Inflammatory bowel disease and risk of urinary cancers: a systematic review and pooled analysis of population-based studies
Introduction
Inflammatory bowel disease (IBD), traditionally classified into Crohn’s disease (CD) and ulcerative colitis (UC), is an idiopathic intestinal inflammatory disease involving the ileum, rectum, and colon (1). IBD is a multifactorial disorder, and it is well-known that the chronic inflammatory response caused by the abnormal reaction of the intestinal mucosal immune system plays an important role in the pathogenesis of IBD (1). In the latter half of the 20th century, IBD was traditionally considered as a disease of westernized nations (including the USA, Canada, Australia, New Zealand, and all countries in western Europe), due to the significantly increased incidence of UC and CD in the western world (2-4) However, recent studies indicate that IBD has become a global condition with the development of newly industrialized countries whose societies have become more westernized (including South America, eastern Europe, Asia, and Africa) (2,5). IBD has been demonstrated with an increased risk of intestinal and extra-intestinal tumors (6-11). Although recent studies (10,11) showed that IBD patients were at increased risk of bladder cancer (BCa) and prostate cancer (PCa), limited and disparate data were available for incidence of urological malignancies in these patients. To better understand this issue, a meta-analysis of population-based studies was performed to elucidate the risk of urologic cancers in patients with CD or UC. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1358).
Methods
Search strategy
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (12), this literature was identified initially through PubMed, the Cochrane Library, Embase and Web of Science from inception to June 2020 with no language limitation. The Medical Subject Heading (MeSH) terms including “Inflammatory Bowel Diseases”, “Urinary Bladder Neoplasms”, “Prostatic Neoplasms”, “Kidney Neoplasms”, “Genital Neoplasms, Male” and “Urologic Neoplasms”, and corresponding synonyms were combined in search strategy. The complete search strategy was presented in Supplementary Material. We scrutinized references of identified studies manually for all potentially eligible studies to broaden the search.
Study selection
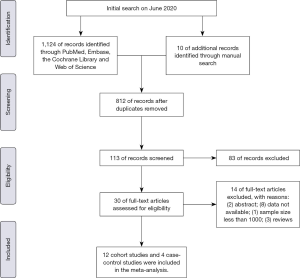
We developed the following inclusion criteria to identify qualified documents: (I) After diagnosis of IBD, regardless of UC or CD, patients were developed with BCa, renal caner (RCa), PCa, or male genital cancer; (II) the sample size included in this study is not less than 1,000; (III) standardized incidence ratio (SIR) or relative risk (RR), with corresponding 95% confidence intervals (CIs), were used to evaluate the association between IBD and risk of urologic cancers (BCa, RCa, PCa or MGCa); (IV) population-based cohort studies or case-control studies; (V) the follow-up should more than one year after diagnosis of IBD. We incorporated the most recent or most informative study if more than one articles studied the same population. Besides, exclusion criteria were as follows: (I) any study which did not satisfy the inclusion criteria; (II) meeting abstracts, review or meta-analysis; (III) data not available. Figure 1 sketches the PRISMA flowchart showing the study selection process of this meta-analysis.
Data extraction and quality assessment
We imported the identified records into the EndNote X9. The initial search results were then screened by two independent authors using prespecified standardized criteria on the basis of title, abstract, and, finally, full text. Any discrepancy was tackled by consensus or a third party. We formulated a unified plan in advance to extract data. Data were independently extracted by two reviewers. The following data were extracted: the first author’s name, year of publication, country, study design, period, sample size, cancer types, outcomes (SIRs or RRs).
The methodological quality of included studies was assessed by two independent reviewers using the Newcastle-Ottawa Scale (NOS) (13). The NOS applied a ‘star system’ to evaluate the quality of study from three perspectives: the selection of the studies, the comparability of studies, and the assessment of outcome. If seven or more of nine stars were received, the study was regarded as to be high-quality. Moreover, two reviewers independently rated the level of evidence of the included articles through the Oxford Centre for Evidence-Based Medicine criteria (14); this scale graded studies from strongest (level 1) to weakest (level 5) strength of evidence according to study design and data quality.
Statistical analysis
The Q and I2-statistics tests were calculated to evaluate the between-study heterogeneity. Pooled SIRs or RRs and corresponding 95% CIs were calculated through fixed-effect model if the heterogeneity was acceptable among studies (P>0.1 or I2≤50%). Otherwise, a random-effect model was used. Moreover, the results of this meta-analysis were reported on the basis of cancer types and IBD subtype. We assessed the potential publication bias according to the asymmetry of funnel plots, and the asymmetry can be quantified by the Egger’s test and Begg’s test. A P value of less than 0.1 was considered as significant publication bias. Furthermore, we performed sensitivity analyses to evaluate the robustness of the pooled results. Statistical significance was set at P<0.05. This meta-analysis was completed by STATA version 14.2 (StataCorp LP, College Station, TX, USA).
Results
Search results
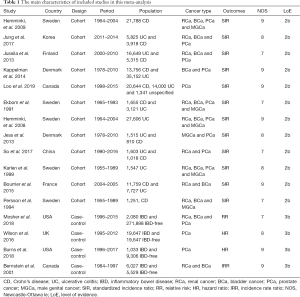
A total of 1,124 records were scrutinized through electronic database and manual search from inception to April 2020. 12 cohort studies (15-26) and 4 case-control studies (27-30) were eligible after screening on the basis of title, abstract, and full text (Figure 1). Ten studies were from Europe, four from North America and two from Asia in the present meta-analysis. A total of 241,969 participants were incorporated in the cohort studies. Specifically, ten studies with 174,094 patients reported on PCa risk, nine studies with 136,502 subjects reporting on BCa risk, nine studies with 94,133 patients reporting on RCa risk and six studies with 58,483 patients reporting on MGCa. Only four case-control articles were available for urologic cancers, including 4 for PCa, 2 for RCa and 2 for BCa. There were 28,787 IBD patients and 306,380 IBD-free subjects in the case-control studies. Table 1 presents the main characteristics of included studies in this meta-analysis.
Full table
Meta-analysis results
Standardized incidence ratio (SIR)
Nine of 12 cohort studies reported on RCa in patients with IBD, showing a significantly increased risk of RCa (pooled SIR: 1.53; 95% CI: 1.25–1.80; I2=42.4%; Figure 2). In the subgroup analysis, RCa risk was higher in patients with CD (pooled SIR: 1.95; 95% CI: 1.45–2.44; I2=39.9%; Figure 2), and a borderline significantly increased risk of RCa was observed in patients with UC (pooled SIR: 1.31; 95% CI:0.94–1.67; I2=48.0%; Figure 2) when compared to the background population. We observed that the risk of PCa in IBD patients were of borderline significant increase (pooled SIR:1.09; 95% CI: 0.95–1.22; I2=61.2%; Figure 2) when compared with the background population, as was the risk of UC subgroup (pooled SIR: 1.13; 95% CI: 0.93–1.33; I2=73.5%; Figure 2) and CD subgroup (pooled SIR: 1.07; 95% CI: 0.93–1.20; I2=15.1%; Figure 2). Besides, we found that no significantly decreased risk of BCa was detected in patients with IBD (pooled SIR:0.99; 95% CI: 0.87–1.12; I2=0%; Figure 2) and UC subgroup (pooled SIR: 0.92; 95% CI: 0.77–1.06; I2=0%; Figure 2), whereas a trend toward an increased risk of BCa was observed in patients with CD subgroup (pooled SIR:1.19; 95% CI: 0.94–1.44; I2=0%; Figure 2). Data from six cohort studies (18-20,24,27,30) showed that IBD or IBD subtype (UC and CD) did not significantly increase the risk of MGCa. The pooled SIRs were 1.30, 1.20, 1.29 for IBD, UC and CD, respectively.
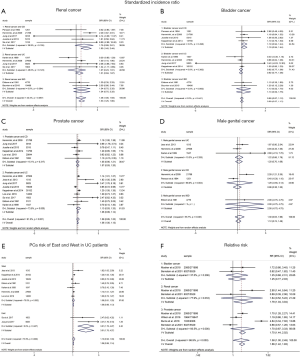
Relative risk (RR)
Only two case-control studies reported the results of RCa risk, resulting no significant difference between IBD group and IBD-free group (pooled RR: 1.64; 95% CI: 0.52–5.22; I2=77.9%; Figure 2). Similarly, the two studies provided data on BCa in UC and CD combined, revealing that IBD patients seemed to have a higher risk of BCa than IBD-free subjects (RR: 1.25; 95% CI: 0.77–2.03; I2=37.5%; Figure 2). Pooled analysis of four case-control studies (27-30) showed there was no significant difference between IBD group and IBD-free group regarding the PCa risk (RR: 1.70; 95% CI:0.87–3.33; Figure 2), while there was significant heterogeneity between studies (I2=93.5%; P=0.000). Subgroup analysis was unlikely to be conducted due to insufficient information.
Publication bias and sensitivity analysis
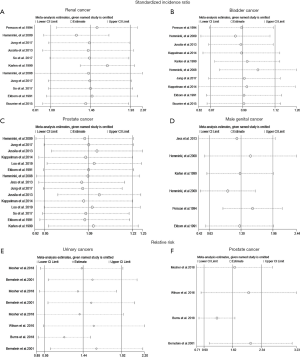
The Egger’s test and Begg’s test were used to quantify potential publication bias. In cohort studies, the p values of Egger’s test and Begg’s test were 0.865 and 0.213 for IBD patients with RCa, respectively. Besides, p values of Egger’s test for PCa, BCa and MGCa were 0.234, 0.194 and 0.276, respectively. In case-control studies, the p values of Egger’s test and Begg’s test were 0.078 and 0.108 for patients with urologic cancers, respectively. Thus, we concluded that there was no significant publication bias in this study. Furthermore, we performed a sensitivity analysis to assess the impact of a single study on the overall effect size through removing each study from the meta-analysis sequentially (Figure 3). As a result, no noticeable changes were observed.
Discussion
In the last century, IBD was mainly concentrated in western developed countries, and little was known about the number of individuals influenced by IBD outside the western world (2). At the turn of the 21st century, newer epidemiological studies found a rapid increase in IBD incidence among newly industrialized countries in Asia, including China and India (2,5). This epidemiological shift occurring with newly industrialized and urbanized countries reflecting the experience reported in the west more than 50 years ago (2). IBD including CD and UC is associated with increased risk of intestinal and extraintestinal cancers (6-11). Local and systemic inflammation were postulated to contribute to the increased risk of cancer in IBD patients (31). Given scarce population-based data about the risk of urinary tumors, we conducted this meta-analysis to illuminate the relationship between IBD and risk of urologic cancers to provide patient consultation and guide clinical practice.
The previous two meta-analysis (10,11) had controversial results regarding PCa risk and UC patients. Pedersen et al. (10) pooled analysis of 4 studies and found that UC patients were not at increased risk of PCa (SIR: 1.14; 95% CI: 0.85–1.52). Conversely, Ge et al. (11) observed IBD patients have significantly elevated PCa risk (SIR: 1.33; 95% CI: 1.03–1.71), especially for UC patients (SIR: 1.58; 95% CI: 1.08–2.30). In the present meta-analysis, pooled data from ten cohort studies indicated that IBD patients were at borderline significantly increased risk of PCa, regardless of UC or CD patients. The difference above might be attributed to geographic region. The 4 studies included in Pedersen’s article were from Western countries, while 2 of 5 studies included in Ge’s article were from Asian countries. Thus, we conducted a subgroup according to geographic region. We observed that IBD patients in Eastern countries have significantly increased risk of PCa (SIR: 2.66; 95% CI: 1.52–3.81; I2=13.6%), especially for UC patients (SIR: 3.01; 95% CI: 1.75–4.27; I2=0.0%; Figure 2). Overall, the incidence of IBD in Asian countries remains lower than that in Western countries, while the rapid increase in disease incidence will also aggravate socioeconomic burden in Asian countries (21). The difference of environmental backgrounds and genetic susceptibility in IBD patients between Asian and Western countries might explain the predisposition of PCa in Eastern patients with IBD.
Unlike the previous meta-analysis (10), our meta-analysis did not observe a significant increase of BCa risk in CD patients. We thought that the sample size and statistical methods contributed to this difference. Pedersen et al. (10) pooled data with less sample size and some data were calculated as the number of person-years of observation divided by the number of patients in the cohort, which might be inappropriate. Besides, our meta-analysis detected that IBD patients, especially CD patients, increased the risk of RCa. When we restricted the analysis to Western countries (data not shown), the pooled data were in accordance with the previous meta-analysis (100. However, due to the limited studies and defective statistical methods in the previous study (10), we were unlikely to make a further conclusion. Similar to the previous study (10), our meta-analysis did not observe an increased MGCa risk in IBD patients. However, all studies pooled for MGCa were from Western countries. Data reporting MGCa risk and IBD patients are still insufficient for Eastern countries.
The use of immunosuppressive or biologic agents might have opposite effects in risk of intestinal and extraintestinal cancers. The widespread use of immunosuppressive or biologic agents may reduce the risk of colorectal cancer by suppressing intestinal inflammation (32), but contribute to the development of extraintestinal cancers (9,33-36). In IBD patients exposed to azathioprine, Pasternak et al. (37) firstly reported that IBD patients with azathioprine were more prone to have urinary tract cancer (UTC) than no users. In the present study, only one study (16) reported the association between thiopurine therapy and risk of urinary cancers in IBD patients. They found that IBD patients receiving thiopurines have a higher risk of UTC than those not receiving thiopurines (Hazard ratio: 2.82; 95% CI: 1.04–7.68; P=0.04) (16). However, they were unable to assess the impact of IBD patients with immunosuppressive therapy prone to undergo imaging techniques of the abdomen, smoking status, and anti-tumor necrosis factor on UTC risk. Therefore, the current evidence on immunosuppressive agents and the risk of UTC is still vastly limited. Despite this, they still advocated an imaging screening strategy (ultrasound or computerized tomography) before initiation of immunosuppressive therapy in elderly patients, especially for male smokers, due to the high prevalence of UTC in this population and the potential facilitating effect of thiopurines on tumor growth (16,38). Besides, we are also unable to estimate other independent risk factors for UTC in patients with IBD as a result of insufficient data. Furthermore, although the rapidly increase in IBD incidence is accelerating the globalization of this disease, epidemiological studies reporting the relationship between IBD and UTI risk are still penurious.
Taken together, our study has preliminarily explored the relationship between IBD patients and risk of urinary cancers, providing a reference for cancer counseling and screening strategy for clinical management of these patients. With the westernization and industrialization of emerging countries, clinicians, especially in outpatient, need to raise awareness of screening patients with IBD for urinary tumors.
Conclusions
Our findings indicate that IBD patients with special reference to CD patients increase the risk of RCa. Besides, IBD patients in Asian countries have significantly increased risk of PCa, especially for UC patients. Further studies are warranted to elucidate the potential mechanism of RCa associated with IBD and the differences of the risk of urinary cancers between Eastern and Western countries.
Acknowledgments
We thank Prof. Gong Juan from the College of Foreign Languages, Sichuan University for her contribution to editing the language of this study.
Funding: This work was supported by Department of Science and Technology of Sichuan Province (2020YFH0099) and the National Natural Science Foundation of China (No. 81370272, 30901621/C1705). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1358
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1358). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet 2007;369:1627-40. [Crossref] [PubMed]
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769-78. [Crossref] [PubMed]
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017;152:313-21.e2. [Crossref] [PubMed]
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-54.e42. [Crossref] [PubMed]
- Mak WY, Zhao M, Ng SC, et al. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol 2020;35:380-9. [Crossref] [PubMed]
- Lutgens MW, van Oijen MG, van der Heijden GJ, et al. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 2013;19:789-99. [Crossref] [PubMed]
- Huang SZ, Liu ZC, Liao WX, et al. Risk of skin cancers in thiopurines-treated and thiopurines-untreated patients with inflammatory bowel disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 2019;34:507-16. [Crossref] [PubMed]
- Singh S, Nagpal SJ, Murad MH, et al. Inflammatory bowel disease is associated with an increased risk of melanoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:210-8. [Crossref] [PubMed]
- Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6- mercaptopurine. Gut 2005;54:1121-5. [Crossref] [PubMed]
- Pedersen N, Duricova D, Elkjaer M, et al. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol 2010;105:1480-7. [Crossref] [PubMed]
- Ge Y, Shi Q, Yao W, et al. The association between inflammatory bowel disease and prostate cancer risk: a meta-analysis. Prostate Cancer Prostatic Dis 2020;23:53-8. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Ottawa Hospital Research Institute. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Evidence-Based Cf. Oxford Centre for evidence-based medicine: levels of evidence, 2009. Available online: http://www.cebm.net/oxford-centreevidence-based-medicine-levels-evidence-march-2009/
- Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001;91:854-62. [Crossref] [PubMed]
- Bourrier A, Carrat F, Colombel JF, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther 2016;43:252-61. [Crossref] [PubMed]
- Burns JA, Weiner AB, Catalona WJ, et al. Inflammatory bowel disease and the risk of prostate cancer. Eur Urol 2019;75:846-52. [Crossref] [PubMed]
- Hemminki K, Li X, Sundquist J, et al. Cancer risks in ulcerative colitis patients. Int J Cancer 2008;123:1417-21. [Crossref] [PubMed]
- Hemminki K, Li X, Sundquist J, et al. Cancer risks in Crohn disease patients. Ann Oncol 2009;20:574-80. [Crossref] [PubMed]
- Jess T, Horvath-Puho E, Fallingborg J, et al. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am J Gastroenterol 2013;108:1869-76. [Crossref] [PubMed]
- Jung YS, Han M, Park S, et al. Cancer risk in the early stages of inflammatory bowel disease in Korean patients: a nationwide population-based study. J Crohns Colitis 2017;11:954-62. [Crossref] [PubMed]
- Jussila A, Virta LJ, Pukkala E, et al. Malignancies in patients with inflammatory bowel disease: a nationwide register study in Finland. Scand J Gastroenterol 2013;48:1405-13. [Crossref] [PubMed]
- Kappelman MD, Farkas DK, Long MD, et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol 2014;12:265-73.e1. [Crossref] [PubMed]
- Karlén P, Loferg R, Brostrom O, et al. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol 1999;94:1047-52. [Crossref] [PubMed]
- Loo SY, Maria V, Alain B, et al. Risk of Malignant Cancers in Inflammatory Bowel Disease. J Crohns Colitis 2019;13:1302-10. [Crossref] [PubMed]
- Mosher CA, Brown GR, Weideman RA, et al. Incidence of colorectal cancer and extracolonic cancers in veteran patients with inflammatory bowel disease. Inflamm Bowel Dis 2018;24:617-23. [Crossref] [PubMed]
- Persson PG, Karlen P, Bernell O, et al. Crohn’s disease and cancer: a population-based cohort study. Gastroenterology 1994;107:1675-9. [Crossref] [PubMed]
- So J, Tang W, Leung WK, et al. Cancer risk in 2621 Chinese patients with inflammatory bowel disease: a population-based cohort study. Inflamm Bowel Dis 2017;23:2061-8. [Crossref] [PubMed]
- Wilson JC, Furlano RI, Jick SS, et al. A population-based study examining the risk of malignancy in patients diagnosed with inflammatory bowel disease. J Gastroenterol 2016;51:1050-62. [Crossref] [PubMed]
- Ekbom A, Helmick C, Zack M, et al. Extracolonic malignancies in inflammatory bowel disease. Cancer 1991;67:2015-9. [Crossref] [PubMed]
- Mak JWY, So J, Tang W, et al. Cancer risk and chemoprevention in Chinese inflammatory bowel disease patients: a population-based cohort study. Scand J Gastroenterol 2020;55:279-86. [Crossref] [PubMed]
- Andersen NN, Jess T. Has the risk of colorectal cancer in inflammatory bowel disease decreased? World J Gastroenterol 2013;19:7561-8. [Crossref] [PubMed]
- Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617-25. [Crossref] [PubMed]
- Armstrong RG, West J, Card TR. Risk of cancer in inflammatory bowel disease treated with azathioprine: a UK population-based case-control study. Am J Gastroenterol 2010;105:1604-9. [Crossref] [PubMed]
- Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011;141:1621-1628.e1621-1625.
- Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012;143:390-399.e1. [Crossref] [PubMed]
- Pasternak B, Svanström H, Schmiegelow K, et al. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol 2013;177:1296-305. [Crossref] [PubMed]
- Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs 2007;67:1167-98. [Crossref] [PubMed]