Rectal neuroendocrine neoplasms: a case report
History
A 68-year-old male patient developed bloody stools without any obvious cause in February 2009. The symptom gradually worsened, but the patient did not seek medical treatment. In March 2009, digital rectal examination during the health check-up in other hospital suggested the possibility of rectal cancer. Other previous disease histories included paroxysmal atrial fibrillation and hypertension for 2 years. During the digital rectal examination, a palpable mass was detected about 4–6 cm from the anal verge, along with blood-stained glove.
Preoperative auxiliary examinations
Abdominal and pelvic CT plain scans (on April 22, 2009, in our hospital)
A mass was found in left lateral rectal wall, and the possibility of rectal cancer with multiple metastases in peripheral lymph nodes was considered. A hypodensic mass was seen in the caudate lobe of liver, with unknown nature.
Electronic colonoscopy (on April 23, 2009, in our hospital)
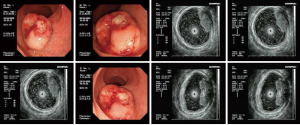
A hemispherical protuberance sized about 3 cm × 3 cm was found in rectum about 4–7 cm from the anal verge. The lesion had a broad base, but with poor activity. The lesion surface was ruptured, which contributed to the formation of ulcers (Figure 1). Rectal carcinoid? Rectal cancer?
Endoscopic ultrasonography (on April 23, 2009, in our hospital)
A space-occupying lesion with medium-level echoes was seen in the intestinal wall where the lesion occurred. The echoes were unevenly distributed, and the lesion borders were unclear. The lesion was mainly located in the mucosa and submucosa layers of the intestinal wall. Parts of the lesion had close relationships with the proper basal layer, without any clear border. The possibility of malignant rectal tumor was considered.
Contrast-enhanced MRI scans of the abdomen (on April 28, 2009, in our hospital)
A tumor sized about 4.9 cm × 3.9 cm was seen in the caudate lobe of liver. The possibility of a hemangioma in caudate lobe of the liver was considered.
Tumor marker detection (on April 4, 2009, in our hospital)
Tumor marker detection showed that the CA19-9, CEA, and TPS levels were normal.
Preoperative diagnosis
- A rectal space-occupying mass, with a high possibility of being malignant;
- Paroxysmal atrial fibrillation;
- Hypertension (grade 2) (high risk).
Treatment
Based on the above findings, a rectal space-occupying mass with high malignant potential was considered.
Since the preoperative CT indicated that the tumor was resectable, open lower anterior resection (AR) of rectal cancer was performed on April 30, 2009. Intraoperative exploration showed that there was no ascites in abdominal cavity, and no space-occupying lesion was palpable in liver and spleen. The tumor (sized about 4 cm) was located beneath in rectal peritoneal folds but did not infiltrate outside the fiber membrane. After 2/3 weeks of invasion, multiple swollen lymph nodes were detected in inferior mesenteric artery. The peritoneal folds were cut open along the left root of sigmoid mesocolon and the descending colon till the rectovesical pouch, where the incision met the lateral incision. After the upper rectal vessels and parts of sigmoid vessels were ligated at the mesenteric root, the posterior wall of the rectum was divided till the level of pelvic floor muscle; meanwhile, the anterior wall was divided till the lower part of the seminal vesicle. The rectum was closed using a stapler 5 cm beneath the tumor; then, the sigmoid colon was transected 15 cm from the upper border of the tumor. End-to-end anastomosis was performed using a stapler. The operation was smooth. The abdominal cavity was flushed after surgery, followed by complete hemostasis. Sustained-release 5-fluorouracil implant (Jeny1 g) was implanted in the pelvic cavity. The amounts of equipment were correct. The abdominal drainage tube was indwelled; then, the incision was closed layer-by-layer. The patient was sent back to his ward safely after recovering from anesthesia after the operation.
The patient recovered satisfactorily after gastrointestinal decompression, anti-inflammatory treatment, acid suppression, and supportive rehydration therapy. The gastric tube was withdrawn on the 4th postoperative day; on day 7, the abdominal drainage tube was removed. The incision healed well and the patient was then discharged.
Postoperative pathology
An elevated well-differentiated neuroendocrine carcinoma sized about 2 cm × 2 cm × 1 cm in rectum. Ulcer formation was seen on rectal surface. The mitotic figures were about 2–3/10HPF. Calcifications were present. The tumor invaded deeply into the muscular layer.
Lymph node metastases (5/10).
Immunohistochemistry: AE1/AE3 (focal +), CK20 (−), CD56 (++), Syn (+), CgA (focal +), NSE (++), and CD34 (−).
Pathological review (on June 30, 2015, in our hospital): a neuroendocrine tumor, G2; mitotic figures: 3/10HPF; and Ki-67, <1%.
Postoperative treatment
Ten cycles (one cycle is 2 weeks) of oxaliplatin 150 mg ivtt day 1 + capecitabine 1,500 mg bid day 1–10 + interferon 6,000,000 U im day 2–6 were applied during the period from June 2009 to October 2009.
Postoperative follow-up
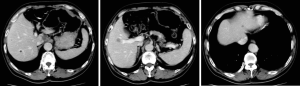
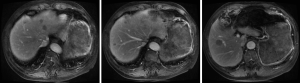
The patient was followed up regularly at 6-month intervals by abdominal/pelvic CT until to April 23, 2014 when some of intrahepatic hypodense nodules became larger, which were found on 31, 2009 initially and considered as hepatic cysts. And then the patient performed CT examination at 6-month intervals continually. And postoperative examinations were normal until intrahepatic multiple metastases were first discovered on April 23, 2014 (Figures 2-4).
Current treatment
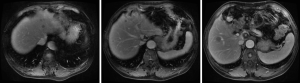
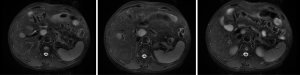
Based on the multidisciplinary treatment (MDT) consultations, sandostatin treatment was recommended. The patient received fours cycles of sandostatin treatment from July 2015 to November 2015. The patient performed MRI examination on November 17, 2015 after the above treatment (Figure 5).
Discussion
Incidence of rectal neuroendocrine neoplasms (NENs)
Gastrointestinal neuroendocrine neoplasms (GI-NENs) include NENs in stomach, duodenum, small intestine, appendix, colon, and rectum, among which NENs in ileum, rectum, and appendix are most common (1). The incidences of NENs are on the rise in recent years. According to a data source in Japan, the annual incidence of ileum/rectum NENs is only 0.20 per 100,000 in Asian populations and the rectal NENs account for 60–89% of all GI-NENs (2), which are dramatically different from the findings in Caucasian populations. No relevant information has been available in China. In 2012, Guo et al. reviewed all the relevant literature published between 1954 and 2011 and found a total of 11,671 GEP-NENs cases, among which pancreatic NENs p-NENs were most common (accounting for 49.80%), followed by rectal NENs (24.3%) and appendiceal NENs (11.1%); the proportions of NENs in other sites did not exceed 10% (3).
Clinical manifestations and laboratory/imaging findings of rectal NENs
The clinical manifestations dramatically differ among GE-NENs (4) (Table 1). Most rectal NENs are non-functioning and have no symptoms related to hormone secretion (as seen in carcinoid syndrome). Patients with rectal NENs may experience non-specific symptoms such as pain, perianal bulge, anemia, and bloody stools (5). Rectal NENs lack specific biochemical markers. Elevated serum chromogranin a (CgA) level has been seen in 60–80% of GEP-NENs. Interpretation of this marker in clinical settings should consider the influence of long-term use of proton pump inhibitors (PPIs), proliferation of enterochromaffin-like cells due to chronic gastritis, and chronic dysfunction. Neuron-specific enolase (NSE) has a low sensitivity and specificity in diagnosing NENs. Other serological markers such as chromogranin B (CgB), pancreatic polypeptide (PP), and human chorionic gonadotropin-p (HCG-p) may also increase in a few rectal NEN cases (6). In this case, the patient developed bloody stools without symptoms related to hormone secretion and tumor marker detection showed that the CA19-9, CEA, and TPS levels were normal. Endoscopy plays an important role in the diagnosis of rectal NENs. After EUS, Ishii N performed endoscopic resection in 22 patients with rectal NENs. The tumor diameters measured by EUS showed no significant difference with those measured during postoperative pathology. EUS had an accuracy of 100% in determining the depth of NEN invasion (7). By EUS the lesion of this patient was mainly located in the mucosa and submucosa layers of the intestinal wall and parts of the lesion had close relationships with the proper basal layer.
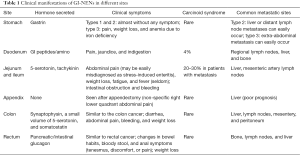
Full table
Surgical treatment of rectal NENs
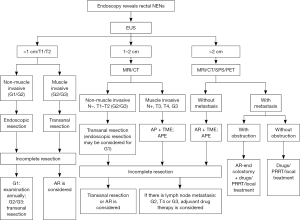
According to the European Society for Medical Oncology (ESMO) guidelines, surgery is the preferred treatment for GI-NENs (8). Complete resection should be achieved for localized tumors. Currently, the commonly used surgical procedures include the endoscopic excision, transanal local excision, transanal endoscopic microsurgery (TEM), AR, and abdominoperineal resection (APR) of the rectum. Selection of a specific procedure depends on the biological behavior of tumors. Tumor size is the marker that has the closest relationship with biological behaviors and meanwhile is also the most easily measured indicator. Tumors with a diameter of <1 cm seldom shows metastasis (<3%); tumors with a diameter of 1–2 cm have a 10–15% probability of metastasis; and tumors with a diameter of >2 cm have a much higher probability (60–80%) of metastasis. Other factors that are associated with tumor metastasis include: poor differentiation, myometrial invasion, lymphatic/vascular/nerval infiltration, high mitotic figures, high Ki-67 index, and invagination/ulceration/unusual color under endoscope. Treatments for rectal NENs with a diameter of >2 cm, T3/G4, G3, and/or with regional lymph node metastasis are same as those for rectal adenocarcinoma (6). For rectal NENs with a diameter of >2 cm but without distant metastasis, AR of the rectum with total mesorectal excision (TME) or abdominoperineal extirpation (APE) of the rectum may be considered. For tumors sized <2 cm, however, local resection is recommended (4) (Figure 6). In this case a hemispherical protuberance sized about 19.5 mm × 11.3 mm was found in rectum by EUS. For the diameter was in close proximity to 2 cm and take measuring error into consideration, we performed an open lower AR of rectal cancer for this patient.
Treatment of liver metastasis
While liver metastasis from rectal NENs seldom occurs, its surgical treatment applies the same principles as that for rectal adenocarcinoma (6). One- or two-stage resection of the liver metastasis may be performed based on the operator’s training level. If patients with advanced tumors develop intolerable carcinoid syndrome symptoms, surgical resection, radiofrequency ablation, and/or other measures may be applied to lower the tumor burden and thus alleviate the symptoms. No evidence has shown that primary lesion resection alone may bring more benefits to the patients (9,10). The 5-year survival in patients who have undergone liver transplantation to treat liver metastasis from NENs is below 50% (11). Liver transplantation can only be applied in patients unresponsive to other treatments; it is contraindicated in patients with systemic multiple metastases and/or G3 tumors. In addition, transcatheter arterial embolization (TAE) or transcatheter arterial chemoembolization (TACE) may be feasible for patients with high liver tumor burden; however, the efficacies of these two procedures require further investigations (8). For this patient, intrahepatic multiple metastases deprived the chance of one- or two-stage resection of the liver metastasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Modlin I, Zikusoka M, Kidd M, et al. The history and epidemiology of neuroendocrine tumors. In: Caplin M, Kvols L, editors. Handbook of Neuroendocrine Tumors. 1st ed. Bristol, UK: BioScientifica, 2006:7-37.
- Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol 2010;45:234-43. [Crossref] [PubMed]
- Guo L, Tang C. Current Status of Clinical Research on Gastroenteropancreatic Neuroendocrine Tumors in China. Chin J Gastroenterol 2012;17:276-8.
- The experts committees of CSCO on NENS. Chinese consensus on gastroenteropancreatic neuroendocrine plasms. Chinese Clinical Oncology 2013;18:815-32.
- Vogelsang H, Siewert JR. Endocrine tumours of the hindgut. Best Pract Res Clin Gastroenterol 2005;19:739-51. [Crossref] [PubMed]
- Zhou X, Xie H, Xie L, et al. Current diagnosis and treatment of rectal neuroendocrine neoplasms. Chin J Clinicians 2013;7:6049-51. (Electronic Edition).
- Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc 2010;24:1413-9. [Crossref] [PubMed]
- Oberg K, Akerström G, Rindi G, et al. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v223-7. [Crossref] [PubMed]
- Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg 2005;241:776-83; discussion 783-5. [Crossref] [PubMed]
- House MG, Cameron JL, Lillemoe KD, et al. Differences in survival for patients with resectable versus unresectable metastases from pancreatic islet cell cancer. J Gastrointest Surg 2006;10:138-45. [Crossref] [PubMed]
- Castaldo ET, Pinson CW. Liver transplantation for non-hepatocellular carcinoma malignancy. HPB (Oxford) 2007;9:98-103. [Crossref] [PubMed]
Cite this article as: Su H, Zhou H. Rectal neuroendocrine neoplasms: a case report. Transl Gastroenterol Hepatol 2016;1:49.