
Progress in studies on pathological changes and future treatment strategies of obesity-associated female stress urinary incontinence: a narrative review
Introduction
The incidence and morbidity of obesity are increasing continuously across worldwide (1) Obesity is closely related to various diseases, especially chronic diseases. Previous studies have identified old age, maternal childbirth injury, and menopause as the main risk factors for female stress urinary incontinence (FSUI) (2). However, the number of FSUI patients among young and middle-aged women who have a high body mass index (BMI) and are not pregnant is increasing. As a result, the pathogenic effect of obesity in FSUI is gradually becoming clearer (3). The epidemiological trends of both obesity and FSUI are also in line with the above finding, and current studies have confirmed that obesity is an independent risk factor for FSUI.
Normal urinary function and control are maintained by urinary control tissues and organs that are structurally intact. However, obesity can cause injury to tissues such as striated muscle, ligaments, nerves, and blood vessels, and compromise the normal anatomical position and function of the urethra and bladder. As a result, the damaged tissues and organs are unable to manage increased intra-abdominal pressure brought on by general movement, resulting in urinary incontinence (4). Current guidelines recommend weight loss as a first-line treatment for obesity-associated FSUI (OA-FSUI) (5). Weight loss can eliminate the cause of OA-FSUI and should be regarded as a fundamental treatment. Alternative therapeutic strategies for OA-FSUI include functional exercise, drug therapy, and various surgical procedures, all of which are used to treat other types of FSUI (6). Although current methods appear effective in treating OA-FSUI, however, lack of evidence to restore pathological changes in OA-FSUI and various drawbacks and injuries associated with these methods may significantly impact the patient’s quality of life.
Regenerative medicine is an emerging interdisciplinary field incorporating life sciences and engineering, and focuses on repairing, replacing, or regenerating injured tissues and organs to restore functionality (7). As an important representative of regenerative medicine, studies on micro-energy therapy, such as such as low-intensity pulsed ultrasound (LIPUS), low-intensity extracorporeal shock wave therapy (Li-ESWT), and pulsed electromagnetic field (PEMF), promotes the regeneration of damaged tissue by activating ESCs, regulating the secretion of various growth factors, and inhibiting inflammation. These effects have attracted widespread attention in recent years, especially as the clinical application of micro-energy therapy in the treatment of various diseases continues to advance.
Aims of this review to explore the possible pathogenic mechanisms of OA-FSUI, discuss the effects and shortcomings of existing treatments and to provide novel ideas and potential therapy methods for OA-FSUI in the future. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1217).
Methods
We performed a literature search on PubMed. Our keyword search terms included: “obesity”, “urinary incontinence”, “stress urinary incontinence”, “obesity and urinary incontinence”, “obesity and stress urinary incontinence”, “stress urinary incontinence and pathological injury”, “stress urinary incontinence therapy”, and “stress urinary incontinence treatment”. Our search of these basic terms generated results reporting on the repair effect of Li-ESWT, LIPUS, and gene therapy, among others. We then searched the PUBMED database using the terms: “Li-ESWT and stress urinary incontinence”, “LIPUS and stress urinary incontinence”, “gene therapy and stress urinary incontinence”, and “stem cell therapy and stress urinary incontinence”. Our literature search covered English-language clinical or basic research articles published from January 1, 1966 to May 1, 2020, as well as related reviews and meta analyses.
Discussion
Obesity and FSUI
Human adipose tissue generally contains about 300–350 billion adipocytes. Obesity is the consequence of adipocytes increasing in number and size. The body mass index (BMI) is the metric most commonly used to assess an individual’s total body fat as a proportion of their body weight, and is calculated by dividing weight by height squared (kg/m2). Generally speaking, adults with BMI ≥25 are considered to be overweight, while BMI ≥30 is considered obese (1). In the past 40 years, the global prevalence of obesity has nearly tripled (1). In the United States of America (USA), the prevalence of obesity among adults is close to 40% (8), and in terms of the number of obese people, China ranks first in the world, with 46.4 million Chinese women classed as obese (9), and the incidence is increasing every year (10). Obesity can damage almost all body organs and systems and cause variety of related diseases, for example limited lung activity, dyspnea (11), hypertension and atherosclerosis (12), diabetes mellitus, coronary heart disease (13,14) and urinary incontinence (UI), through a series of complex mechanisms.
It is estimated that almost a quarter of women worldwide will suffer from UI in their lifetime (15), and the cost of UI treatment in the USA alone is between $19.5–$76 billion (16). As the most common type, accounting for approximately half of UI, SUI is characterized by involuntary urination due to increased intra-abdominal pressure, which significantly impacts the individual’s ability to exercise, as well as their mental health (4,5,17). In 2006, the largest epidemiological study of FSUI among Chinese women reported that FSUI patients accounted for 18.9% of 19,024 samples, and that concentric obesity (waistline ≥80 cm) was a significant risk factor for FSUI (18). However, many researchers believe that due to economic, cultural, and other factors in different regions of China, some women are reluctant to seek medical attention and the actual incidence rate may be higher than the findings of this study suggest. Furthermore, the incidence of FSUI is higher in the USA, with approximately 20–40% of adult women suffering from this condition (19).
Data suggest that in addition to high-intensity exercise (20-22), pregnant and older women (23), obesity can also lead to exceed intra-abdominal pressure (21), damage the structure and anatomical position of the pelvis and urethral, and even nerve injury (22,23). A number of large-scale epidemiological studies involving different ethnic groups in the USA, Europe, Japan, and Egypt have consistently shown that obesity is an independent risk factor for FSUI (19). The National Health Service (NHS) conducted a prospective cohort study of 83,355 participants and reported that obese women had a 3% increased risk of urinary leakage for every 1 kg overweight, and for every 1 kg/m2 increase in BMI, the risk of urinary leakage increased by 7% (24). A study by Mishra et al. showed that obesity (BMI ≥30) increases the risk of FSUI, regardless of age [20 years old: odds ratio (OR) 1.32, 95% confidence interval (CI): 0.66–2.66; 26 years old: OR 1.73, 95% CI: 0.93–3.22; 36 years old: OR 1.79, 95% CI: 1.16–2.74; 43 years old: OR 1.59, 95% CI: 1.15–2.20] (25). Therefore, OA-FSUI has been widely accepted as a common type of SUI.
Pathogenic mechanism of OA-FSUI
Currently, the structure and function of female micturition are contentious. The mainstream view is that normal urinary control in women (to coordinate the pressure in different parts of the lower urinary tract) is realized by the respective functions and complex interactions of related tissues. Firstly, the bladder neck and internal orifice of the urethra remain closed during the bladder-filling period, benefiting from the maintenance of their normal anatomical position and the contraction of the internal sphincter of the urethra. Moreover, the supporting structures of the pelvic floor play a key role in inhibiting overactivity of the bladder and urethra. Pelvic floor striated muscles, such as levator anus and coccyx muscles, are innervated by autonomic and motor nerves including the levator anus and pudendal nerves. Generally, autonomic nerves maintain the long-term tension of type I muscle fibers to compress pelvic organs and connective tissue to the pubic bone, fixing the urethra and bladder neck to keep a certain bending angle, thus maintaining the resistance of urine from the bladder to the urethra. When the abdominal pressure increases (for instance, during coughing and sneezing), type II muscle fibers are activated by motor nerves to participate in maintaining the anatomical position and also help to reduce the pressure on the urethra (26,27). The internal urethral sphincter, regulated by the visceral autonomic nerve (which is located at the internal orifice of the urethra), is formed by the continuation of smooth muscle fibers of the bladder neck and remains in a state of contraction during the non-voiding phase (28). The external urethral sphincter (EUS) consists of a longitudinal and transverse circular arrangement of striated muscles and is innervated by the pudendal motor nerve. It is involved in the conscious regulation of urination and maintains about one-third of its maximum urethral closure pressure (MUCP). Studies have discovered that almost all women with SUI have varying degrees of EUS damage (27,29). Lastly, urethral mucosa-related studies reported that there is an abundant vascular plexus in the submucosal tissue of the urethra that far exceeds the need for nutritional support of the urethra (30,31). Consequently, some researchers speculate that the cavernous vascular sinus tissues in the urethral mucosa and submucosa are helpful in the closure of the urethral cavity, and contribute approximately one-third of the MUCP (32).
Numerous pathogenic consequences of obesity can lead to unconscious leakage of urine in women. For instance, obesity is widely understood to lead to a significant increase in abdominal pressure. Taking the maximum bladder capacity as the standard, every 1 kg/m2 increase in BMI or a 2 cm increase in abdominal circumference in women will lead to a 0.4 cmH2O increase in abdominal pressure (33). This elevated pressure directly translates to bladder pressure (3). However, the obesity-induced impairment of the tissues and organs involved in urinary control may be the more damaging consequence. Clinical and anatomical studies have found that long-term increases in abdominal pressure can lead to atrophy and deformation of pelvic floor muscles and weak connective tissue (3,34), leading to excessive movement of the urethra and bladder neck. Additionally, lipid droplet deposition and several other causes can also lead to muscle dysfunction and other injuries.
In a recent animal experiment, the urethral striated muscles of female Zucker fatty (ZF) rats and Zucker lean (ZL) rats were separated and stained. More lipids were found to be deposited in the striated muscle fibers of the ZF rats, which caused thinning and atrophy, resulting in the reduction or disappearance of the contractile properties of the urethral striated muscle (35). At the same time, the maximum bladder capacity and urine leak point pressure (LPP) of the ZF rats decreased (35). Researchers found that the increased lipid deposition in muscle cells of ZF rats limited the contractile characteristics of the urethral striated muscle (36).
Additionally, adipose tissue can secrete various inflammatory factors, including interleukin (IL)-1, IL-6, and IL-18, tumor necrosis factor-α (TNF-α), and reactive oxygen species (ROS) (37). Increased inflammatory factors and ROS may impair the pelvic floor nerve, visceral autonomic nerve, and the motor nerve (38,39). They can also damage bladder and urethral epithelial cells, reduce bladder compliance (38,39), and obstruct the blood flow of submucosal vessels (40). Finally, obesity can also decrease the number of ESCs from different sources in the urethra and pelvic floor, which prevents stem cells from repairing damaged tissue and promotes OA-FSUI progression (36). In fact, ESCs are also a potential target for SUI therapy.
Current treatment of OA-FSUI
Weight loss
Reducing body fat content is the most fundamental approach to treating OA-FSUI. Current studies suggest that weight loss provides long-term benefits for OA-FSUI and improves quality of life (41). However, symptomatic relief through weight loss is limited in the short term. Effective methods for losing weight include reducing calorie intake, proper exercise, weight loss medications, and surgery. Of all the approaches to weight loss, the role of reasonable diet and exercise in improving obesity-related diseases has been widely established and recognized. Compared with lifestyle adjustment, drug and surgical interventions could lead to more rapid weight loss (41,42).
At present, liraglutide and metformin are reported as effective weight-loss drugs (43,44); however, the potential risk of severe hypoglycemia has limited their use as weight-loss agents. Surgical treatments include Roux-en-Y gastric bypass surgery (RYGB), sleeve gastrectomy, duodenotomy and cholangiopancreatic shunt, gastric banding implantation, and intermittent vagus nerve block (44). However, the safety of surgical procedures should be carefully evaluated, because obese patients often have numerous risk factors for adverse surgical outcomes. These include poor heart function, hypertension, and diabetes, which may aggravate perioperative infection, present difficulties in wound recovery, and an overall increased risk during the operation and anesthesia (44). Following surgery, emphasis should be placed on reducing the intake of high-calorie foods and proper exercise; otherwise, the effect of the surgery will be difficult to maintain.
Conservative treatments
At present, conservative treatments, including pelvic floor muscle exercise, pelvic floor electrical stimulation, acupuncture, laser therapy, and drug therapy, are mainly utilized in patients with mild and moderate FSUI. Studies have shown that restricting caffeine and alcohol can reduce urgent urination symptoms, but cannot slow the progression of urinary incontinence symptoms (45). Pelvic floor muscle exercises can enhance the tension and contractility of pelvic floor muscle tissue, and are considered to have an A-grade recommendation for the treatment of SUI in females (23). Basically, these exercises involve repeated contraction of the levator anus muscle for at least 3 seconds, and then relaxing it, 150–200 times a day. Typically, this is continued as a course of treatment for 6–8 weeks (46). However, the effect is difficult to maintain over the long term.
Overall, electrical stimulation is not reliably effective in the treatment of FSUI (47), and it commonly causes pain (48). Acupuncture in traditional medicine has also achieved some results in the treatment of urinary incontinence (49). In traditional medicine, acupuncture is believed to improve neuromuscular excitability and muscle contractility. Furthermore, vaginal laser procedure plays a role in promoting urethral mucosal hyperplasia (50), although its safety has not been widely confirmed. Finally, the Food and Drug Administration (FDA) listed drug therapy as a second-line treatment, particularly muscarinic receptor blockers. However, the long-term effects are insufficient and may lead to a series of adverse events, such as arrhythmia and increased blood pressure (51).
Surgical treatments
Surgical correction of OA-FSUI is mostly employed in patients with moderate to severe urinary incontinence, where non-operative treatment has failed. The primary purpose of surgical intervention is to restore the normal anatomical position of the organs, so as to support and close the urethra, particularly in periods of elevated abdominal pressure. The common surgical treatments include: middle urethral sling, injection of fillings around the bladder neck, and artificial sphincter implantation (52). Firstly, by fixing the middle urethra to the ligament or pubic bone, the urethral suspension sling can support the urethral-bladder junction. The overall success rate of this procedure in FSUI caused by urethral overactivity is 80%, while the efficacy for other types of FSUI is poor (53). Also, injecting synthetic or autologous implants into the mucosa around the bladder neck (under the guidance of a cystoscope) can enhance mucosal tightness of the urethral sphincter and improve the symptoms of FSUI; however, the implant material could be absorbed and may need to be injected repeatedly (23). In addition to the aforementioned procedures, other surgical methods are used in the treatment of FSUI (52).
However, it is important to recognize that surgical treatment of OA-FSUI is the most invasive option, and despite surgical treatment being generally effective initially, the long-term therapeutic effect depends on the surgical process and may diminish over time. Recent studies have found that obese patients have a higher incidence of surgical complications, such as persistent pain, bladder perforation, and pelvic and urethral infection (33), and the treatment effectiveness for obese patients is worse than that of FSUI patients of normal weight (54).
Although the above treatments can relieve some of the symptoms of OA-FSUI, they each have their own side effects and a limited effectiveness in improving the pathological injury of FSUI.
OA-FSUI potential regeneration therapy
In theory, regeneration therapy can fundamentally restore the urine control function of patients by treating the underlying pathological injury of OA-FSUI. Related technologies and methods include, but are not limited to, gene therapy, stem cell transplantation, and micro-energy therapy (55,56).
Early exploration of regenerative therapy
With the development of gene technology and stem cell transplantation technology, the effect of regenerative therapy in repairing pathological defects and improving organ function at the molecular and tissue level have been recognized in basic experiments.
Insulin-like growth factor (IGF)-β1 gene transfection therapy can promote the recovery of injured muscles and nerves (57), the proliferation of fibroblasts and endothelial cells, and the expression of extracellular matrix (58). However, the safety of gene therapy is highly controversial, especially considering the off-target effect of the vector and the gene-editing tool itself, as well as the uncertainty of the long-term impact of gene changes on patients.
Stem cell transplantation is a technique in which exogenous stem cells cultured in vitro are injected into the body. These cells then participate in histopathological repair through stem-cell homing and multi-directional differentiation. Recent SUI studies have shown that transplantation of autologous and allogeneic adipose stem cells, amniotic fluid stem cells, and bone marrow mesenchymal stem cells could significantly improve bladder capacity (BC) and LPP, as well as repair injured striated muscle and peripheral nerve fibers in the urethra (59-62). Moreover, a clinical study that involved injecting autologous adipose stem cells into the damaged pelvic floor tissue of 10 women with SUI showed that their symptoms improved in the short term (63). Theoretically, stem cell transplantation can treat FSUI, or at least relieve related symptoms; however, the safety of this therapy remains a concern. Transplanted stem cells, even autologous cells, still carry the risk of immunity and tumorigenesis that may result in serious adverse effects (64). Moreover, many reports may have been subject to commercial and human interests, and so the safety conclusions of the treatment are biased (65). Therefore, its clinical transformation should be cautious.
Promising method of regenerative therapy
Since the risks of the aforementioned methods are difficult to overcome, researchers have been exploring alternative options. In recent years, ESCs, which exist in mammalian organs and participate in tissue replenishment and repair, have attracted a great deal of attention. These cells have significant advantages, including that they do not involve immune rejection and do not raise ethical or other serious issues. Treatment with micro-energy plays a key therapeutic role through activating ESCs, such as skeletal muscle satellite cells, endothelial stem cells, and Schwann cells, to restore injured tissue. This method also avoids the complications involved with stem cell acquisition, preparation, and transplantation.
In 2019, Yang et al. (66) established a FSUI rat model with different parameters of LIPUS. The results showed that LIPUS with appropriate parameters promoted the proliferation and myogenic differentiation of skeletal muscle satellite cells in FSUI rats. This regeneration significantly increased the thickness and integrity of urethral striated muscles. Moreover, the BC and LPP of the rat model were restored to the basic normal level. Furthermore, Kang et al. (67) treated the OA-FSUI rat model with micro-energy acoustic pulses (MAPs). Firstly, 5-ethynyl-2’-deoxyuridine (EdU) labeling was carried out on 10 newborn ZF and 10 newborn ZL female rats to track possible ESCs. The 20 rats were subsequently divided into 4 groups (the ZL control, ZL MAP, ZF control, and ZF MAP groups). At 8 weeks of age, MAPs (0.033 mJ/mm2, 3 Hz for 500 pulses, twice a week for 2 weeks) were applied to the treatment area, including to the urethral and pelvic floor muscles. Following the final MPA treatment, urethral and pelvic floor muscle cells were isolated and incubated with antibodies conjugated with paired box 7 (Pax-7), Integrin α-7, and phosphor-histone H3 (H3P), and then analyzed by flow cytometry. The expression and co-expression level of EdU, integrin α-7, and Pax-7, and the percentage of differentiation of EdU+ cells into skeletal muscle satellite cells were calculated. The results showed that EdU+ and skeletal muscle satellite cells were reduced in the pelvic floor muscles and urethras of the ZF rats. MAP treatment can reverse the above condition, and increase the total number of EdU+, integrin α-7, and Pax-7+ cells significantly.
Importantly, the co-expression of EdU+/integrin α-7+, and EdU+/Pax-7+ increased in both the ZL and ZF rats, which means that MAP promoted the differentiation of EdU+ into skeletal muscle satellite cells. Combined with the previous experimental results (36), researchers proposed that obesity could damage the urethra and pelvic floor striated muscle (through muscle fiber lipid deposition), adversely affect tissue resident ESCs, and damage tissue regeneration. MAP can restore the striated muscle thickness and contractile function of ZF rats by increasing the number of skeletal muscle satellite cells and promoting their differentiation, ultimately restoring urine control.
Meanwhile, another study (68) treated animal models of erectile dysfunction caused by bilateral cavernous and pudendal neurovascular injury using Li-ESWT. The results showed that Li-ESWT could recruit ESCs to the treatment area and activate them to restore neurovascular injury. In vitro studies also found that Li-ESWT and PEMF could repair injured nerve fibers and axons by promoting the proliferation and differentiation of Schwann cells as well as the secretion of neurotrophic factors (such as S100, brain-derived neurotrophic factor (BDNF), and IGF-1) (69,70). Furthermore, compared with the control, the application of Li-ESWT caused vascular endothelial stem cells to form a solid tubular network in vitro, and the tube length and branching point increased by 42% and 43%, respectively (71). The repair effect of micro-energy on nerves and blood vessels further demonstrates its effect on reversing the pathogenic changes of OA-FSUI. Finally, LIPUS can also treat chronic inflammation and urinary system pain by inhibiting the secretion of proinflammatory factors and ROS synthesis (72).
The above results show that micro-energy therapy may play a key role in restoring a variety of pathogenic injures. Although there are few clinical studies of OA-FSUI treatment with micro-energy, the safety of this therapy has been sufficiently established from current clinical research into conditions such as fracture nonunion and male erectile dysfunction (73,74). Thus, micro-energy presents a promising potential therapeutic option.
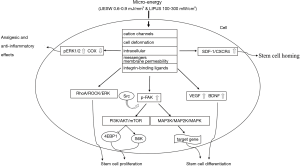
The mechanism of micro-energy regulating the biological behavior of stem cells has not been fully illuminated. Previous studies have suggested that one of the mechanisms may be that non-invasive mechanical stimulation triggers cell deformation, and the related signals are transmitted to the nucleus through the cytoskeleton (Actin) to regulate the expression of related genes, such as BDNF, S100, endothelial nitric oxide synthase (eNOS), and vascular endothelial growth factor (VEGF). Another possible mechanism is that mechanical force signals sent by LIPUS or LESW to local tissue cells can change the components of the extracellular matrix and induce related regulatory proteins in cells, such as talin and kindlin proteins, to bind to the intracellular regions of cytoskeleton proteins and integrins. This would change the conformation of integrins from a folded to an extended state, and then increase ligand affinity and integrin-ligand binding. Integrin-binding ligands (such as fibrin, laminin, and vitrein) can further induce the conformational change and aggregation of integrin on the cell membrane surface, thus activating multiple protein kinases and related downstream signal pathways. Moreover, it may also activate stem cells by regulating cation channels, cell membrane permeability, and even intracellular messengers through electric field forces (75). The downstream signal pathway is more complex. For example, extracellular signal-related kinases 1 and 2 (ERK1/2) are related to the promotion of Schwann cell proliferation by micro-energy (71), and others have also been gradually discovered by recent studies (Figure 1).
Conclusions
Obesity can damage urine control structures and cause SUI in females. Current treatments of OA-FSUI are not ideal for relieving symptoms and improving quality of life, and also have no ability to reverse or repair the underlying pathological changes. However, micro-energy therapy, such as such as LIPUS, Li-ESWT, and PEMF, showed the potential to activate ESCs and other complex mechanisms to restore damaged tissues, making it a novel treatment option for OA-FSUI patients in future, which might be considered to be a promising method for the treatment of OA-FSUI in clinical.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grants 81671450).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1217
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1217). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO—10 Facts on Obesity. Available online: (accessed on 13 October 2018).http://www.who.int/features/factfiles/obesity/en/
- Lamerton TJ, Torquati L, Brown WJ. Overweight and obesity as major, modifiable risk factors for urinary incontinence in young to mid-aged women: a systematic review and meta-analysis. Obes Rev 2018;19:1735-45. [Crossref] [PubMed]
- Fuselier A, Hanberry J, Margaret Lovin J. Obesity and Stress Urinary Incontinence: Impact on Pathophysiology and Treatment. Curr Urol Rep 2018;19:10. [Crossref] [PubMed]
- Osman NI, Li Marzi V, Cornu JN. Evaluation and Classification of Stress Urinary Incontinence: Current Concepts and Future Directions. Eur Urol Focus 2016;2:238-44. [Crossref] [PubMed]
- National Institute for Health and Clinical Excellence. Urinary incontinence in women: management. London: NICE, 2015: 21-28.
- Padmanabhan P. Urinary incontinence in women: a comprehensive review of the pathophysiology, diagnosis and treatment. Minerva Ginecol 2014;66:469-78. [PubMed]
- Mao AS, Mooney DJ. Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci U S A 2015;112:14452-9. [Crossref] [PubMed]
- Hales CM, Carroll MD, Fryar CD. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief 2017.1-8. [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017;390:2627-42. [Crossref] [PubMed]
- Lu J, Bi Y. Curbing the obesity epidemic in China. Lancet Diabetes Endocrinol 2016;4:470-1. [Crossref] [PubMed]
- Masa JF, Pépin JL, Borel JC, et al. Obesity hypoventilation syndrome. Eur Respir Rev 2019;28:180097. [Crossref] [PubMed]
- Seravalle G. Obesity and hypertension. Pharmacol Res 2017;122:1-7. [Crossref] [PubMed]
- Wannamethee SG. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc 2015;74:405-412. [Crossref] [PubMed]
- Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care 2016;22:s176-85. [PubMed]
- Committee on Practice Bulletins—Gynecology and American Urogynecologic Society. ACOG Practice bulletin no. 155: urinary incontinence in women. Obstet Gynecol 2015;126:e66-81. [Crossref] [PubMed]
- Coyne KS, Wein A, Nicholson S, et al. Economic burden of urgency urinary incontinence in the United States: a systematic review. J Manag Care Pharm 2014;20:130-40. [Crossref] [PubMed]
- Mota RL. Female urinary incontinence and sexuality. Int Braz J Urol 2017;43:20-8. [Crossref] [PubMed]
- Zhu L, Lang J, Liu C, et al. The epidemiological study of women with urinary incontinence and risk factors for stress urinary incontinence in China. Menopause 2009;16:831-6. [Crossref] [PubMed]
- Gordon B, Shorter B, Isoldi KK, et al. Obesity with Comorbid Stress Urinary Incontinence in Women: A Narrative Review to Inform Dietetics Practice. J Acad Nutr Diet 2017;117:889-907. [Crossref] [PubMed]
- Dias N, Peng Y, Khavari R, et al. Pelvic floor dynamics during high-impact athletic activities: A computational modeling study. Clin Biomech (Bristol, Avon) 2017;41:20-7. [Crossref] [PubMed]
- Jiang YH, Wang CC, Chuang FC, et al. Positioning of a suburethral sling at the bladder neck is associated with a higher recurrence rate of stress urinary incontinence. J Ultrasound Med 2013;32:239-45. [Crossref] [PubMed]
- Fozzatti C, Riccetto C, Herrmann V, et al. Prevalence study of stress urinary incontinence in women who perform high-impact exercises. Int Urogynecol J 2012;23:1687-91. [Crossref] [PubMed]
- Lasak AM, Jean-Michel M, Le PU, et al. The Role of Pelvic Floor Muscle Training in the Conservative and Surgical Management of Female Stress Urinary Incontinence: Does the Strength of the Pelvic Floor Muscles Matter? PM R 2018;10:1198-210. [Crossref] [PubMed]
- Townsend MK, Danforth KN, Rosner B, et al. Body mass index, weight gain, and incident urinary incontinence in middle-aged women. Obstet Gynecol 2007;110:346-53. [Crossref] [PubMed]
- Mishra GD, Hardy R, Cardozo L, et al. Body weight through adult life and risk of urinary incontinence in middle-aged women: Results from a British prospective cohort. Int J Obes (Lond) 2008;32:1415-22. [Crossref] [PubMed]
- Abelson B, Sun D, Que L, et al. Sex differences in lower urinary tract biology and physiology. Biol Sex Differ 2018;9:45. [Crossref] [PubMed]
- Mirto-Aguilar N, Palacios JL, Munoz A, et al. Urethral regions with differential tissular composition may underlie urinary continence and voiding function in female rats. Neurourol Urodyn 2019;38:893-901. [Crossref] [PubMed]
- de Groat WC. Anatomy and physiology of the lower urinary tract. Handb Clin Neurol 2015;130:61-108. [Crossref] [PubMed]
- Wei JT. Functional anatomy of the pelvic floor and lower urinary tract. Clin Obstet Gynecol 2004;47:3-17. [Crossref] [PubMed]
- Wallner C, Dabhoiwala NF, DeRuiter MC, et al. The anatomical components of urinary continence. Eur Urol 2009;55:932-43. [Crossref] [PubMed]
- Yucel S, Baskin LS. An anatomical description of the male and female urethral sphincter complex. J Urol 2004;171:1890-7. [Crossref] [PubMed]
- Yang JM, Yang SH, Huang WC. Functional correlates of Doppler flow study of the female urethral pressure in women. Ultrasound Obstet Gynecol 2006;28:96-102. [Crossref] [PubMed]
- Richter HE, Creasman JM, Myers DL, et al. Urodynamic characterization of obese women with urinary incontinence undergoing a weight loss program: the Program to Reduce Incontinence by Diet and Exercise (PRIDE) trial. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:1653-8. [Crossref] [PubMed]
- Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol 2009;182:S2-7. [Crossref] [PubMed]
- Wang L, Lin G, Lee Y C, et al. Transgenic Animal Model for Studying the Mechanism of Obesity-Associated Stress Urinary Incontinence. BJU International 2017;119:317. [Crossref] [PubMed]
- Wang B, Ruan Y, Zhou T, et al. The effects of microenergy acoustic pulses on an animal model of obesity‐associated stress urinary incontinence. Part 1: Functional and histologic studies. Neurourol Urodyn 2019;38:2130-9. [Crossref] [PubMed]
- Stolarczyk E. Adipose tissue inflammation in obesity: a metabolic or immune response? Curr Opin Pharmacol 2017;37:35-40. [Crossref] [PubMed]
- Grover S, Srivastava A, Lee R, et al. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 2011;3:19-33. [Crossref] [PubMed]
- Andersson KE. Oxidative stress and its possible relation to lower urinary tract functional pathology. BJU Int 2018;121:527-33. [Crossref] [PubMed]
- Marcelissen T, Anding R, Averbeck M, et al. Exploring the relation between obesity and urinary incontinence: Pathophysiology, clinical implications, and the effect of weight reduction, ICI-RS 2018. Neurourol Urodyn 2019;38 Suppl 5:S18-24. [Crossref] [PubMed]
- Wing RR, West DS, Grady D, et al. Effect of weight loss on urinary incontinence in overweight and obese women: results at 12 and 18 months. J Urol 2010;184:1005-10. [Crossref] [PubMed]
- Paul L, van der Heiden C. Cognitive behavioral therapy and predictors of weight loss in bariatric surgery patients. Curr Opin Psychiatry 2017;30:474-9. [Crossref] [PubMed]
- O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018;392:637-49. [Crossref] [PubMed]
- Wolfe BM, Kvach E, Eckel RH. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ Res 2016;118:1844-55. [Crossref] [PubMed]
- Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol 2005;174:187-9. [Crossref] [PubMed]
- Paiva LL, Ferla L, Darski C, et al. Pelvic floor muscle training in groups versus individual or home treatment of women with urinary incontinence: systematic review and meta-analysis. Int Urogynecol J 2017;28:351-9. [Crossref] [PubMed]
- Stewart F, Berghmans B, Bø K. Electrical stimulation with non-implanted devices for stress urinary incontinence in women. Cochrane Database Syst Rev 2017;12:CD012390. [Crossref] [PubMed]
- Filocamo MT, Li MV, Del PG, et al. Effectiveness of early pelvic floor rehabilitation treatment for post-prostatectomy incontinence. Eur Urol 2005;48:734-8. [Crossref] [PubMed]
- Wang M. Acupuncture for Stress Urinary Incontinence. JAMA 2017;318:1500. [Crossref] [PubMed]
- Conté C, Jauffret T, Vieillefosse S, et al. Laser procedure for female urinary stress incontinence: A review of the literature. Prog Urol 2017;27:1076-83. [Crossref] [PubMed]
- Lukacz ES, Santiago-Lastra Y, Albo ME, et al. Urinary Incontinence in Women: A Review. JAMA 2017;318:1592-604. [Crossref] [PubMed]
- Garely AD, Noor N. Diagnosis and surgical treatment of stress urinary incontinence. Obstet Gynecol 2014;124:1011-27. [Crossref] [PubMed]
- Lim YN, Dwyer PL. Effectiveness of midurethral slings in intrinsic sphincteric-related stress urinary incontinence. Curr Opin Obstet Gynecol 2009;21:428-33. [Crossref] [PubMed]
- Elshatanoufy S, Matthews A, Yousif M, et al. Effect of Morbid Obesity on Midurethral Sling Efficacy for the Management of Stress Urinary Incontinence. Female Pelvic Med Reconstr Surg 2019;25:448-452. [Crossref] [PubMed]
- Jacques E, Suuronen EJ. The Progression of Regenerative Medicine and its Impact on Therapy Translation. Clin Transl Sci 2020;13:440-50. [Crossref] [PubMed]
- Trohatou O, Roubelakis MG. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell Reprogram 2017;19:217-24. [Crossref] [PubMed]
- Bellini MJ, Hereñú CB, Goya RG. Insulin-like growth factor-I gene delivery to astrocytes reduces their inflammatory response to lipopolysaccharide. J Neuroinflammation 2011;8:21. [Crossref] [PubMed]
- Grol MW. Gene therapy for repair and regeneration of bone and cartilage. Curr Opin Pharmacol 2018;40:59-66. [Crossref] [PubMed]
- Li M, Li G, Lei H, et al. Therapeutic potential of adipose-derived stem cell-based microtissues in a rat model of stress urinary incontinence. Urology 2016;97:277.e1-7. [Crossref] [PubMed]
- Ni J, Li H, Zhou Y, et al. Therapeutic potential of human adipose-derived stem cell exosomes in stress urinary incontinence-an in vitro and in vivo study. Cell Physiol Biochem 2018;48:1710-22. [Crossref] [PubMed]
- Chun SY, Cho DH, Chae SY, et al. Human amniotic fluid stem cell-derived muscle progenitor cell therapy for stress urinary incontinence. J Korean Med Sci 2012;27:1300-7. [Crossref] [PubMed]
- Jin M, Chen Y, Zhou Y, et al. Transplantation of bone marrow-derived mesenchymal stem cells expressing elastin alleviates pelvic floor dysfunction. Stem Cell Res Ther 2016;7:51. [Crossref] [PubMed]
- Arjmand B, Safavi M, Heidari R, et al. Concomitant Transurethral and Transvaginal-Periurethral Injection of Autologous Adipose Derived Stem Cells for Treatment of Female Stress Urinary Incontinence: A Phase One Clinical Trial. Acta Med Iran 2017;55:368-74. [PubMed]
- Marks PW, Witten CM. Clarifying Stem-Cell Therapy's Benefits and Risks. N Engl J Med 2017;376:1007-9. [Crossref] [PubMed]
- Stenudd M, Sabelström H, Frisén J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol 2015;72:235-7. [Crossref] [PubMed]
- Yang B, Li M, Lei H. Low Intensity Pulsed Ultrasound Influences the Myogenic Differentiation of Muscle Satellite Cells in a Stress Urinary Incontinence Rat Model. Urology 2019;123:297.e1-8. [Crossref] [PubMed]
- Kang N, Peng D, Wang B, et al. The effects of microenergy acoustic pulses on animal model of obesity-associated stress urinary incontinence. Part2: In situ activation of pelvic floor and urethral striated muscle progenitor cells. Neurourol Urodyn 2019;38:2140-50. [Crossref] [PubMed]
- Li H, Matheu MP, Sun F, et al. Low-energy Shock Wave Therapy Ameliorates Erectile Dysfunction in a Pelvic Neurovascular Injuries Rat Model. J Sex Med 2016;13:22-32. [Crossref] [PubMed]
- Wu AK, Zhang X, Wang J, et al. Treatment of stress urinary incontinence with low-intensity extracorporeal shock wave therapy in a vaginal balloon dilation induced rat model. Transl Androl Urol 2018;7:S7-16. [Crossref] [PubMed]
- Hei WH, Byun SH, Kim JS, et al. Effects of electromagnetic field (PEMF) exposure at different frequency and duration on the peripheral nerve regeneration: in vitro and in vivo study. Int J Neurosci 2016;126:739-48. [PubMed]
- Lin G, Reed-Maldonado AB, Wang B, et al. In Situ Activation of Penile Progenitor Cells With Low-Intensity Extracorporeal Shockwave Therapy. J Sex Med 2017;14:493-501. [Crossref] [PubMed]
- Xin Z, Lin G, Lei H, et al. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl Androl Urol 2016;5:255-66. [Crossref] [PubMed]
- Bayat M, Virdi A, Jalalifirouzkouhi R. Comparison of effects of LLLT and LIPUS on fracture healing in animal models and patients: A systematic review. Prog Biophys Mol Biol 2018;132:3-22. [Crossref] [PubMed]
- Cui W, Li H, Guan R, et al. Efficacy and safety of novel low-intensity pulsed ultrasound (LIPUS) in treating mild to moderate erectile dysfunction: a multicenter, randomized, double-blind, sham-controlled clinical study. Transl Androl Urol 2019;8:307-19. [Crossref] [PubMed]
- Chen Y, Cai Q, Pan J, et al. Role and mechanism of micro-energy treatment in regenerative medicine. Transl Androl Urol 2020;9:690-701. [Crossref] [PubMed]


