
Application of full lateral decubitus position with cephalic parallel approach in robotic-assisted minimally invasive esophagectomy
Introduction
After it was introduced at the University Medical Center Utrecht in The Netherlands in 2003 (1), robot-assisted minimally invasive esophagectomy (RAMIE) has been used for esophageal surgery due to its increased magnification, dexterity, and 3D visual clarity (2). Although several studies believed that RAMIE is equivalent or even superior to video-assisted thoracoscopic minimally invasive esophagectomy (VAMIE) in facilitating thoracoscopic procedures such as lymphadenectomy (3-5), it still needs to overcome several shortcomings.
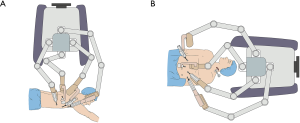
Several studies reported about RAMIE mobilization of the esophagus and mediastinal lymphadenectomy in the prone position, with the patient cart of the robot system (da Vinci S or Si System, Intuitive Surgical Inc., Sunnyvale, California, USA) standing behind the patient’s back and extending its arms in a direction crossing the longitudinal axis of the patient (back-crossing approach) (Figure 1A) (3). Thus, the patient cart must be repositioned in front of the patient’s head in the subsequent abdominal phase because the robotic arms should extend in a direction parallel to the longitudinal axis of the patient (Figure 1B). Robot repositioning is time and labor consuming. Also, the vertebral column often blocks the view of the operation field in prone position. To overcome these shortcomings, we designed a full lateral position with cephalic-parallel approach for RAMIE mobilization of the esophagus and mediastinal lymphadenectomy to allow the completion of thoracic phase. In this approach, the robot stands in front of the patient’s head and extends its arms in a direction parallel to the longitudinal axis of the patient. In this paper, we describe the characteristics of this modified approach for RAMIE and demonstrate its convenience on the basis of our practical experience.
Methods
Patients
We performed 80 cases of RAMIE since April 2016. The first 70 cases were completed by back-crossing approach with prone position, whereas the last 10 consecutive cases were conducted using full lateral decubitus position with cephalic-parallel approach. All 10 patients preoperatively underwent endoscopy, chest computerized tomography (CT), abdominal CT, cervical ultrasonography, and pulmonary function and blood testing routinely. They were also evaluated for resectable thoracic esophageal cancer preoperatively (cT1-3N0-2). All patients were staged according to the eighth edition of TNM staging for esophageal cancer (6). Our study was approved by the Ethics Committee of West China Hospital, Sichuan University (approval number: 2017239)
Surgical technique of cephalic-parallel approach
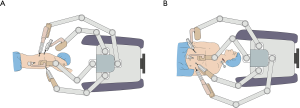
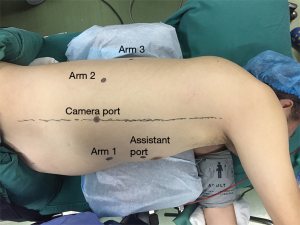
After intubation with a left-side double-lumen tube, the patient was placed in standard left lateral decubitus position. The patient cart of the robotic system (da Vinci Si System, Intuitive Surgical Inc., Sunnyvale, California) was positioned on the cephalic side of the patient, and its arms extended parallel to the longitudinal axis of the patient’s body (Figure 2A). The patient cart was still positioned at the cephalic side of the patient (Figure 2B). Five ports were used during thoracic phase (Figure 3). A camera port (12 mm) was placed in the eighth intercostal space at the mid-axillary line. Three robotic ports (8 mm) were placed in the seventh intercostal space near the costal arch (arm 1), in the ninth intercostal space at the posterior axillary line (arm 2), and in the eighth intercostal space at the subscapular line approximating the tip of the scapula (arm 3). An assistant port was placed at the fifth intercostal space between the anterior axillary line and the mid-clavicular line with a 12 mm trocar. Artificial pneumothorax by 8 mmHg CO2 insufflation was established through the nonrobotic assistant port to facilitate mediastinal dissection and keep the lung out of the operative field. Afterward, the patient cart arms were docked to the ports, and the robotic camera was introduced with a 30° down-facing orientation.
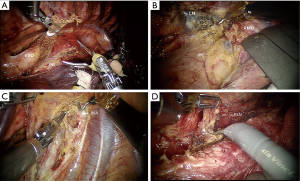
Mediastinal dissection was started far from the tumor. Different surgical procedures were performed according to the location and T stage of esophageal cancer. For either upper or lower esophageal cancer, regardless of the T stage, we dissected the middle segment of the esophagus circumferentially first and then started dissecting at the level of the diaphragm and progressed in a cephalad direction. The dissection for middle-segment esophageal cancer with T1 or T2 disease started from the lower segment of the esophagus and progressed caudally to cranially. Dissection for the T3 mid-esophageal cancer was alternatively started from the supra-azygos area or from the lower segment of the esophagus to avoid dissection of the most difficult part at the beginning. The subsequent dissection was directed to go downwards or upwards accordingly. During this procedure, a band tape was used to retract the esophagus to facilitate dissection (Figure 4A). The subcarinal lymph node (Figure 4B) and lymph nodes along bilateral recurrent laryngeal nerves (RLNs) (Figure 4C,D) were carefully dissected. The whole thoracic part of the esophagus and paraesophageal lymph nodes were mobilized subsequently. Selective en masse ligation of the thoracic duct was performed as previously described (7) to prevent postoperative chylothorax. To dissect the abdominal phase, the patient was placed in a supine position. The lesser omentum was opened and transected using a harmonic scalpel until the left crus of the diaphragm was reached. The greater gastric curvature was also dissected, and the left gastric artery was ligated with Hem-o-lok and transected. An abdominal lymphadenectomy was also conducted on the lymph nodes surrounding the celiac trunk, along the left gastric and splenic artery, and on the lesser omental lymph nodes. The subxiphoid trocar port was subsequently widened to a 3 cm incision. A 2 cm-wide gastric conduit was created by linear stapler extracorporeally. A jejunostomy feeding tube was also placed for postoperative feeding. A left-side incision was made along the sternocleidomastoid muscle to create a cervical hand-sewn end-to-side anastomosis between the gastric conduit and the cervical esophagus.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data for analysis
Pathological outcomes, including pathology type, TNM stage, and lymph node yields, were collected and analyzed. Lymph node yields included the total number of dissected lymph nodes and positive lymph nodes. Perioperative data concerning operation time, blood loss, major complications (including severe pneumonia, anastomotic leakage, RLN paralysis, and postoperative chylothorax), and 30-day mortality were also reviewed and analyzed. All major complications were evaluated according to the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons joint definitions (8). Severe pneumonia was defined as grade 3 (tracheostomy or intubation with mechanical ventilation) and higher by using the Clavien-Dindo classification (9).
Results
R0 resection was performed on all 10 cases, and none of them experienced conversion. The cases were all squamous cell carcinoma, and six (60%) cases were at stage T3. The mean durations of the thoracic phase, abdominal phase, and entire RAMIE procedure were 120.0±25.1, 149.3±29.8, and 381.0±57.5 min, respectively. The mean volume of intraoperative blood loss was 102.5±12.8 mL. No postoperative mortality and severe complications, such as anastomotic leak and pneumonia, were observed. Using the selective en masse ligation technique, we detected and treated intraoperative thoracic duct injury in two cases, and no postoperative chylothorax was detected. Two patients suffered from postoperative hoarseness, which may be due to postoperative RLN paralysis, and recovered four months after the operation. The mean total number of lymph nodes (mediastinum and abdomen) dissected was 22.4±4.0, and the mean number of positive lymph nodes dissected was 2.0±2.7. The perioperative data are shown in Table 1.
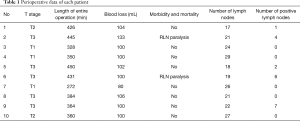
Full table
Discussion
Recently, RAMIE approaches have been increasingly described, with early studies reporting varying techniques and outcomes (3,10-12). This technique remains a complicated procedure in thoracic surgery.
Almost all of the reported average operation time for RAMIE exceeded more than 430 min (4,5), which is longer than that in our report. According to our previous data, the average operative time of cases using the former approach is 414.9±71.9 min. which is also longer than that of the optimized approach. We consider robotic surgery more time consuming than open esophagectomy and VAMIE due to the following reasons: RAMIE needs two dockings in each operation, and the robotic carts should be repositioned when the thoracic phase is over and when the abdominal phase begins. With this technique, the patient cart of the robotic system is constantly located at the patient’s cephalic side, whether performing thoracic or abdominal phase. Neither the patient cart nor the surgical bed needs direction changing or repositioning during the entire operation, thereby simplifying the RAMIE surgical procedure. Unfortunately, as a retrospective study, we do not have data on the duration of re-docking for comparison. The recent randomized controlled trial in RAMIE reported a total operating time of 349 minutes (13). It shows that in a high-volume center where the operation team is well experienced and routinely performs the procedure, the operation time is reduced. We think this may have a certain correlation with the learning curve, which is an issue that must be faced in the early stages of all new technologies. Operation time can be reduced after more patients are studied. Additionally, the cephalic positioning of the patient cart may hamper the airway control of the anesthetist. Dislocation of the double lumen tube during the procedure does occur. When the tube is accidentally removed completely, the robot on the cephalic side must be removed immediately to facilitate rescue. However, in most cases, anesthetists can also adjust the location of the tube from the ventral side of the patient when dislocation occurs because the patient is placed in a lateral decubitus position.
Several studies found that the lymph node yields of RAMIE and VAMIE are comparable (4,14,15). Nevertheless, to the best of our knowledge, most RAMIE procedures are performed in a semi-prone position, which was initially designed for VAMIE to overcome view blockage from vertebral column and lung and to facilitate endoscopic instrument in order to dissect the posterior or left side of the esophagus from the right hemithorax. However, EndoWrist robotic instruments are helping surgeons to overcome these difficulties, which have made prone position dispensable for RAMIE. Moreover, the full left lateral decubitus position exposes more chest surface in deploying robotic arms than that of prone position. Consequently, three robotic arms can be used during the whole procedure with little mutual restriction among them. With the assistance of three 270° flexible EndoWrist instruments, we can perform the bilateral laryngeal lymph node dissection more easily and meticulously than when using the prone position RAMIE or VAMIE (Figure 3).
In dissecting the thoracic esophagus, the middle or lower segment of the esophagus is usually selected as the pointcut. Therefore, dissection can start from the para-pericardium part, which is anatomically simple. Subsequently, full mobilization of the esophagus and passing a retracting tape to surround it become easy, thereby facilitating the subsequent procedures, such as the complex paratracheal, para-carina part, and meticulous bilateral RLN lymph node dissections. We did not perform the appropriate examination to determine which side of the RLN palsy was present for the two postoperative hoarseness cases because we focused on the integrity of the RLN during the operation. These two cases of RLN palsy were both involved with lymph node metastasis around the RLN. The RLN was skeletal after lymph nodes were dissected, so it is difficult to avoid a period of its dysfunction. Moreover, based on our experience, nerve palsy could not be completely avoided if the lymph nodes are thoroughly dissected. However, we believe that keeping the RLN physically intact may be essential in avoiding postoperative hoarseness or having a quick recovery from it.
With 3D visualization and extreme precision of instruments, RAMIE is very useful in a multimodal approach. The latest result of NEOCRTEC5010 showed that neoadjuvant chemoradiotherapy plus surgery improves survival with acceptable and manageable adverse events over surgery alone among patients with locally advanced esophageal squamous cell carcinoma (16). However, one patient underwent induction treatments among the five patients with stage T3 disease because the tumors of the other four cases were resectable and did not have significant invasion according to the results of preoperative imaging examination. Despite this, we should pay more attention to the role of neoadjuvant chemoradiotherapy in the future.
During the early stages of our practice in using RAMIE, we explored different approaches and techniques to make the procedure highly convenient and easy. Comparing the current optimized approach with the original is difficult because the approach we applied currently was not developed in one step. It was gradually developed during each surgical procedure instead of a sudden leap. Despite this, the lack of effective comparison and small sample size are the limitations of our study.
In summary, full lateral position with cephalic-parallel approach is technically feasible and safe. Although we still lack data to prove its advantages, we believe our design may be a valuable alternative to facilitate the RAMIE procedure and that the robotic surgical platform will improve with the advancement of robotic technology.
Acknowledgments
Funding: This work was supported by the Applied Basic Research Program of Sichuan province, China (2016JY0100 to Senyi Deng).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our study was approved by the Ethics Committee of West China Hospital, Sichuan University (approval number: 2017239).
References
- van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2006;20:1435-9. [Crossref] [PubMed]
- Kernstine KH, DeArmond DT, Karimi M, et al. The robotic, 2-stage, 3-field esophagolymphadenectomy. J Thorac Cardiovasc Surg 2004;127:1847-9. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Park S, Hwang Y, Lee HJ, et al. Comparison of robot-assisted esophagectomy and thoracoscopic esophagectomy in esophageal squamous cell carcinoma. J Thorac Dis 2016;8:2853-61. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Lin Y, Li Z, Li G, et al. Selective En Masse Ligation of the Thoracic Duct to Prevent Chyle Leak After Esophagectomy. Ann Thorac Surg 2017;103:1802-7. [Crossref] [PubMed]
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Finley DJ, et al. Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development. Eur J Cardiothorac Surg 2013;43:e107-15. [Crossref] [PubMed]
- Cerfolio RJ, Wei B, Hawn MT, et al. Robotic Esophagectomy for Cancer: Early Results and Lessons Learned. Semin Thorac Cardiovasc Surg 2016;28:160-9. [Crossref] [PubMed]
- Anderson C, Hellan M, Kernstine K, et al. Robotic surgery for gastrointestinal malignancies. Int J Med Robot 2007;3:297-300. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Yang CJ, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Suda K, Ishida Y, Kawamura Y, et al. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short-term outcomes. World J Surg 2012;36:1608-16. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]


