The safety of thymic vein sealing with ultrasonic energy in video-assisted thoracoscopic surgery thymectomy
Introduction
Extended thymectomy is the main surgical treatment for myasthenia gravis and thymoma. Since Watanabe et al. and Blalock et al. reported the first case of thymectomy with median sternotomy in the treatment of myasthenia gravis in 1939, median sternotomy thymectomy has gradually become the standard operation for myasthenia gravis and thymoma (1,2), In 2002, Hsu reported the first case of thymectomy by video-assisted thoracoscopic surgery (VATS) (3). Thymectomy is also needed for thymic tumors. For thymectomy, the protection of the phrenic nerve, the dissociation of the innominate vein, and the management of thymic veins are the key points. It is reported that 67% of thymic veins originate from the left innominate vein, 14.7% from the right innominate vein, 11.5% from the inferior thyroid vein, and 6.6% from the superior vena cava. Most of the thymic veins are located behind the thymus. The number of thymic veins fluctuates from 1 to 3, and single thymic veins are common (83.6%) (4,5). The traditional treatment of a thymic vein is to cut off the distal and proximal end of the thymic vein after clamping the Ham-Lock separately. Beginning in 2016, our team has effectively used an ultrasound scalpel to cut off thymic veins in open surgery. Thereafter, we have applied this method in thoracoscopic thymectomy, and there has been no conversion to open operation, intraoperative massive hemorrhage, post-operative hemorrhage, and secondary operation. The characteristics of patients and operative data of 169 patients undergoing thymectomy in our department were retrospectively analyzed to evaluate the safety of the transection of thymic veins by ultrasonic energy in thymectomy under video-assisted thoracoscopy.
Methods
We retrospectively reviewed the recorded data of patients who underwent thymectomy under video-assisted thoracoscopy between July 2012 and December 2018 at Jiangxi Provincial People’s Hospital Affiliated to Nanchang University, in the Department of Cardio-thoracic Surgery. Authorized relatives of all the patients were consulted preoperatively to inform them of the disease information, the pros and cons of surgical procedure, and the possible complications during and after the operation. Informed consent was obtained from all patients. All patients were confirmed to have no invasion of the innominate vein, pericardium, lung and tumor’s diameter greater than 5 cm by enhanced chest computed tomography (CT) within 1 week before the operation. All patients had no history of previous thoracic surgery.
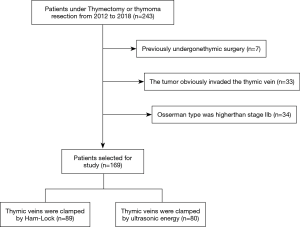
Patients were excluded if they had previously undergone thymic surgery, the tumor obviously invaded the thymic vein, or Osserman type was higher than stage IIb, but not inclusive of IIb (Figure 1).
Patient characteristics
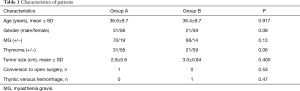
Between July 2012 and December 2018, there were 169 patients who underwent thymectomy with VATS. Among them, 33 patients with simple thymoma, 19 patients with thymoma combined with myasthenia gravis, and 115 patients with myasthenia gravis combined with thymic hyperplasia, were included. Additionally, 89 patients had a thymic vein resection by Ham-Lock (Group A), with one of these being converted to thoracotomy due to left anonymous venous hemorrhage. Meanwhile, 80 patients had thymic veins resected by ultrasonic energy (Group B). One patient in group B developed bleeding after disconnection of the thymic vein, but the bleeding was successfully staunched by ultrasonic energy under thoracoscopy. Details of all patients above are given in Table 1.
Full table
Surgical technique
All patients received single-lumen endotracheal intubation, inhalation, and intravenous general anesthesia. After satisfactory anesthesia, the patient’s legs were spread. The operator stood in the middle of the patient's legs, the armrest stood on the right side of the patient, and the display was placed on the patient's left shoulder. A straight incision about 3 cm below the xiphoid process was made as the observation hole, and the xiphoid cartilage was not damaged. Oval forceps bluntly dissociated the soft tissue spaces around the observation hole, behind the sternum and on both sides of the observation hole to form a “tunnel” behind the sternum of the anterior mediastinum. A 0.5 cm incision was made at the middle line of the clavicle at the lower edge of the left and right rib arches. The two incisions were used as operating holes and a 5-mm endoscopic puncture device was placed under the guidance of the hand. A 1-cm laparoscopic trocar was placed in the incision under the xiphoid process, and a thoracoscope was placed. The pressure was set to 8–12 cm water column to form an artificial pneumothorax. Laparoscopic graspers were placed in tracer at the lower edge of the right costal arch, and ultrasound scalpels were placed on the left side (which can be adjusted according to the operator’s habits). The surgeon fully freed the posterior sternal tissue, enlarged the tunnel, incised the left and right mediastinal pleura, cleared the right diaphragmatic angle fat, and opened the phrenic nerve 0.5 cm up to separate the right thymic lobe. Separation of the bilateral supramolecular thymus and complete thymectomy are preferred if there are thymic tumors. After thorough exposure of thymic veins and free thymic veins, the proximal and distal thymic veins were clamped by Ham-Lock in group A, and then the vessels were severed by ultrasound scalpel. In group B, the vessels were interrupted after three seconds of MAX grade with ultrasound scalpel and then resected in the middle by the ultrasound scalpel of MIN grade (Figure 2).
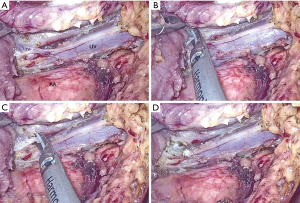
Then the left diaphragm angle fat and thymus was removed, so thymic tumors and thymus were completely resected. The trocar was removed from the observation hole. The specimen bag was placed in the right thoracic cavity through the incision under the xiphoid process. The thymus, thymic tumors, and adipose tissue were put into the specimen bag and removed. The drainage tube was placed in the left and right thoracic cavity and connected with the drainage bottle. The operation was thus completed (6). A video clip of our 3-minute operation is available in the attachment (Figure 3).

Data collection
The objective of the present study was to determine the feasibility and safety of thymic vein sealing with ultrasonic energy in VATS thymectomy. Information pertaining to age, gender, accompanying disease, tumor size, blood transfusion, drainage, duration of the operation, and duration of postoperative hospital stays, was collected retrospectively. One patient in group A converted to open surgery due to left innominate vein hemorrhage, and one patient in group B developed thymic venous hemorrhage but succeeded in hemostasis. Postoperative follow-ups were routinely carried out for all patients.
Statistical analysis
The patients’ characteristics were compared using Fisher’s exact test, Pearson’s χ2 test for categorical variables, and the Mann-Whitney U test for continuous variables. Pearson’s χ2 test was used to compare the differences between combined thymoma or myasthenia gravis between the two groups, with a value of P<0.05 being considered statistically significant.
Results
There were 56 (33.1%) male and 113 (66.9%) female patients, and the mean age of the patients was 36.5±9.7 years (range, 21–52 years). The mean size of the thymoma was 2.9±0.7 cm (range, 1.5–5.0 cm). The mean length of postoperative stay was 8.4±2.6 days (range, 4–12 days). No patient had reoperation or any other complications in either group. However, one patient in group A converted to open surgery due to left innominate vein hemorrhage; we tried to repair the blood vessel under thoracoscopy, but failed because of poor visual field. Finally, we performed hemi-sternotomy to complete the blood vessel repair. Another patient in group B received hemostasis under thoracoscope with an ultrasound scalpel, because the thymic vein was not completely cut off. The bleeding was about 50 mL during the operation. There were 136 (80.5%) patients with myasthenia gravis, and no patients underwent plasma exchange perioperatively. Furthermore, there was no significant difference between the two groups in terms of myasthenia gravis, thymoma proportion, and tumor size. Complete resection was achieved in all patients. The characteristics of patients in both groups are presented in Table 1.
All patients recovered and left the hospital. There was no significant difference in operative data such as intraoperative bleeding, operation time, postoperative drainage, and hospitalization time between the two groups (Table 2).

Full table
No procedure-related mortality occurred. All patients were followed up one month after discharge, and no pleural effusion was observed. However, two of the patients received post-operative chemotherapy for the diagnosis of thymic carcinoma. No tumor recurrence or metastasis was seen in any patients.
Discussion
Epidemiological survey shows that thymoma accounts for about 10–20% of all mediastinal tumors, and the incidence of myasthenia gravis worldwide is 0.53–3/10,000 annually (8,9); thus, there are a large number of patients with thymoma and myasthenia gravis. Thymectomy is recognized as an effective treatment for patients with thymoma and myasthenia gravis. With the continuous development of endoscopic technology, thoracoscopic thymectomy has become the main surgical method to treat the above diseases, because it can help patients by reducing operation time, bleeding, hospital stays, pain, and other negatives (10,11).
Thymectomy under VATS is a safe surgical procedure. In this surgery, it is crucial to protect the bilateral phrenic nerves and avoid injury of the innominate vein and thymic veins. The common causes leading to the conversion to thoracotomy are bleeding of the innominate vein and thymic veins. The location and quantity of thymic veins vary greatly. If not handled properly, they often cause bleeding. About 67% of thymic veins originate from the left innominate vein, 14.7% from right innominate vein, 11.5% from the inferior thyroid vein, and 6.6% from the superior vena cava. Most of the thymic veins are located behind the thymus. The number of thymic veins fluctuates from 1 to 3, and single thymic veins are common (83.6%) (4,5). The traditional method of thymic vein cutting is to clamp the Ham-Lock at the proximal and distal end of the thymic vein, and then cut it in the middle. Considering that the pressure of thymic veins is low and the wall of thymic veins is thin, the ultrasound scalpel is safe and reliable in treating vessels less than 5 mm (12). Our department tries to cut off the proximal and distal thymic veins with an ultrasound scalpel, without obvious bleeding, further treatment, and secondary operation. In recent years, our department has applied this technique to thymoma and myasthenia gravis thymectomy under thoracoscope, with very reliable and safe results. In order to compare the reliability and safety of sealing with ultrasonic energy, we retrospectively analyzed the data of patients who had undergone thoracoscopic thymectomy in our department in the past 7 years. Tables 1,2 show that there were no significant differences in sex, age, myasthenia gravis, thymoma, and tumor diameter between the two groups, and the operation time and diameter were similar. There were no significant differences in bleeding volume, drainage volume, and hospitalization time. We believe that the treatment of thymic veins with a diameter less than 5 mm by ultrasound scalpel is reliable and effective when compared to Ham-Lock which requires a thorough dissociation of the thymic veins (1).
Previously, when using Ham-Lock to block thymic veins, thorough free thymic veins were needed before closure. It took time to free the veins and often resulted in bleeding by injuring the innominate veins and thymic veins, leading to prolonged operation time or conversion to thoracotomy. The advantage of using an ultrasound scalpel to cut thymic veins directly is that for proper blood vessels, you can use an ultrasound scalpel only after freeing the approximate contour of a thymic vein. It can avoid replacing Ham-Lock instruments in operation; the Ham-Lock may also fall off, especially when a thymic vein is thin or improperly used. From our study, we can see that only one case of intravenous hemorrhage occurred in group B. Since the thymic vein was not completely resected by ultrasound scalpel, the remaining part of the thymic vein was treated by ultrasound scalpel and the hemorrhage stopped. There was no evidence of hemorrhage during subsequent hospitalization and follow-up.
Conclusions
In conclusion, we believe that it is safe and reliable to use an ultrasound scalpel to cut off thymic veins in thoracoscopic thymectomy. However, the study lacks large-sampling and long-term follow-up. Multi-center research is needed to validate the results.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee and the Institutional Review Board of the Jiangxi Provincial People’s Hospital (IRB number: 2019125) and written informed consent was obtained from all patients.
References
- Watanabe Y, Kurashima Y, Madani A, et al. Surgeons have knowledge gaps in the safe use of energy devices: a multicenter cross-sectional study. Surg Endosc 2016;30:588-92. [Crossref] [PubMed]
- Blalock A, Mason MF, Morgan HJ, et al. Myasthenia gravis and tumors of the thymic region: report of a case in which the tumor was removed. Ann Surg 1939;110:544-61. [Crossref] [PubMed]
- Hsu CP. Subxiphoid approach for thoracoscopic thymectomy. Surg Endosc 2002;16:1105. [Crossref] [PubMed]
- Abbas P, Holder-Haynes J, Taylor DJ, et al. More than a camera holder: teaching surgical skills to medical students. J Surg Res 2015;195:385-9. [Crossref] [PubMed]
- Bennett A, Birch DW, Menzes C, et al. Assessment of medical student laparoscopic camera skills and the impact of formal camera training. Am J Surg 2011;201:655-9. [Crossref] [PubMed]
- Numanami H, Yano M, Yamaji M, et al. Thoracoscopic Thymectomy Using a Subxiphoid Approach for Anterior Mediastinal Tumors. Ann Thorac Cardiovasc Surg 2018;24:65-72. [Crossref] [PubMed]
- Wu H, Lin Q, Liu Y, et al. A video of thymic vein sealed with ultrasonic energy in VATS thymectomy. Asvide 2019;6:247. Available online: http://www.asvide.com/watch/32932
- McGrogan A, Sneddon S, de Vries CS. The incidence of myasthenia gravis: a systematic literature review. Neuroepidemiology 2010;34:171-83. [Crossref] [PubMed]
- Carr AS, Cardwell CR, McCarron PO, et al. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol 2010;10:46. [Crossref] [PubMed]
- Raza A, Woo E. Video-assisted thoracoscopic surgery versus sternotomy in thymectomy for thymoma and myasthenia gravis. Ann Cardiothorac Surg 2016;5:33-7. [PubMed]
- Caronia FP, Fiorelli A, Santini M, et al. Uniportal bilateral video-assisted thoracoscopic extended thymectomy for myasthenia gravis: A case report. J Thorac Cardiovasc Surg 2015;150:e1-3. [Crossref] [PubMed]
- Caronia FP, Arrigo E, Trovato S, et al. Uniportal bilateral video-assisted sequential thoracoscopic extended thymectomy. J Vis Surg 2017;3:69. [Crossref] [PubMed]