
Prostate specific membrane antigen (PSMA) expression in vena cava tumour thrombi of clear cell renal cell carcinoma suggests a role for PSMA-driven tumour neoangiogenesis
Introduction
Kidney cancer accounts for approximately 2% of all malignant neoplasms (1). Most malignant kidney tumours are renal cell carcinoma (RCC). Of these neoplasms, clear cell RCC (ccRCC) accounts for 60–70% of all RCC and represents the most common subtype (2). Nearly 50% of all ccRCC cases are of clinical stage T3, or greater at diagnosis, making prognosis of this group of malignant neoplasms unfavorable (3). Clinical stage T3b, a stage that consists of contiguous extension of tumour into the infradiaphragmatic portion of the inferior vena cava (IVC), represents 4–10% of cases (4). Presence of intravascular tumour extension may represent a useful model for studying contributors to the aggressive invasive front of neoangiogenesis in locally advanced ccRCC.
For this investigation, prostate-specific membrane antigen (PSMA) and CD34 were selected as immunohistochemical (IHC) biomarkers. CD34 is a vascular marker of widespread usage in surgical pathology. It is present in endothelial cells of vessels of all sizes, including capillaries. CD34 positivity highlights well-differentiated endothelial cells of most normal tissues and various vascular neoplasms (5-7). PSMA is a glycoprotein that was first discovered in normal and neoplastic prostatic epithelium (8-12). It has been utilized extensively in prostate cancer for diagnostic and prognostic ancillary techniques (13). For example, 68Gallium bound HBED-CC PSMA binding ligand (14), 18Fluorine-DCFPyL PSMA binding ligand (15,16) or 111indium-labelled 7E11 conjugate (17) are currently used as radiotracers for prostate cancer imaging with positron emission tomography (PET).
Despite its name referring to a prostatic origin, PSMA has been identified in many other tumour types, including transitional cell carcinoma, malignant melanoma, lung cancer soft tissue sarcomas, and renal tumours (18-20). Previous studies have demonstrated that there is PSMA expression in normal kidney proximal tubular epithelial cells and in RCC neovasculature (21-23). There is no recorded expression of PSMA in vessels of normal kidney tissue. This discrimination supports its role as a marker of neoangiogenesis in different types of kidney epithelial neoplasms (22,23). The hypothesis of the current study was that PSMA expression, a marker of neoangiogenesis, is expressed differently in ccRCC vena cava neoplastic thrombi (invasive front) when compared to the paired renal tumour mass.
Methods
Patients
Between 2013 and 2015, 202 patients diagnosed with RCC were surgically treated at Princess Alexandra Hospital (Queensland, Australia). Of these cases, 131 consisted of ccRCC, of which ten presented with IVC tumour extension. Normal paired kidney tissue, renal tumour and leading edge of the neoplastic thrombus (invasive front) were collected from these patients. Approval for this research was obtained from the human research ethics committees of Princess Alexandra Hospital and Greenslopes Private Hospital (HREC/05/QPAH/95 and protocol 13/23) and informed patient consent was obtained. The surgical specimens were fixed in formalin, paraffin embedded, step-sectioned at 3 to 4 µm intervals and mounted on treated glass slides. Hematoxylin and eosin (H&E) staining was routinely performed and assessed microscopically.
Immunohistochemistry
CD34 was selected as the preferred pan endothelial IHC marker. It highlights a higher percentage of endothelial cells compared to other endothelial markers such as CD31, CD105 or factor VIII-related antigen (6,7). Primary antibody for CD34 was mouse anti-human CD34 MAb, clone QBend-10 Cat No. Vector VP-C345 or Dako M7165, diluted 1:150 in Tris-buffered saline (TBS). Primary antibody for PSMA was Dako Flex pre-diluted mouse anti-human PSMA, clone: 3E6 cat. No. IR089. Positive and negative control sections were used for IHC and batch staining of the slides was utilized. IHC for CD34 and PSMA was performed using standard protocols. Briefly, the slides were dewaxed and rehydrated through descending graded alcohols to TBS. Endogenous peroxidase activity was then blocked by incubating the sections in 1.0% hydrogen peroxide (H2O2) in TBS for 10 minutes. For antigen retrieval (AR), the samples were transferred to Dako Target Retrieval Solution pH 9.0 and submitted to heat (30 minutes at 100 °C for CD34 and 20 minutes at 97 °C for PSMA) using the Biocare Medical Decloaking Chamber. After AR was completed, the slides were removed and allowed to cool for a further 20 minutes before transferring back to TBS for washing. Biocare Medical Background Sniper with 1.0% bovine serum albumin/BSA for CD34 and 2.0% BSA for PSMA was used for 20 minutes to block non-specific antibody binding. The slides were then incubated with the primary antibodies for CD34 or PSMA for 60 minutes. Signals were developed in 3,3'-diaminobenzidine hydrochloride (DAB) with H2O2 as substrate for 5 minutes. Additional copper sulphate enhancement for 5 minutes was used for PSMA. The slides were washed in distilled water, counterstained with Mayer’s hematoxylin, dehydrated, and mounted with Depex mounting medium.
Morphometric analysis with Aperio ImageScope
There is much controversy around the subject of intratumoural microvascular density (iMVD) measurements (23-26). While some groups, like Weidner et al., proposed specific effective methods for measuring iMVD in some neoplasms (7), other researchers, such as Nico et al. (24), carried out a systematic review detailing several different possible methods for iMVD assessment in various tumours, either manually, using vessel number and vascular density classification such as grades 0 to 3, or by computerized image, using pan endothelial cell markers. The present study adopted specific methods for morphometric analysis of iMVD, which included subjective (qualitative) methods and objective (quantitative) methods. The qualitative method considered PSMA and CD34 vascular expression. Strong (diffuse strong) staining was scored 3, moderate (diffuse weak or focal strong) staining was scored 2, weak staining (weak staining in less than 5% of vessels) was scored 1, and the absence of staining was scored 0. Positive PSMA in tumour-associated neovasculature was defined as a staining score of 2 or greater. Objective tumoural neovasculature morphological patterns were assessed by counting vessel number in 10 high power microscope fields (HPF) and measuring total vascular area in each field (sum of the measurements of each vessel area). The averages of all values obtained in 10 HPF were then obtained, as well as the measurements in the highest vascular density areas (vascular “hot spots”). Positive pixel analysis was used both for CD34 and PSMA, comparing the whole sections, highest positivity areas (hot spots) and selected areas of 10 paired HPF between tumour mass and thrombus, excluding areas of necrosis and hemosiderin deposition. The results were analyzed by paired Student’s t-test, with a P<0.05 taken as significant.
Results
Clinical and histopathological data
Clinical and histopathological data are shown in Table 1. The mean age of the patients was 59 years with eight of the ten patients being male. WHO/ISUP grade 3 and tumour diameter varied from 65 to 95 mm in the majority of cases. All of the patients were staged as pT3 or higher and had unilateral tumours with no signs of lymph node metastasis (pN0) or distant metastasis (pM0). When comparing adjacent normal kidney tissue, only one patient showed signs of chronic kidney fibrosis, and no alterations in adjacent kidney parenchyma correlated with any histological patterns in the tumour mass or thrombus.
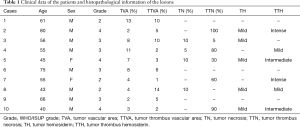
Full table
Immunohistochemistry and quantification
When comparing the morphological patterns of the neoplasm and neoplastic thrombus, there was no clear distinction between vascular patterns, vascular density, and tumour necrosis or hemosiderin deposition. CD34 IHC showed a diffuse strong staining in all vessels, both in tumour and tumour thrombi samples. PSMA IHC showed a predominantly weak to moderate diffuse staining in most samples, with a subjective impression of increased expression in the vena cava tumour thrombi when compared to tumour tissue (Figure 1). This impression is proven objectively using a PSMA/CD34 positive pixel expression ratio, both in 10 HPF average, excluding areas of necrosis and hemosiderin deposition (Figure 2) and in full slide digital analysis (Figure 3). This ratio was used to rule out the great variation in iMVD observed due to intertumoural and intratumoural heterogeneity. There was a 57% increase in the PSMA/CD34 ratio using 10 HPF average and a 60% increase using total slide digital analysis. The P value for both comparisons was <0.01 (Table 2).
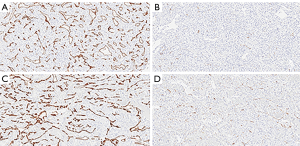
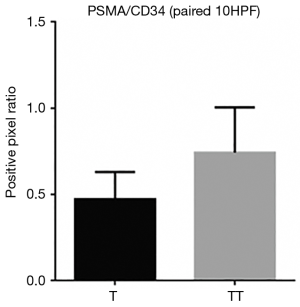
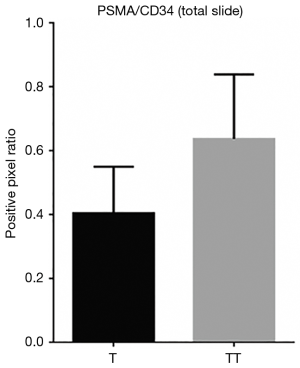
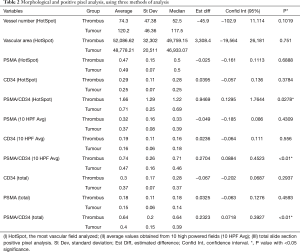
Full table
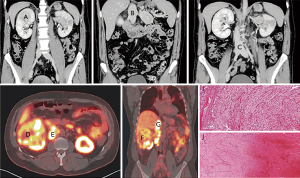
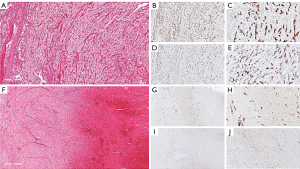
Of the 10 cases, one case showed peculiar preoperative radiological and tumoural IHC characteristics. The preoperative contrasted computerized tomography (CT) revealed a right kidney neoplasm (Figure 4) extensive IVC tumour which appeared to infiltrate the IVC both superiorly (Figure 4B) and inferiorly (Figure 4C). 68Gallium PSMA-PET-CT Scan study showed PSMA avidity in the right renal neoplasm associated with intense PSMA avidity in the IVC (Figure 4D,E,F and G). This PSMA avidity was more obvious in the suprarenal portion of the IVC (Figure 4G) than the infra renal portion (Figure 4H). Following surgery, the superior part of the thrombus was composed exclusively by viable tumour thrombus (Figure 4I and Figure 5), while the inferior part was entirely bland thrombus, without any viable neoplastic cells (Figure 4J and Figure 5F). On IHC staining, there were CD34 positive vessels in both tumour thrombus (superior leading front) and also in the bland thrombus (inferior portion of IVC thrombus) (Figure 5B,C,G and H). However, there were significant numbers of PSMA positive vessels in the superior neoplastic thrombus portion (Figure 5D,E) and focal weak PSMA staining noted in the inferior IVC bland thrombus portion (Figure 5I,J).
Discussion
The current study investigated whether PSMA was expressed differently in ccRCC vena cava tumour extensions compared with the paired intrarenal tumour mass. The results showed significantly increased PSMA/CD34 ratio in the intravascular tumour extension versus intrarenal tumour tissue. We propose that PSMA activity in ccRCC tumour thrombi may be a useful marker for neoangiogenesis and help provide better understanding of the invasive nature of the kidney tumour. Despite no clear morphological difference of microvascular characteristics of tumour thrombi evaluated by light microscopy using H&E stained sections, PSMA expression paired with CD34+ vessels revealed increased expression in the invasive front. This phenomenon strengthens the hypothesis that PSMA is involved in tumour neoangiogenesis, a fact that may lead to future clinical implications such as targeting PSMA for treatment of cancers (27). We have also confirmed that there is a peripheral pattern of neovascularization in the tumour that is evident with the increased PSMA intensity of neovascularization in the invasive front in the vena cava tumour extension.
The one clinical case that demonstrated increased diffuse PSMA IHC staining in the neovessels of the IVC tumour extension compared with focal weak PSMA IHC staining in the bland thrombus portion of the IVC, provides further evidence that PSMA is involved with tumour neovascularization. This was also highlighted with the preoperative PSMA PET scan that showed PSMA avidity in the portion of the IVC thrombus that was confirmed histologically as contiguous tumour extension. The PSMA PET image of this case was published by Rhee et al. (20). The present study provides further evidence showing the corresponding PSMA PET avidity with PSMA positivity on histology of the superior portion of IVC tumour thrombus.
The findings have important implications for future research in areas of tumour neoangiogenesis and invasiveness (24). First, the PSMA IHC demonstrated its usefulness in highlighting neovascularization (23), a differentiation that cannot be made by CD34 alone. This may have implications for further characterizing neoplasms histologically. Second, in understanding the molecular mechanisms of angiogenesis, PSMA expression is gaining interest as an important enzyme related to neovascularization. Conway et al. have shown previously that enzymatic activity of PSMA regulates angiogenesis by modulating integrin signal transduction, resulting in endothelial cell migration and invasion (25). Nguyen et al. demonstrated that PSMA was not expressed in human umbilical endothelial cells (HUVEC), a commonly used cell line for in vitro models of angiogenesis. However, in culturing the cells with conditioned media from PSMA-expressing cells, the HUVEC cells expressed PSMA, as well as achieving a tubular morphology. The authors concluded that tumour-related factors were responsible for neovasculature formation and PSMA expression (26). Recent adoption of PSMA imaging in a variety of cancers, increased interest in molecular cancer research, and PSMA-targeted therapies (27,28) make this present study timely. Further increase in knowledge in these areas is likely to help deliver PSMA targeted therapies for a variety of cancers.
This is the first study to highlight the increased expression of PSMA in vena cava tumour thrombi originating from ccRCC. This novel result suggests the role of PSMA-driven neoangiogenesis in ccRCC leading to their progression and metastasis. Future research into the mechanism of PSMA in neoangiogenesis can, hopefully, lead to improved diagnostic and therapeutic pathways.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval for this research was obtained from the ethics committees of Princess Alexandra Hospital and Greenslopes Private Hospital (HREC/05/QPAH/95 and protocol 13/23) and patient consent obtained.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Samaratunga H, Gianduzzo T, Delahunt B. The ISUP system of staging, grading and classification of renal cell neoplasia. J Kidney Cancer VHL 2014;1:26-39. [Crossref] [PubMed]
- Delahunt B, Samaratunga H, Martignoni G, et al. Percutaneous renal tumour biopsy. Histopathology 2014;65:295-308. [Crossref] [PubMed]
- Bissada NK, Yakout HH, Babanouri A, et al. Long-term experience with management of renal cell carcinoma involving the inferior vena cava. Urology 2003;61:89-92. [Crossref] [PubMed]
- Poblet E, Gonzalez-Palacios F, Jimenez FJ. Different immunoreactivity of endothelial markers in well and poorly differentiated areas of angiosarcomas. Virchows Arch 1996;428:217-21. [PubMed]
- Traweek ST, Kandalaft PL, Mehta P, et al. The human hematopoietic progenitor cell antigen (CD34) in vascular neoplasia. Am J Clin Pathol 1991;96:25-31. [Crossref] [PubMed]
- Weidner N. Chapter 4: Measuring intratumoural microvessel density. Methods Enzymol 2008;444:305-23. [Crossref] [PubMed]
- O’Keefe DS, Su SL, Bacich DJ, et al. Mapping, genomic organization and promoter analysis of the human prostate specific membrane antigen gene. Biochim Biophys Acta 1998;1443:113-27. [Crossref] [PubMed]
- Israeli RS, Powell CT, Fair WR, et al. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res 1993;53:227-30. [PubMed]
- Wright GL, Haley C, Beckett ML, et al. Expression of prostate-specific membrane antigen in normal, benign and malignant prostate tissues. Urol Oncol 1995;1:18-28. [Crossref] [PubMed]
- Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial cells and serum of prostatic cancer patients. Anticancer Res 1987;7:927-35. [PubMed]
- Mukherjee A, Darlington T, Baldwin R, et al. Development and screening of a series of antibody-conjugated and silica-coated iron oxide nanoparticles for targeting the prostate-specific membrane antigen. ChemMedChem 2014;9:1356-60. [Crossref] [PubMed]
- Ristau BT, O'Keefe DS, Bacich DJ. The prostate-specific membrane antigen: lessons and current clinical implications from 20 years of research. Urol Oncol 2014;32:272-9. [Crossref] [PubMed]
- Eiber M, Herrmann K. From NETTER to PETTER: PSMA-targeted radioligand therapy. J Nucl Med 2017;58:9-10. [Crossref] [PubMed]
- Gorin MA, Rowe SP, Hooper JE, et al. PSMA-targeted 18F-DCFPyL PET/CT imaging of clear cell renal cell carcinoma: results from a rapid autopsy. Eur Urol 2017;71:145-6. [Crossref] [PubMed]
- Rowe SP, Gorin MA, Pomper MG. Imaging of prostate-specific membrane antigen using 18F-DCFPyL. PET Clin 2017;12:289-96. [Crossref] [PubMed]
- Rauscher I, Maurer T, Souvatzoglou M, et al. Intrapatient comparison of 111In-PSMA SPECT/CT and hybrid 68Ga-HBED-CC PSMA PET in patients with early recurrent prostate cancer. Clin Nucl Med 2016;41:e397-402. [Crossref] [PubMed]
- Chang SS, Reuter VE, Heston WDW, et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumour-associated neovasculature. Cancer Res 1999;59:3192-8. [PubMed]
- Wang HL, Wang SS, Song WH, et al. Expression of prostate-specific membrane antigen in lung cancer cells and tumour neovasculature endothelial cells and its clinical significance. PLoS One 2015;10:e0125924. [Crossref] [PubMed]
- Rhee H, Blazak J, Tham CM, et al. Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res 2016;6:76. [Crossref] [PubMed]
- Chang SS, Reuter VE, Heston WDW, et al. Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology 2001;57:801-5. [Crossref] [PubMed]
- Rhee H, Ng KL, Tse BW, et al. Using prostate specific membrane antigen (PSMA) expression in clear cell renal cell carcinoma for imaging advanced disease. Pathology 2016;48:613-6. [Crossref] [PubMed]
- Baccala A, Sercia L, Li J, et al. Expression of prostate-specific membrane antigen in tumour-associated neovasculature of renal neoplasms. Urology 2007;70:385-90. [Crossref] [PubMed]
- Nico B, Benagiano V, Mangieri D, et al. Evaluation of microvascular density in tumours: pro and contra. Histol Histopathol 2008;23:601-7. [PubMed]
- Conway RE, Petrovic N, Li Z, et al. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol 2006;26:5310-24. [Crossref] [PubMed]
- Nguyen DP, Xiong PL, Liu H, et al. Induction of PSMA and Internalization of an anti-PSMA mAb in the vascular compartment. Mol Cancer Res 2016;14:1045-53. [Crossref] [PubMed]
- Wang X, Yin L, Rao P, et al. Targeted treatment of prostate cancer. J Cell Biochem 2007;102:571-9. [Crossref] [PubMed]
- Crowley MJ, Scognamiglio T, Liu YF, et al. Prostate-specific membrane antigen is a potential antiangiogenic target in adrenocortical carcinoma. J Clin Endocrinol Metab 2016;101:981-7. [Crossref] [PubMed]


