A comprehensive protocol for physiokinesis therapy and enhanced recovery after surgery in patients undergoing video-assisted thoracoscopic surgery lobectomy
Introduction
Enhanced recovery after surgery (ERAS) is a multimodal approach to perioperative care that includes specific pathways to promote an early recovery after surgical procedures. It focuses on maintaining an adequate pre- and post-operative organ function and on reducing the stress response following surgery. The cornerstones of ERAS are: preoperative counselling, optimization of nutrition, standardization of anesthetic and analgesic regimens, physiotherapy (PT) rehabilitation and early mobilization.
Video-assisted thoracoscopic surgery (VATS) lobectomy is the gold standard treatment for early stage, non-small cell lung cancer (NSCLC) in eligible candidates. However, this procedure may be associated, similarly as in other surgical operations on the chest, with the development of postoperative pulmonary complications (PPCs) such as atelectasis, pneumonia and pleural complications (1,2). These complications may have an impact on patients’ early recovery after surgery and on their long-term quality of life. PPCs are one of the most frequent causes of postoperative mortality following pulmonary resections, accounting for up to 84% of all deaths (3).
In 1949, Leithauser et al. acknowledged that “early ambulation was essential for the well-being and safety of patients undergoing surgery” (4). In addition, he proposed that “early mobilization has proven to be able to save lives by preserving them from protracted hospitalization, thus preventing many fatal complications” (5). Unfortunately, bed rest has been historically adopted for its expected benefits derived from patient comfort (6,7). Today, even short-term immobility is widely recognized as a potential cause of many complications, including thromboembolic events (8). For this reason, some kind of motor and respiratory therapy after surgery of the chest has been adopted by most of the physicians of the thoracic surgical community, based on their benefit on patients (9-12).
PT is universally considered as a fundamental support; it may prevent the development of PPCs that may be associated with a significant clinical and economic impact; it may also contribute to prevent post-operative respiratory failure, as extensively reported in the literature (13-16), and to promote patients’ recovery.
It is widely recognized that all patients should be included into a perioperative rehabilitation protocol after thoracic surgical operations. However, there is consistent heterogeneity on PT programs and the timing for their implementation (before and/or after surgery). Evidence on the effectiveness of PT is not homogeneous, probably because of the variability of the programs’ contents and the quality of the design of the studies. In addition, there is still just a limited number of studies focused on the effectiveness of physical therapy in patients undergoing VATS lobectomy for lung cancer and, therefore, the evidence-based recommendations are scarce (17).
The aim of this paper is to provide a synopsis of a motor and respiratory PT protocol within the ERAS project promoted by the Italian VATS Group. This study proposes a guide for standardized treatment criteria and suggests an adequate timing and methodology in respect to each individual center expertise and resources.
ERAS and VATS lobectomy: what is the evidence?
The literature on ERAS for lung resections is very scant. A systematic review by Fiore and colleagues only identified six studies involving lung resections, and only one of them was a randomized trial defining fast track protocols and analyzing outcomes (18). The incidence of postoperative complications with the use of ERAS was reduced in one of the three studies. Two studies only evaluated the incidence of readmissions and reported discordant results: one showed no differences between ERAS and non-ERAS patients (19) and the other reported a 3-fold increase of readmissions in the fast-tracked patients (20).
These non-univocal findings are even harder to interpret given the fact that the majority of the studies on ERAS for thoracic surgery did not include patients undergoing VATS, but were retrospective analyses of series antecedent the wide application of VATS lobectomy.
Minimally invasive surgery is considered one of the mainstays of ERAS. In particular, compared to open surgery, VATS has shown to reduce pain, the incidence of complications, hospital mortality and length of stay (LOS); to improve functional recovery and the quality of life (21-26). The beneficial effects of VATS over open surgery are even more evident in high-risk patients (27,28).
Another recent retrospective, single-center study compared patients undergoing VATS lobectomy before and after the initiation of a formal ERAS protocol, without showing substantial differences in terms of early mortality rates and complications between groups. The possible explanation for this finding was that the pre-ERAS “standard of care” already included several ERAS components that may have contributed to provide good outcomes to VATS patients (29).
It is matter of debate whether the inclusion of aggressive perioperative PT protocols would be effective to provide better outcomes, in particular for patients undergoing VATS lobectomies who typically present an uncomplicated recovery.
Accordingly, VATS itself is the mainstay of ERAS in thoracic surgery, even though it may mask the effects of other components of ERAS on patients’ outcome.
Training and rehabilitation: acquiring targets
Clearance of bronchial secretions, chest expansion exercises, postural correction and shoulder range of motion are all exercises that are traditionally included in PT schedules. Technical advances in surgery and pulmonary PT are bringing new perspectives to rehabilitation for candidates to surgery. As reported in the literature, the perioperative implementation of traditional PT targets such as exercise training, combined or not with inspiratory muscle training (IMT) and intended as a recovery and a maintenance approach, suggested beneficial results (30,31). IMT has been widely studied in pulmonary rehabilitation programs especially in COPD patients (32,33). It has been demonstrated that IMT may provide clinical improvements through a better inspiratory muscle endurance and strength, reduction of dyspnea and better quality of life. In addition, respiratory muscle training displays better results compared to endurance training, and it can be easily performed at home with a specific device for inspiratory resistive breathing and threshold loading (34).
In order to prevent PPC, it is important to promote an adequate expansion of the lung and to remove bronchial secretions. Thus, the inclusion of deep breathing exercises with bronchial clearance and early patient mobilization within the PT program is of utmost importance.
A recent randomized control trial (RCT) investigated postoperative IMT in addition to breathing exercises and early mobilization in high-risk patients after lung cancer surgery, failing to show a significant difference in pulmonary function outcomes. Only a significant reduction in hypoxemia was observed in patient who underwent training. This is a unique study considering the postoperative effects and associated risks of IMT in patients undergoing lung surgery. The lack of significant results could be related to the low load level during IMT (35). Unfortunately, there are very few studies investigating respiratory muscle training in surgical patients, particularly in pulmonary surgery, either preoperatively or postoperatively.
The mainstays of PT: where does evidence stand?
Preoperative conditioning
Preoperative PT is considered a mainstay in patients undergoing surgery of the chest, due to its preparatory efficacy, but there is a lack of knowledge about its actual impact (36). Many institutions provide pre-operative PT treatments, but the studies on their efficacy are surprisingly scarce and methodologically limited (37,38). Recent studies of preoperative pulmonary rehabilitation performed on surgical candidates showed a correlation between adherence to pre-surgical exercise and relative pulmonary function improvement (39-41).
Aerobic and muscle training
According to Pouwels et al., preoperative exercise therapy (PET) may be a valuable approach to reduce PPC, mortality and LOS, to improve physical fitness and quality of life (42). Patients with severe COPD, who did not meet the inclusion criteria for lung surgery due to their reduced maximal oxygen consumption (VO2max), were included in a four-week program of muscle training on a cycle-ergometer with a progressive load of their maximum work on CPET. Patients were also encouraged to practice breathing exercises and incentive spirometry (IS) twice a day. The aerobic capacity was significantly improved after training, reaching the inclusion criteria for lung surgery, despite the absence of lung function improvements.
Respiratory training circuit
A randomized, controlled clinical trial compared the effect of IS muscle training (started two weeks before surgery and maintained for three months) with a control group including patients who did not receive any respiratory training, and showed a significant improvement in FEV1 and FVC before surgery. This improvement became even more pronounced at the end of the observation period (43). Another study on COPD patients considered preoperative IS training in combination with a 5,000 step/day walk for two weeks prior to pulmonary surgery, with similar results in terms of FEV1 and FVC. Candidates were also instructed about pursed-lip breathing, diaphragmatic breathing exercises, huffing and coughing for 15 min after nebulization with bronchodilation five times a day (44). A recent RCT examined the effect of a PT program (started one week before surgery and continued during all hospital stay), assessing a large number of variables (heart rate, performance in the six-minute walk test, FVC, FEV1, DLCO, oxygen arterial pressure, and carbon dioxide arterial pressure). In the treatment group, all the parameters were improved compared to the control group. PPC and LOS were both also significantly reduced in the intervention group (43). A smoking cessation program and the promotion of a healthy lifestyle, optimization of pharmacological therapy, nutritional counselling, stress management, bronchial hygiene and an exercise training program should be recommended in addition to other multidisciplinary interventions before lung-volume reduction surgery (45).
Immediate postoperative period
PT has been routinely used for a long time as a perioperative treatment (46) but has been only recently recommended by the European Society of Respiratory Society, the European Society of Thoracic Surgeons and the American College of Chest Physicians because of its proven efficacy in achieving functional benefits (47-50). More recently, a large non-randomized study investigated the effects of PT treatment, including deep breathing and early mobilization with a static cycling or treadmill, and confirmed the global reduction of pulmonary morbidity (51).
Several surgical centers are adopting a precise, measurable and achievable pathway in order to optimize cardiopulmonary function and improve surgical outcomes (52). Although it is difficult to verify patients’ compliance to treatment, the achievement of target activities during the postoperative course may influence the outcome. PT treatment in the perioperative period has been the topic of many studies on lung surgery. Unfortunately, most of them showed methodological flaws, such as the absence of a control group.
Routine respiratory PT
One of the few RCTs including a control group that did not receive any respiratory PT program assessed the occurrence of PPC and the LOS in patients undergoing different types of lung resection with a thoracotomy approach. The authors concluded that routine respiratory PT seems to be unnecessary in such patients, even if the control group was not totally blinded towards PT, due to preoperative patient teaching on breathing, coughing and shoulder exercises, early ambulation and mobilization. Additionally, the exercise program in the treatment group had a limited duration and all patients included in this sample had an acceptable baseline pulmonary function (2). Another interesting study by Novoa et al. applied a propensity matched analysis on data made available by Varela et al. (14) that were of limited interest due to methodological flaws. Compared to Varela’s conclusions, the authors showed, using a solid statistical analysis, that an intensive PT program significantly reduced patients’ morbidity, avoided PPC, and improved the recovery process of patients undergoing lung surgery (51).
Respiratory devices
Routine PT programs in surgical patients are supported by scientific evidence, but some controversies still remain regarding the effective advantages of external respiratory devices, such as IS or the “PEP-mask” device, over traditional chest PT interventions. The inclusion of IS devices into a PT program has been questioned by randomized control trials in which patients undergoing lobectomy were divided into an “intervention” group (receiving PT and the volumetric IS device), and a “control” group (receiving PT only), both following a program lasting from pre-operatively until hospital discharge. The study concluded that PT with or without IS was equally effective in reducing PPCs and in improving pulmonary function (53). These findings were corroborated in a systematic review on the routine use of IS devices in patients undergoing thoracic surgery, which also stated that IS cannot replace or significantly improve the physiotherapist’s work (54). The results reported in the literature about the use of other external devices within PT treatment protocols did not reach a definitive conclusion on advantages and costs.
Many devices are available for PT treatments. A systematic review compared the effectiveness of using simple devices (blow-bottle system and the “EP-mask”) to promote breathing exercises with positive expiratory pressure (PEP) after thoracic operations. Depending on the type of respiratory technique, the PEP can be used either to increase or to reduce lung volume, but none of the studies included in this review referred to the pressure applied or to the breathing pattern (55). Well-designed studies would be required in order to clarify the benefits of using these external devices in patients undergoing lung resection.
NIV therapy
A popular review of non-invasive ventilation (NIV) associated with chest PT after lung surgery showed that, in five “best evidence” trials, NIV improved the outcomes after lung resection (56). Perrin and colleagues applied NIV in 39 patients, 7 days before and 3 days after lobectomy. The 14 patients receiving NIV had a higher PaO2 and lower PaCO2. It was concluded that pre- and post-operative NIV may significantly reduce pulmonary dysfunction after lung resection (57).
Early mobilization and upper segmental exercises
Reeve et al. carried out a study in which all patients undergoing lung resection were stimulated to early mobilization and ambulation, frequent changes in position during bed rest, and performing upper limb and breathing exercises. The intervention group underwent a daily PT program, including actively-assisted shoulder mobilization. Patients were evaluated before surgery, on the day of hospital discharge, and one and three months after discharge. The intervention group had less shoulder and chest pain. The same results were observed one month and three months after discharge. Shoulder function was also significantly improved in this group, without significant differences in mobility, strength and quality of life (58).
A recent retrospective study considered 36 patients with non-small-cell lung cancer undergoing lobectomy who were encouraged to walk 4 hours after surgery, sit up on their beds 3.5 hours after surgery and maintain a sitting position for 30 min. Dressings were used to stabilize the chest tube and to minimize pain caused by movements. The results suggested that walking 4 h after surgery may achieve a better recovery of pulmonary function than conventional mobilization (walking the day after surgery) (59).
Another prospective, propensity-matched study clearly demonstrated that patients undergoing VATS lobectomy developed less PPC and had improved outcomes compared to patients undergoing thoracotomy. A better adherence to rehabilitation exercises during the perioperative period was noticed. In fact, patients were significantly more mobile at an earlier stage, required half of the PT and developed less pulmonary and mobility issues (17).
Postoperative rehabilitation
Dyspnea, pain, fatigue and limitation in daily activities often occur in patients undergoing pulmonary surgery and may determine significant loss of their quality of life after discharge. Based on these considerations, many studies have focused on validating a PT program after-discharge. For example, one study defined a program where a supervised incremental cycle ergometer exercise provided 30 minutes of continuous stress at sub-maximal load achieved in CPET at baseline. Different exercises to fit abdominal muscles, upper and lower limb and inspiratory muscle were included, combined with educational sessions on nutrition, relaxation and stress management, principles of energy conservation, and breathing control. A PT program accelerated functional recovery in the interventional group, improvement of the symptoms of dyspnea and exercise tolerance (60). Lung function seems to recover in the first three months, while exercise tolerance grows slower, regaining a maximum after about one year. Long-term recovery of lung function and exercise capacities appear to have a different timing, because the recovery of pulmonary function is directly related to the progressive reduction of pain (61). Some studies support the beneficial effects of exercise training with a specific methodology, such as the one from Spruit et al. (31). An eight-week rehabilitation program through a non-randomized pilot study included patients undergoing surgical resection and adjuvant cancer therapy. The study was focused on the application of general exercise training preconized for COPD patients. None of the patient displayed an improvement of lung function after eight weeks, but conversely all showed an actual improvement in exercise capacity (37). A wide variety of patients with malignancy seems to have a beneficial impact from exercise programs, as described by McMillan et al. (62). Unfortunately, lung cancer patients are not included in any of these studies. Considering lung cancer, it might be expected that an improvement of physical training would be seen because, in parallel with other factors such as COPD and cardiac overload, pulmonary surgical resections also tend to result in a decreased exercise tolerance.
PT and analgesia: an issue of teamwork
Postoperative pain control is critical, together with PT, to achieve a rapid improvement in pulmonary function. According to the principles of ERAS, analgesic drugs such as opioids are preferably avoided and analgesia with ropivacaine and sufentanil via thoracic epidural anesthesia (TEA) is preferred over intravenous morphine administration, resulting in a significant improvement of pulmonary function after lung surgery (63). Patients with epidural pain control showed a faster improvement in lung volumes, reducing the incidence of pulmonary complications such as atelectasis and pneumonia. Considering the disadvantages of TEA, including hypotension, urinary and bowel retention, respiratory muscle weakness, and neurological injury risk, other safe analgesic alternatives have been used, such as thoracic paravertebral block. A recent meta-analysis compared these two approaches, in terms of pain control efficacy and opioid consumption, and no differences were found except for a lower incidence of urinary retention and hypotension in the paravertebral block group (64). Analgesia by means of other techniques such as transcutaneous electrical nerve stimulation (TENS) (65) or serratus anterior plane (SAP) block (66) may be used as a technical adjunct to avoid or control severe pain. The concept of thoracic anesthesia and analgesia must be coherently conceived in an era of minimally invasive operations, which allow a consistent reduction of post-surgical pain.
ERAS—PT protocol in VATS lobectomy patients
A rehabilitation program is crucial for a successful recovery process, and should be coupled to the selection of appropriate outcome measures. The purpose of a “PT protocol in patients undergoing VATS Lobectomy” is to reduce the risk of complications in patients undergoing anatomic lung resections with a minimally invasive approach. There only are a few viable studies that may allow the definition of the best PT strategy, and none are focused on VATS. The “Italian VATS Group” is planning on a carefully designed clinical trial to apply ERAS to all patients accessing the VATS group national lobectomy database, in order to match operative outcomes with all the data acquired form the ERAS-PT Protocol. The results of the trial could outline potential surgery-related factors that may have an actual impact on respiratory and other physiologic parameters, independently from the patient’s status.
The main respiratory complications occurring after surgery are:
- Compression atelectasis;
- Infections;
- Pulmonary embolisms;
- Acute bronchospasm;
- Retention of bronchial secretions;
- Respiratory failure (ultimately leading to ARDS).
The main non-respiratory complications occurring after surgery are:
- Bed rest syndrome (stiffness/joint blocks, shortening/muscle retraction, decubitus wounds);
- Central nervous system alterations (coma, hemiplegia/paresis, paraplegia, tetraplegia);
- Peripheral nervous system alterations (brachial plexus stretching, ulnar and median nerve compression, sacral plexus compression, Sciatic-Popliteal external syndrome).
Using a multi-disciplinary approach, the physiotherapist will inspect clinical patient data with the assistance of medical and nursing staff, if necessary. The following contraindications to PT will be taken into account, according the international clinical evidence and good practice:
- Absolute contraindications:
- Active pneumothorax;
- Inability to tolerate highest respiratory load.
- Relative contraindications:
- Hemodynamic instability;
- Acute asthma;
- Acute pulmonary embolism;
- Active airway or gastrointestinal bleeding;
- Unstable coronary artery disease, unstable angina;
- Gastroesophageal reflux;
- Tracheoesophageal fistula;
- Nausea or vomiting;
- Intracranial pressure >20 mmHg.
Contraindications to motor PT (in addition to those indicated for respiratory PT):
- Severe respiratory impairment;
- Unaddressed atrial fibrillation;
- Severe hemodynamic instability;
- Dialysis in progress.
Preoperative approach
Key-points
- Motor and respiratory preoperative evaluations (estimated session time: 30 minutes) are performed to improve patient recovery after surgery: by the physiotherapists during the pre-assessment visit, during the pre-operative Day Hospital admission (if applicable) or ultimately the day before surgery;
- An empathic approach to patients, a thorough explanation of the role of the physiotherapist and emphasis over the importance of motor and respiratory treatments in the perioperative period are mandatory;
- Physiotherapists attend routinely to surgeons’ or anesthesiologists’ rounds and patients’ evaluation;
- The Rehabilitation Service Brochure and all operative devices are also delivered to patients at this time, together with a with accurate teaching about the PT program;
- A scoreboard should be used including a customizable checklist displaying information about disability or comorbidity such as: advanced age, obesity, malnutrition, cardiorespiratory diseases, neuromuscular disabilities, previous operations. It may be helpful to identify and to prevent possible complications after surgery;
- If no particular issue arises from the patient’s assessment process, a single session of information, education and training will be performed. Conversely, physiotherapists will consider the opportunity of additional sessions in order to schedule specific treatments.
Aims
Physiotherapists should encourage an active and conscious participation of the patient and his relatives to the rehabilitation program. Information about the physiology of the chest wall and lungs are provided.
Methods
The patient will be informed about the effects of surgery on lung function. The role of bronchial clearance and early mobilization will be stressed: as soon as possible (immediately after awakening from surgery), the patient will be asked to sit at the bedside or in a chair. The patient is instructed on postural passages and on the protection of the surgical site using hands; the efficacy of cough is evaluated.
Physiotherapists will show how to perform deep breathing exercises: starting with deep tidal volume breaths until reaching the total lung capacity (TLC), then maintaining a short apnea and performing one or two coughs or forced expiration technique (FET). The patient will learn the use of “volume-based” respiratory incentive devices (Coach and/or Voldyne) or “flow-based” respiratory incentive devices (Triflo) to allow a deep, open glottis inspiration. This preoperative assessment will give a baseline evaluation of the deep breathing capacity that will be available for comparison with postoperative data.
PEP, PEP-Bottle or Acapella devices are also presented, in order to prevent sputum retention and atelectasis. The activity of the inspiratory/expiratory chest musculature is explained, stressing to keep a good articular range of both thoracic and scapular crawlers with a traditional PT.
The importance of a good peripheral oxygenation is also emphasized by suggesting active limb exercises. A more appropriate ventilation mechanism can be pursued by stretching the inspiratory accessory muscles, together with muscles relaxation techniques.
Postoperative approach
Key-points
- The treatment must be carried out postoperatively between 4 and 12 hours after recovery form general anesthesia. The estimated session time is 30 minutes, from 2 up to 3 daily sessions;
- Physiotherapists will fill a postoperative evaluation sheet based on chest examination, respiratory pattern evaluation, recognition of parameters (HR, RR, NIBP, possible signs of hypoxia and hypercapnia) and taking into account biochemical data, ABG, chest X-ray, therapeutic and monitoring prescriptions;
- The rehabilitation program defined in the preoperative assessment will be followed by a physiotherapist;
- A modification of the clinical status may require extra-protocol rehabilitation measures, due to standard functional mobilization impairments.
Aims
The rehabilitation Criteria are focused on adequate pulmonary ventilation, through the recruitment of lung segments with atelectasis and bronchial secretions washout. Recovering an optimal articulation and stimulating an early walking and daily life activity is also essential. Patients’ relatives should also be involved in the rehabilitation exercises and in the entire process of care.
Methods
All the steps of the protocol may be modified according to individual clinical outcomes. For example, a longer duration of chest tube drainage, obstructive persistent bronchial secretions, pain from coughing or mobilization, hypo mobility, fatigue and desaturation, should induce quantitative and qualitative adjustments (according to multidisciplinary discussion with the surgeon or anesthesiologists).
The rehabilitation program will provide progressively growing goals of care. The physiotherapist will perform the techniques that he believes to be most effective in achieving defined targets. For motor PT, a target ≥80 meters at least twice a day, independently or with walking aid will be adopted. For respiratory PT, the evaluation of the recovery of pulmonary volumes is performed with the use of flow or volume respiratory devices.
The protocol is summarized in Figure 1, starting from postoperative day zero (POD 0) to the following sessions, until patient discharge from rehabilitation service.
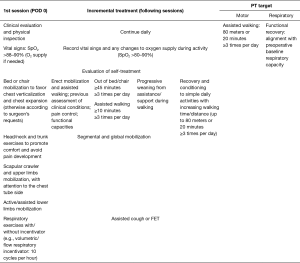
Physiotherapists will set up self-treatment program aimed to restore balanced and symmetric costal-diaphragmatic kinetics (cautious solicitation of upper and lower rib expansions, in supine or seated decubitus). Therefore, the program will be focused on avoiding stiffness and reduction of muscle strength (working mainly on the scapula fixator muscles: latissimus dorsi, serratus, rhomboid and trapezium, through active-assisted cervical spine/back/lumbar PT). The patient will be spurred to perform exercises alone, in between rehabilitation sessions with the physiotherapist, and to report the results on a personal scoreboard sheet as in Table 1.

Full table
PT service discharge and self-treatment program
The patient concludes the hospital rehabilitation treatment when he is able to walk and to perform self-treatment exercises, possibly without assistance and possibly without oxygen therapy, without evidence of residual complications or disabilities. PT treatment goals are: walking ≥80 meters or ≥20 minutes at least 3 times per day; realigning respiratory capacity with pre-operative values. The patient continues with a personal self-treatment program received from the physiotherapists on the day of PT service discharge. Daily routine should be recorded on the personal scoreboard sheet as in Table 1.
Discussion
In our opinion, the ERAS-PT protocol should be individually designed according to the patient’s needs, considering all the issues related to surgery for lung cancer. The schedule of the treatment should be divided into three different phases according to the process of surgery: pre-operative, post-operative and maintenance. During the pre-operative treatment, the attention is dedicated to promoting a healthy lifestyle, respiratory expansion exercises, and bronchial hygiene. The role of patients’ compliance to PT is a mainstay for a successful rehabilitation across all the three phases of the treatment, in order to reduce PPCs and hospitalization. Exercise training plays an important role to improve cardiopulmonary load, and may allow to consider for surgery even candidates with poor VO2max (67). The postoperative PT aims to promote a faster recovery through maximal inspiratory maneuvers, coughing and mobilization exercises of the upper and lower limbs, postural changes and shoulder impairment (68). In this phase, PT is focused on balancing the transition between the preoperative and the maintenance treatments, because as soon as the pain is reduced, the pulmonary function recovers. Patients’ attitude to exercise may take longer to improve, mostly in patients who still have to undergo co-adjuvant therapies. This is another reason to stress the importance of an early mobilization approach, such as early ambulation or mobilization supported by static cycling and treadmill. Mobilization is considered the most critical step of a PT program and the one with, prospectively, the most productive outcomes.
A possible limitation of the ERAS rehabilitation program for pulmonary surgery is related to the physical limitations hindering patients from walking (for example, the severity of disease, worsened clinical conditions, ventilation, sedation). However, in some institutions, even ventilated patients are encouraged to walk to improve their outcome (69,70): an extreme and not easily reproducible trail.
One interesting message emerging from the literature emphasizes the idea that pre- and post-operative motor and respiratory PT may benefit from a synergistic effect in terms of reduced morbidity, improved functional capacity and better overall outcomes in patients undergoing lung resection. Conversely, unimodal treatments lose the beneficial impact on post-surgical pulmonary complications and length of hospitalization (71,72). Therefore, considering the inherent economic costs, programs of PT intervention based on a robust scientific evidence are required.
All the “Italian VATS Group” participants will be asked to adhere to this ERAS-PT protocol for all patients who may tolerate the rehabilitation approach. All PT data will be collected into the National VATS Group Database. The prospective analysis on this data should help defining which is the best and safest PT treatment in patients undergoing VATS lobectomy, and understanding the best exercise trails, devices and timing of intervention. The results of this study will be provided on behalf of “Italian VATS Group” after an adequate follow-up period.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Magnusson L, Spahn DR. New concepts of atelectasis during general anaesthesia. Br J Anaesth 2003;91:61-72. [Crossref] [PubMed]
- Reeve JC, Nicol K, Stiller K, et al. Does physiotherapy reduce the incidence of postoperative pulmonary complications following pulmonary resection via open thoracotomy? A preliminary randomized single-blind clinical trial. Eur J Cardiothorac Surg 2010;37:1158-66. [Crossref] [PubMed]
- Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815-8. [Crossref] [PubMed]
- Leithauser DJ. Rational principles of early ambulation. J Int Coll Surg 1949;12:368-74. [PubMed]
- Beniaminovitz A, Lang CC, La Manca J, et al. Selective low-level leg muscle training alleviates dyspnea in patients with heart failure. J Am Coll Cardiol 2002;40:1602-8. [Crossref] [PubMed]
- Eichemberger A, Proietti S, Wicky S, et al. Morbid obesity and postoperative pulmonary atelectasis: an understimated problem. Anest Analg 2002;95:1788-92. [Crossref]
- Schatz C. Enhanced recovery in a minimally invasive thoracic surgery program. AORN J 2015;102:482-92. [Crossref] [PubMed]
- Gregor JI, Schwenk W, Mall J, et al. "Fast-track" rehabilitation in thoracic surgery. First experiences with a multimodal, interdisciplinary, and proven perioperative treatment course. Chirurg 2008;79:657-64. [Crossref] [PubMed]
- Yie JC, Yang JT, Wu CY, et al. Patient-controlled analgesia (PCA) following video-assisted thoracoscopic lobectomy: Comparison of epidural PCA and intravenous PCA. Acta Anaesthesiol Taiwan 2012;50:92-5. [Crossref] [PubMed]
- Bjerregaard LS, Jensen K, Petersen RH, et al. Early chest tube removal after video-assisted thoracic surgery lobectomy with serous fluid production up to 500 ml/day. Eur J Cardiothorac Surg 2014;45:241-6. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Does minimally invasive thoracic surgery warrant fast tracking of thoracic surgical patients? Thorac Surg Clin 2008;18:301-4. [Crossref] [PubMed]
- Sokouti M, Aghdam B, Golzari S, et al. A comparative study of postoperative pulmonary complications using fast track regimen and conservative analgesic treatment: a randomized clinical trial. Tanaffos 2011;10:12-9. [PubMed]
- Schwarzbach MH, Ronellenfitsch U, Wang Q, et al. Effects of a clinical pathway for video-assisted thoracoscopic surgery (VATS) on quality and cost of care. Langenbecks Arch Surg 2010;395:333-40. [Crossref] [PubMed]
- Varela G, Ballesteros E, Jiménez MF, et al. Cost-effectiveness analysis of prophylactic respiratory physiotherapy in pulmonary lobectomy. Eur J Cardiothorac Surg 2006;29:216-20. [Crossref] [PubMed]
- Sekine Y, Chiyo M, Iwata T, et al. Perioperative rehabilitation and physiotherapy for lung cancer patients with chronic obstructive pulmonary disease. Jpn J Thorac Cardiovasc Surg 2005;53:237-43. [Crossref] [PubMed]
- Sebio R, Yañez MI, Gimenez E. Impact of a pre-operative pulmonary rehabilitation program on functional performance in patients undergoing video-assisted thoracic surgery for lung cancer. Arch Bronconeumol 2016;52:231-2. [PubMed]
- Agostini P, Lugg ST, Adams K, et al. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: a propensity score matched study of patients undergoing video-assisted and thoracic surgery. Interact Cardiovasc Thorac Surg 2017;24:931-7. [Crossref] [PubMed]
- Fiore JF Jr, Bejjani J, Conrad K, et al. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J Thorac Cardiovasc Surg 2016;151:708-715.e6. [Crossref] [PubMed]
- Salati M, Brunelli A, Xiume F, et al. Does fast-tracking increase the readmission rate after pulmonary resection? A case-matched study. Eur J Cardiothorac Surg 2012;41:1083-7. [Crossref] [PubMed]
- Numan RC, Klomp HM, Li W, et al. A clinical audit in a multidisciplinary care path for thoracic surgery: an instrument for continuous quality improvement. Lung Cancer 2012;78:270-5. [Crossref] [PubMed]
- Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000;70:1644-6. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28. [Crossref] [PubMed]
- Bertani A, Ferrari PA, De Monte L, et al. Video-assisted thoracic surgery lobectomy in patients with reduced pulmonary function: a single-center series. Future Oncol 2016;12:39-45. [Crossref] [PubMed]
- Brunelli A, Thomas C, Dinesh P, et al. Enhanced recovery Pathway versus standard care in patients undergoing video assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2017;154:2084-90. [PubMed]
- Varela G, Novoa NM, Ballesteros E, et al. Chest physiotherapy in lung resection patients: state of the art. Semin Thorac Cardiovasc Surg 2011;23:297-306. [Crossref] [PubMed]
- Spruit MA, Janssen PP, Willemsen SC, et al. Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: a pilot study. Lung Cancer 2006;52:257-60. [Crossref] [PubMed]
- Shoemaker MJ, Donker S, Lapoe A. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: the state of the evidence. Cardiopulm Phys Ther J 2009;20:5-15. [PubMed]
- Petrovic M, Reiter M, Zipko H, et al. Effects of inspiratory muscle training on dynamic hyperinflation in patients with COPD. Int J Chron Obstruct Pulmon Dis 2012;7:797-805. [Crossref] [PubMed]
- Gosselink R, De Vos J, Van den Heuvel SP, et al. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J 2011;37:416-25. [Crossref] [PubMed]
- Brocki BC, Andreasen JJ, Langer D, et al. Postoperative inspiratory muscle training in addition to breathing exercises and early mobilization improves oxygenation in high-risk patients after lung cancer surgery: a randomized controlled trial. Eur J Cardiothorac Surg 2016;49:1483-91. [Crossref] [PubMed]
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007;131:4S-42S. [Crossref] [PubMed]
- Ambrosino N, Gabbrielli L. Physiotherapy in the perioperative period. Best Pract Res Clin Anaesthesiol 2010;24:283-9. [Crossref] [PubMed]
- Pasquina P, Tramèr MR, Walder B. Prophylactic respiratory physiotherapy after cardiac surgery: Systematic review. BMJ 2003;327:1379-84. [Crossref] [PubMed]
- Cesario A, Ferri L, Galetta D, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer 2007;57:118-9. [Crossref] [PubMed]
- Jones LW, Peddle CJ, Eves ND, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer 2007;110:590-8. [Crossref] [PubMed]
- Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg 2008;33:95-8. [Crossref] [PubMed]
- Pouwels S, Fiddelaers J, Eijink JA, et al. Preoperative exercise therapy in lung surgery patients: a systematic review. Respir Med 2015;109:1495-504. [Crossref] [PubMed]
- Pehlivan E, Turna A, Gurses A, et al. The effects of preoperative short-term intense physical therapy in lung cancer patients: a randomized controlled trial. Ann Thorac Cardiovasc Surg 2011;17:461-8. [Crossref] [PubMed]
- Weiner P, Man A, Weiner M, et al. The effect of incentive spirometry and inspiratory muscle training on pulmonary function after lung resection. J Thorac Cardiovasc Surg 1997;113:552-7. [Crossref] [PubMed]
- Bartels MN, Kim H, Whiteson JH, et al. Pulmonary rehabilitation in patients undergoing lung-volume reduction surgery . Arch Phys Med Rehabil 2006;87:S84-8. [Crossref] [PubMed]
- Harms CA, Setter TJ, St Croix CM, et al. Effects of respiratory muscle work on exercise performance. J Appl Physiol (1985) 2000;89:131-8. [Crossref] [PubMed]
- McConnell TR, Mandak JS, Sykes JS, et al. Exercise training for heart failure patients improves respiratory muscle endurance, exercise tolerance, breathlessness, and quality of life. J Cardiopulm Rehabil 2003;23:10-6. [Crossref] [PubMed]
- McCool FD. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:48S-53S. [Crossref] [PubMed]
- Navalesi P, Barbarico N. Coinvolgimento respiratorio nelle malattie neuromuscolari. In: Donner CF, Sanguinetti CM. editors. Trattato di pneumologia. Edi-Aipo Scientifica, 2001.
- Navalesi P, Frigerio P. Cought Assistance in mechanically ventilated neuromuscolar patients. In: Vencent J. editors. Yearbook of intensive care and emergency medicine. New York: Springer, 2004:345-52.
- Novoa N, Ballesteros E, Jiménez MF. Chest physiotherapy revisited: evaluation of its influence on the pulmonary morbidity after pulmonary resection. Eur J Cardiothorac Surg 2011;40:130-4. [Crossref] [PubMed]
- Ozalevli S, Ilgin D, Karaali HK, et al. The effect of in-patient chest physiotherapy in lung cancer patients. Support Care Cancer 2010;18:351-8. [Crossref] [PubMed]
- Gosselink R, Schrever K, Cops P, et al. Incentive spirometry does not enhance recovery after thoracic surgery . Crit Care Med 2000;28:679-83. [Crossref] [PubMed]
- Agostini P, Singh S. Incentive spirometry following thoracic surgery: what should we be doing? Physiotherapy 2009;95:76-82. [Crossref] [PubMed]
- Orman J, Westerdahl E. Chest physiotherapy with positive expiratory pressure breathing after abdominal and thoracic surgery: a systematic review. Acta Anaesthesiol Scand 2010;54:261-7. [Crossref] [PubMed]
- Freynet A, Falcoz PE. Does non-invasive ventilation associated with chest physiotherapy improve outcome after lung resection? Interact Cardiovasc Thorac Surg 2008;7:1152-4. [Crossref] [PubMed]
- Perrin C, Jullien V, Venissac N, et al. Prophylactic use of noninvasive ventilation in patients undergoing lung resectional surgery. Respir Med 2007;101:1572-8. [Crossref] [PubMed]
- Reeve J, Stiller K, Nicol K, et al. A postoperative shoulder exercise program improves function and decreases pain following open thoracotomy: a randomised trial. J Physiother 2010;56:245-52. [Crossref] [PubMed]
- Kaneda H, Saito Y, Okamoto M, et al. Early postoperative mobilization with walking at 4 hours after lobectomy in lung cancer patients. Gen Thorac Cardiovasc Surg 2007;55:493-8. [Crossref] [PubMed]
- Cesario A, Ferri L, Galetta D, et al. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung Cancer 2007;57:175-80. [Crossref] [PubMed]
- Nagamatsu Y, Maeshiro K, Kimura NY, et al. Long-term recovery of exercise capacity and pulmonary function after lobectomy. J Thorac Cardiovasc Surg 2007;134:1273-8. [Crossref] [PubMed]
- McMillan EM, Newhouse IJ. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: a metanalysis. Appl Physiol Nutr Metab 2011;36:892-903. [Crossref] [PubMed]
- Bauer C, Hentz JG, Ducrocq X, et al. Lung function after lobectomy: a randomized, double-blinded trial comparing thoracic epidural ropivacaine/sufentanil and intravenous morphine for patient-controlled analgesia. Anesth Analg 2007;105:238-44. [Crossref] [PubMed]
- Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2014;18:626-35. [Crossref] [PubMed]
- Freynet A, Falcoz PE. Is transcutaneous electrical nerve stimulation effective in relieving postoperative pain after thoracotomy? Interact Cardiovasc Thorac Surg 2010;10:283-8. [Crossref] [PubMed]
- Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound guided thoracic wall nerve block Anaesthesia 2013;68:1107-13. [Crossref] [PubMed]
- Rodriguez-Larrad A, Lascurain-Aguirrebena I, Abecia-Inchaurregui LC, et al. Perioperative physiotherapy in patients undergoing lung cancer resection. Interact Cardiovasc Thorac Surg 2014;19:269-81. [Crossref] [PubMed]
- Nomori H, Kobayashi R, Fuyuno G, et al. Preoperative respiratory muscle training: assessment in thoracic surgery patients with special reference to postoperative pulmonary complications. Chest 1994;105:1782-8. [Crossref] [PubMed]
- Reeve J, Denehy L, Stiller K. The physiotherapy management of patients undergoing thoracic surgery: a survey of current practice in Australia and New Zealand. Physiother Res Int 2007;12:59-71. [Crossref] [PubMed]
- Morano MT, Araújo AS, Nascimento FB. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil 2013;94:53-8. [Crossref] [PubMed]
- Madani A, Fiore JF, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899-908. [Crossref] [PubMed]
- Padilla Alarcón J, Peñalver Cuesta JC. Experience with lung resection in a fast-track surgery program. Arch Bronconeumol 2013;49:89-93. [PubMed]


