibroblast growth factor receptor 1 (FGFR1) signaling in NSCLC without the EGFR T790M mutation (31). Histological transformation to small cell lung cancer in lung cancers bearing EGFR driver genes is another mechanism of resistance to EGFR-TKI treatment (32-34). A multicenter retrospective study revealed that anlotinib showed better efficacy in these patients after SCLC transformation than platinum-etoposide, which was the most common treatment regimen (35). Considering the above evidence, we hypothesized that combining third-generation EGFR-TKIs with anlotinib in untreated EGFR-mutant patients with advanced NSCLC might postpone drug resistance and further prolong their survival benefits. Besides, both drugs are orally administered, which is convenient for medication and could improve the patients’ compliance with treatment. Accordingly, we conducted a phase II trial to assess the efficacy and safety of anlotinib plus osimertinib or aumolertinib in untreated patients with EGFR mutations and advanced NSCLC. We present this article in accordance with the TREND reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-175/rc).
Methods
Patients
Eligible patients were aged 18 to 75 years old, had untreated stage IIIB–IV NSCLC (according to the 8th edition American Joint Committee on Cancer staging system), adequate organ function, a life expectancy of 3 months or more, and an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or lower. The primary inclusion criteria were as follows: (I) EGFR mutation-positive, with or without T790M mutations; (II) at least one measurable lesion based on the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Patients were excluded if they had previously received chemotherapy, radiotherapy, or EGFR and/or VEGF and VEGFR inhibitors; if they had coagulation disorders or spontaneous bleeding; or contraindications to third-generation EGFR-TKIs or anlotinib. Patients with asymptomatic brain metastases were considered eligible to enroll in the study. Written informed consent was obtained from all enrolled patients. The reported ORRs were 80% of osimertinib alone, and 73.8% of aumolertinib alone. The average ORR was 76.9%. Thirty-one events were deemed necessary to detect an ORR of 95.1% of the third generation EGFR-TKIs plus anlotinib, with a two-sided significance level of 0.05 and a power of 0.8. The target sample size was set at 35, allowing for dropouts, calculated by SPSS (IBM Corp., Armonk, NY, USA).
Study design
This study was a nonrandomized, single-center, single-arm, open-label, and prospective phase II clinical trial (Figure S1). Patients who voluntarily chose to participate were enrolled from the Department of Thoracic Oncology at the Cancer Center of the Fifth Affiliated Hospital of Sun Yat-sen University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Board of the Fifth Affiliated Hospital of Sun Yat-sen University (No. ZDWY-2020-K46-1). All eligible patients received anlotinib and a third-generation EGFR-TKI (osimertinib or aumolertinib) simultaneously until progressive disease (PD) or unacceptable toxicity occurred. Osimertinib was orally administrated at a dose of 80 mg/day and aumolertinib at 110 mg/day. From our clinical observations, almost 50% of patients who received standard-dose anlotinib treatment required dose adjustments; thus, in the combined regimen, anlotinib was given at a dose of 12 mg once every other day to reduce related toxicity. The drugs were prescribed by trained specialists. The estimated time for enrollment was 1 and 2 years for study completion.
The primary end point of the study was the objective response rate (ORR). Secondary endpoints included the safety of the combined treatment and the additional efficacy endpoints of disease control rate (DCR), OS, and PFS.
Study assessments
During treatment, computed tomography imaging was performed every 6 weeks and evaluated by RECIST 1.1. The safety of the treatment was assessed by the occurrence of AEs, and the investigators graded the severity of the AEs according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. Treatment discontinuation occurred at the time of radiographically identified PD, unacceptable toxicity, withdrawal of consent, or the investigators’ decision. After combined anlotinib and third-generation EGFR-TKI treatment was completed, patients were followed up for safety evaluation of subsequent EGFR-TKI treatment as an additional endpoint.
Statistical analysis
Demographic and safety data and AE occurrence were collected on all patients. Toxic effects were analyzed using descriptive statistics. Enrollment was ceased due to treatment-related AEs (trAEs) after 11 of 35 planned patients were treated. Consequently, 11 eligible patients were enrolled in the trial in total, while two patients were lost to follow-up. The ORR (complete response + partial response), DCR (complete response + partial response + stable disease), PFS, and OS were calculated in the per-protocol population of nine patients. The data cutoff was February 2, 2021, for all analyses.
Results
Patient characteristics
Between July 2020 and February 2021, a total of 11 eligible patients with adenocarcinoma were enrolled in the trial. The baseline clinical characteristics are provided in Table 1. Their median age was 68 years (range, 47–73 years). Four (36.4%) were male, 2 (18.2%) were smokers, 5 (45.5%) had EGFR 19DEL mutations, 6 (54.5%) had EGFR L858R mutations, 9 (81.8%) received osimertinib and anlotinib, and 2 (18.2%) received aumolertinib and anlotinib (Table S1). Brain metastases were present in 9 patients (81.8%). Two (18.2%) patients were lost to follow-up before the first imaging assessment. A total of nine patients were eventually included in the final analysis. At the time of data cutoff, the median duration of follow-up was 84 days, and four patients remained on the combined therapy.
Table 1
| No. | Age (years) | Gender | Smoker | Histology | Clinical stage | CNS metastases | ECOG | EGFR mutation | EGFR-TKI choice | trAEs [grade] | Best response† | Time on trial (m) | TKI after trial | TKI-related AEs [grade] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | M | No | ADC | IVB | No | 1 | 19DEL | Osimertinib | Stomachache [3], rash [2] | PR | 2.0 | Yes | Rash [1] |
| 2 | 47 | F | No | ADC | IVB | Yes | 2 | 19DEL | Osimertinib | Rash [3] | PR | 2.7 | Yes | None |
| 3 | 72 | F | No | ADC | IVB | Yes | 2 | L858R | Osimertinib | Diarrhea [2], hyponatremia [3] | SD | 4.3 | Yes | Hyponatremia [1] |
| 4 | 68 | F | No | ADC | IIIC | No | 1 | L858R | Osimertinib | N/A | N/A | N/A | N/A | N/A |
| 5 | 70 | F | No | ADC | IVB | Yes | 2 | L858R | Osimertinib | None | SD | OT | N/A | N/A |
| 6 | 67 | M | No | ADC | IVB | Yes | 2 | L858R | Osimertinib | N/A | N/A | N/A | N/A | N/A |
| 7 | 73 | F | No | ADC | IVB | Yes | 2 | L858R | Osimertinib | None | SD | OT | N/A | N/A |
| 8 | 62 | F | Yes | ADC | IVB | Yes | 2 | 19DEL | Osimertinib | None | PR | OT | N/A | N/A |
| 9 | 69 | M | No | ADC | IVB | Yes | 2 | 19DEL | Aumolertinib | Pulmonary embolism [4] | N/A | 0.8 | Yes | None |
| 10 | 71 | F | No | ADC | IVB | Yes | 1 | 19DEL | Osimertinib | None | PR | OT | N/A | N/A |
| 11 | 68 | M | Yes | ADC | IVB | Yes | 1 | L858R | Aumolertinib | Interstitial pneumonia [3] | SD | 2.0 | Yes | None |
†, the time of assessment of the endpoints was from August 2021 to September 2021. CNS, central nerve system; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; trAEs, treatment-related adverse events; AEs, adverse events; M, male; ADC, adenocarcinoma; DEL, deletion; PR, partial response; F, female; SD, stable disease; N/A, not applicable; OT, continues on trial.
Efficacy
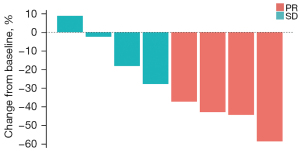
At the time of data cutoff, one patient out of the nine included had discontinued the trial due to trAEs before the first imaging assessment but did show evidence of tumor regression on the CT scan (the first imaging assessment was performed after 6 weeks of medication according to the study design, while this patient took a CT scan due to dyspnea caused by pulmonary embolism only after 4 weeks of medication). Of the remaining eight patients, confirmed partial response was the best response in four patients, while four exhibited stable disease. None showed disease progression (Figure 1). The ORR for the combined therapy was 44.4%, and the DCR was 88.9%. Neither median PFS nor OS was reached at the time of data cutoff.
Safety
Fifty-six percent (5/9) of treated patients experienced trAEs of grade 3 or higher with the combined therapy, including stomachache, rash, hyponatremia, pulmonary embolism, and interstitial pneumonia, which led to trial discontinuation in these five patients (Table 1, Table S2). Among these five patients, three received osimertinib (3 of 7, 7 patients in total in this study received osimertinib and anlotinib), and two received aumolertinib (2 of 2, 2 patients in total received aumolertinib and anlotinib) in the combined regimen. The only grade 4 trAE was pulmonary embolism, and there was no grade 5 trAEs reported with the combined therapy. All trAEs were reversible after trial discontinuation.
Decision regarding study discontinuation
This trial was designed as a small exploratory study to assess the feasibility of combining third-generation EGFR-TKIs with anlotinib in untreated EGFR-mutant patients with advanced NSCLC. Despite the 44.4% ORR and 88.9% DCR, the trial was discontinued for more than half of the patients due to trAEs. Therefore, the study was closed to further enrollment based on toxicity, with an enrollment of only 11 of 35 planned patients.
Safety of subsequent EGFR-TKI treatment after trial discontinuation
All five patients who discontinued the trial received subsequent EGFR-TKI treatment. Three patients who had previously received osimertinib and anlotinib were treated with osimertinib alone afterward. Likewise, the remaining two patients received subsequent aumolertinib treatment, one of whom eventually switched to icotinib due to the recurrence of interstitial pneumonia. All patients demonstrated good tolerance to ongoing EGFR-TKI treatment. One patient developed a grade 1 rash, and another developed grade 1 hyponatremia (Table 1). There was no grade 2 or higher trAEs reported. The four patients who showed no signs of trAEs in the trial continued using the study drug combo.
Discussion
To our knowledge, this is the first phase II study combining third-generation EGFR-TKIs and anlotinib as a first-line treatment for EGFR-mutant advanced NSCLC. In this study, we selected two third-generation EGFR-TKIs, osimertinib and aumolertinib, to combine with anlotinib. Both drugs have been proven safe and effective as monotherapy in EGFR-mutant advanced NSCLC. However, adding anlotinib to the third-generation EGFR-TKIs significantly increased the incidence of trAEs compared with monotherapy of the third-generation EGFR-TKIs. The trial was discontinued for more than half of the patients due to trAEs. Although the estimated ORR and DCR of the combined regimen were lower than osimertinib alone, as reported in the FLAURA trial (9), it is still too early to assert that the combined therapy was inferior to monotherapy. First, the number of patients treated in the trial was limited due to premature closure for significant toxicity. And the one patient who discontinued the trial before the first imaging assessment had evidence of tumor regression on their CT scan. Second, patients with an ECOG performance status of 2 or lower were eligible for this study, and the ECOG performance status of most patients (67%) was 2, whereas in the FLAURA trial, such patients were excluded. Meanwhile, 81.8% of our patients had CNS metastases, in contrast to only 19% of patients in the FLAURA trial. Finally, subgroup analyses of the FLAURA trial revealed that osimertinib was more effective in patients with the exon 19 deletion than those with the L858R mutation (10). Thirty-five percent of patients in the FLAURA trial had L858R mutations, and 57% had exon 19 deletions. However, in our trial, more than half of the patients had L858R mutations.
The reported trAEs of the combined therapy included stomachache, rash, diarrhea, hyponatremia, pulmonary embolism, and interstitial pneumonia, consistent with those reported for third-generation EGFR-TKIs and anlotinib. However, trAEs of grade 3 or higher were observed in more patients in our trial than those receiving osimertinib monotherapy in the FLAURA trial (55.5% vs. 35%). The possible reasons might include an overlap of the toxicity of the third-generation EGFR-TKIs and anlotinib, heterogeneity in the patients’ performance status and CNS metastases status, etc. Five patients went off the trial due to grade 3 or higher trAEs, but there was no treatment-related death. All trAEs were reversible after trial discontinuation. The rate and severity of trAEs were significantly decreased in the subsequent EGFR-TKI treatment.
However, limitations of the study design including single arm, single center and unblinded etc. weakened the stability and credibility of experimental results to some extent.
Conclusions
In summary, our preliminary data suggested that combining third-generation EGFR-TKIs with anlotinib as the initial treatment might significantly increase toxicity in EGFR-mutant patients with advanced NSCLC. Therefore, we conclude that the combined treatment strategy is not an appropriate therapeutic choice in this setting.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-175/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-175/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-175/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-175/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Board of the Fifth Affiliated Hospital of Sun Yat-sen University (No. ZDWY-2020-K46-1). Written informed consent was obtained from all enrolled patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol 2011;6:49-69. [Crossref] [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Singh N, Temin S, Baker S Jr, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO Living Guideline. J Clin Oncol 2022;40:3310-22. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Lu S, Dong X, Jian H, et al. AENEAS: A Randomized Phase III Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or MetastaticNon-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations. J Clin Oncol 2022;40:3162-71. [Crossref] [PubMed]
- Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B 2015;5:390-401. [Crossref] [PubMed]
- Petit AM, Rak J, Hung MC, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol 1997;151:1523-30. [PubMed]
- Hirata A, Ogawa S, Kometani T, et al. ZD1839 (Iressa) induces antiangiogenic effects through inhibition of epidermal growth factor receptor tyrosine kinase. Cancer Res 2002;62:2554-60. [PubMed]
- Ciardiello F, Caputo R, Damiano V, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res 2003;9:1546-56. [PubMed]
- De Luca A, Carotenuto A, Rachiglio A, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol 2008;214:559-67. [Crossref] [PubMed]
- Masuda C, Yanagisawa M, Yorozu K, et al. Bevacizumab counteracts VEGF-dependent resistance to erlotinib in an EGFR-mutated NSCLC xenograft model. Int J Oncol 2017;51:425-34. [Crossref] [PubMed]
- Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 2009;15:3484-94. [Crossref] [PubMed]
- Ichihara E, Hotta K, Nogami N, et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol 2015;10:486-91. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019;20:625-35. [Crossref] [PubMed]
- Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:1655-69. [Crossref] [PubMed]
- Jiang T, Zhang Y, Li X, et al. EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur J Cancer 2019;121:98-108. [Crossref] [PubMed]
- Yu HA, Schoenfeld AJ, Makhnin A, et al. Effect of Osimertinib and Bevacizumab on Progression-Free Survival for Patients With Metastatic EGFR-Mutant Lung Cancers: A Phase 1/2 Single-Group Open-Label Trial. JAMA Oncol 2020;6:1048-54. [Crossref] [PubMed]
- Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [Crossref] [PubMed]
- Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Li T, Qian Y, Zhang C, et al. Anlotinib combined with gefitinib can significantly improve the proliferation of epidermal growth factor receptor-mutant advanced non-small cell lung cancer in vitro and in vivo. Transl Lung Cancer Res 2021;10:1873-88. [Crossref] [PubMed]
- Zhang C, Cao H, Cui Y, et al. Concurrent use of anlotinib overcomes acquired resistance to EGFR-TKI in patients with advanced EGFR-mutant non-small cell lung cancer. Thorac Cancer 2021;12:2574-84. [Crossref] [PubMed]
- Zhou R, Song L, Zhang W, et al. Combination of Osimertinib and Anlotinib May Overcome the Resistance Mediated by in cis EGFR T790M-C797S in NSCLC: A Case Report. Onco Targets Ther 2021;14:2847-51. [Crossref] [PubMed]
- Lian Z, Du W, Zhang Y, et al. Anlotinib can overcome acquired resistance to EGFR-TKIs via FGFR1 signaling in non-small cell lung cancer without harboring EGFR T790M mutation. Thorac Cancer 2020;11:1934-43. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Marcoux N, Gettinger SN, O'Kane G, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol 2019;37:278-85. [Crossref] [PubMed]
- Wang W, Xu C, Chen H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: A multicenter retrospective study. Lung Cancer 2021;155:20-7. [Crossref] [PubMed]


