Neural respiratory drive and cardiac function in patients with obesity hypoventilation syndrome following initiation of non-invasive ventilation
Introduction
The prevalence of obesity has increased continuously over recent decades reaching epidemic proportions (1). Obesity increases the load on the respiratory system and the work of breathing (2), leads to high levels of neural respiratory drive (NRD) (3), and is associated with a high prevalence of sleep-disordered breathing (4). An imbalance in the load-to-capacity ratio of the respiratory muscles (2) leads to increased levels of NRD (3); in turn, nocturnal loss of NRD may first lead to hypercapnic respiratory failure (HRF) while asleep and, subsequently, breathlessness in patients with obesity hypoventilation syndrome (OHS) while awake (5-7).
Obesity-related respiratory failure, the OHS, is an increasingly common problem (8). Current estimates suggest that between 0.3% to 0.4% of the general population may develop OHS (9). Patients with morbid obesity are more likely to develop significant sleep-disordered breathing and OHS (10). However, it remains unclear why some obese patients develop OHS over time (11), while others remain unaffected: approximately a third of morbidly obese patients develop hypercapnia (12).
In addition to the respiratory constraints, pulmonary hypertension (PH) and cardiac impairment, particularly right ventricular (RV) dysfunction, are associated with adverse health outcomes in OHS (13-16). The prevalence of PH in obese patients is higher than in the general population, 5% of all individuals with a body mass index (BMI) greater than 30 kg/m2 have some degree of PH, while 48% of patients with severe PH are obese (17,18). Moreover, there is a strong correlation between BMI and RV dysfunction, with congestive heart failure being two-fold more common in obese patients (19).
Non-invasive ventilation (NIV) is commonly used to treat HRF in OHS and its impact on mechanical indices of respiratory function and blood gas homeostasis has been previously described (20-24). However, the effect of NIV on NRD and RV function remains largely unexplained (22-24). The aim of this study was to assess the impact of NIV on respiratory and cardiac function in patients with HRF caused by OHS. We hypothesised that NIV improves not only respiratory but also cardiac function, and that this functional improvement would contribute to better oxygenation, quality of life and exercise capacity.
Methods
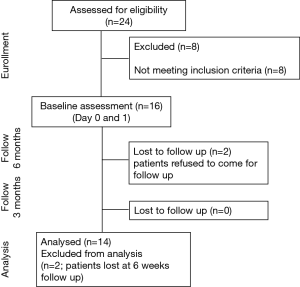
This was a prospective observational study assessing patients with OHS prior to and immediately following NIV setup, followed up at 6 weeks and 3 months; it was approved by London City & East NHS Research Ethics Committee (reference: 15/LO/1,390). OHS was defined as obesity (BMI >30 kg/m2) with associated daytime hypercapnia (pCO2 >6 kPa) and sleep disordered breathing. Patients were recruited at Guy’s and St Thomas’ NHS Foundation Trust, London (UK) from November 2015 to November 2016. Patients were enrolled within 24 hours of admission to the hospital; follow up was arranged at six weeks with an outpatient review and at three months with a planned inpatient review. Informed and written consent was obtained from all patients. The study was registered on ClinicalTrials.gov (CARE-NIV, NCT02699112).
Primary and secondary outcomes
Primary outcome parameters were NRD and tricuspid annular plane systolic excursion (TAPSE) score. These were used to assess the impact of NIV on the cardiorespiratory system, using markers of NRD, as measured by the electromyography of the parasternal intercostals (EMGpara). These were measured prior to, during and after NIV initiation and at 6 weeks and 3 months. In addition, transthoracic echocardiography (TTE) to assess cardiac function was performed at baseline and 3 months. Secondary outcomes were oxygenation (SpO2), arterial blood gas analysis including pO2, pCO2 and pH, quality of life, and exercise capacity.
Inclusion and exclusion criteria
Inclusion criteria were a confirmed diagnosis of OHS with HRF and the need to commence on NIV (pCO2 >6 kPa), age ≥18 and <80 years, BMI >30 kg/m2, confirmed sleep-disordered breathing, and clinical stability without acute deterioration for ≥4 weeks.
Exclusion criteria were patients already established on NIV, patients with an overlap syndrome (OHS and COPD), inability to tolerate NIV, a low compliance with NIV during the hospital stay (usage <4 hours/day), any contraindication to NIV, acute respiratory deterioration, other acute pathology or critical illness, and psychological or social factors that would impair compliance with the protocol (Figure 1).
Measurement of NRD
The EMGpara was recorded whilst the patient was awake, at rest and in semi-recumbent position in bed, as previously described (25). EMG electrodes were applied 3cm from the midline in the 2nd intercostal space; a reference electrode was placed on the right clavicle. The skin was prepared using NuPrep abrasive skin preparation gel (D.O.Weaver, Aurora, CO/USA) and alcohol solution. The signal was amplified and processed using a high differential amplifier with band pass filters set at 10 and 2,000 Hz (1902, Cambridge Electronic Design, Cambridge, UK). Additional analogue 50 Hz notch filter and AC coupling were used. Amplified signals were passed to an analogue to digital convertor (Powerlab, ADInstruments, Chalgrove, UK) and passed to a personal computer. Further digital filtering occurred at 20Hz after data acquisition (LabChart v7.1, ADInstruments, Chalgrove, UK). At baseline, EMGpara was recorded during maximal inspiratory and expiratory manoeuvres: sniff, maximal inspiratory pressure (PImax), maximal expiratory pressure (PEmax), and inspiratory capacity. The average EMGpara of two minutes tidal breathing was calculated (mean EMGpara) with a time constant of 100 ms. EMGpara%max was calculated as the mean of EMGpara divided by the maximum EMGpara ×100%; neural respiratory drive index (NRDI) was obtained from EMGpara%max × respiratory rate.
TTE
All TTEs were performed by the same trained technician of the cardiology department of Guy’s and St Thomas’ NHS Foundation Trust while patients were awake, at rest and supine in bed. The assessment followed the American Society of Echocardiography guidelines (26) using an IE-33 device and an ×5–1 probe (Phillips, Amsterdam/Netherlands). The following cardiac chamber dimensions and pressures were recorded: RV systolic pressure (RVSP), RV diameters [RV basal (RVD1), mid-cavity (RVD2) and longitudinal dimension (RVD3) and RV outflow tract (RVOT)], right atrium major and minor dimension, right atrial pressure (RAP) and inferior vena cava (IVC) diameter. Pulmonary artery (PA) diameter was measured between the valve and the bifurcation point, while the PA pressures [mean, PAPM and systolic, pulmonary artery pressure (PAPS)] were derived by measuring the PA acceleration time and tricuspid regurgitation. RV systolic function was assessed by recording the TAPSE score. Left ventricular (LV) systolic and diastolic functions were defined by the ejection fraction (Simpson’s rule) and E/E’ ratio (between early mitral inflow velocity and mitral annular early diastolic velocity), respectively.
Study protocol
Baseline measurements were taken on the day prior to (day 0) and following NIV set up (day 1), at follow up assessment at six weeks (outpatient; W6) and at three months (inpatient; M3). Demographics and anthropometric parameters were recorded including age, gender, height, weight, BMI, hip, waist and neck circumference, office blood pressure (average of three measurements), oxygen saturation, temperature, respiratory rate and heart rate. The patients filled in the Medical Research Council (MRC) dyspnoea scale (27), the COPD assessment test (CAT) (28), the St George's Respiratory Questionnaire (SGRQ) (29) and the Severe Respiratory Insufficiency Questionnaire (SRI) (30). Electrocardiogram (ECG) and chest X-ray were performed routinely and were reviewed to exclude ischaemic heart disease, arrhythmias, cardiomegaly, signs of acute heart failure and respiratory co-morbidities like fibrosis or emphysema. All subjects underwent standard spirometry (31). Blood tests included the full blood count, the renal profile, thyroid stimulating hormone (TSH), N-terminal pro-brain natriuretic peptide (NT-proBNP), glucose and a radial artery blood gas (ABG) analysis. At baseline, a nocturnal transcutaneous oximetry and capnography (TCM TOSCA, Radiometer Medical ApS, Bronshoj/Denmark) and a walking test (4-metre gait speed) were performed (32). EMGpara and TTE were measured prior to NIV setup.
NIV setup followed established guidelines of the Lane Fox Unit for NIV in OHS, as described elsewhere (33). The aim was to control snoring, avoid chest wall paradox with oxygen desaturations, to maintain oxygen saturation (SpO2) >88% and transcutaneous CO2 <7.0 kPa, while using nocturnal NIV. Oxygen supplementation was applied, if indicated, to maintain SpO2 >88%. NIV modes used were spontaneous/timed (S/T) and volume-assured pressure assistance (AVAPS-AE). A full face mask was used as standard interface. Following the first night of NIV usage (within 4 hours) the ABG was repeated, and EMGpara (EMGpara%max) and NRDI were measured and compared to baseline. At six weeks’ outpatient follow-up, NIV settings and usage were recorded, the patient was clinically re-assessed and NRDI measurements performed. Adherence to NIV (hours/day) and percentage of nights used were retrieved from NIV built-in software at 6 weeks and 3 months follow-up. Anthropometrics, questionnaires, ABG, spirometry and NRDI measurements were repeated. At three months inpatient follow-up, all baseline measurements and an overnight assessment were repeated, including NRDI measurements and TTE.
Sample size calculation
The sample size was calculated with an alpha of 5% and a statistical power of 90%. A standard deviation was available from previous studies using the TAPSE score (standard deviation of 3.5 and a minimum clinical significant difference of 6 mm) (26). With these parameters, a total number of 14 patients were required for this observational pre-/post-comparison study design.
Statistics
Normal distribution of the data was checked with the Shapiro-Wilk test. Data were expressed as mean (± standard deviation) for normally distributed variables, while median (interquartile range with 25th–75th percentile) were used for non-normally distributed data. Comparisons were made using paired t-test, Wilcoxon signed rank test, one way and two way repeated measures ANOVA, and McNemar’s test. Holm-Sidak and Dunn’s post hoc correction methods were used where appropriate. A P value <0.05 was considered significant. Statistical analysis was performed using SigmaPlot V13.0 statistical software (Systat Software Inc., CA, USA).
Results
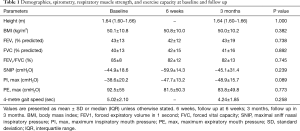
Sixteen patients were recruited at baseline with two drop-outs (Figure 1). The anthropometric, demographic, and respiratory function data (spirometry, respiratory muscle strength, and exercise capacity) of the 14 patients (8 females, age 53.3±9.9 years) who completed the study did not change during the observation period (Tables 1 and S1).
Full table

Full table
Arterial blood gas analysis
The arterial blood gas analysis confirmed chronic compensated HRF at baseline with an improvement in the pCO2 and bicarbonates at 3 months (Table 2).

Full table
Venous blood tests
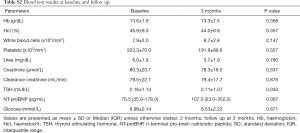
The patients were not anaemic, had no significant infection or renal problems; one patient had an abnormal elevated serum creatinine level. The NTpro BNP levels were increased in 6 patients. Although the TSH changed slightly, it remained within the normal range during the study period (Table S2).
Full table
NIV and nocturnal transcutaneous capnography
At baseline, 12 patients received NIV with an S/T mode, while two patients were set up on AVAPS-AE; the pressure settings were not significantly altered over the three months follow up period. Three patients required supplemental oxygen (1 L/min by day and 2 L/min by night) at initial discharge, but were weaned off oxygen over the follow up period. At six weeks and three months follow up, only 6 patients used NIV longer than 4 hours/day (compliant patients). There were no differences in adherence at 6 weeks and 3 months. Two patients did not use NIV at all at three months, and one patient utilised NIV less than 1 hour daily, while 4 patients used the NIV 3–4 hours daily. One patient was non-compliant at six weeks and became more compliant during further follow up.
Night-time ventilation improved respiratory control over time with higher average oxygenation, whilst oxygen desaturations were diminished. Transcutaneous CO2 monitoring indicated that the maximal tCO2 was lower at three months than the mean tCO2 at baseline (Table 3). Efficacy of the ventilation was also confirmed by the improved daytime ABG results (Table 2) and the weaning of supplemental oxygen in all patients.

Full table
Dyspnoea and quality of life
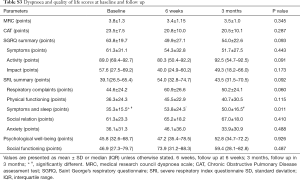
The MRC dyspnoea and SGRQ scores did not change over time, but the SRI indicated an improvement in “attendant symptoms and sleep” at 6 weeks and 3 months (Table S3).
Full table
Parasternal intercostal electromyography
NRDI improved significantly over time (Table 4). NIV successfully offloaded the work of breathing, when comparing the electromyographic activity of the inspiratory muscles on NIV vs. off NIV. EMG raw (D1 P=0.002, W6 P=0.016, M3 P=0.004), EMG %max (D1 P=0.001, W6 P=0.011, M3 P=0.002), and EMG NRDI (D1 P=0.014, W6 P=0.021, M3 P=0.014) significantly improved at all follow up assessments (Figure 2).

Full table
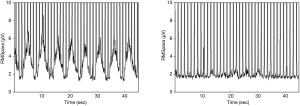
TTE
The systolic pulmonary artery pressure (PAPS) could only be assessed in 7 patients, 3 of which had a PAPS >40 mmHg. Five patients had dilated RV dimensions at baseline and at 3 months, while five had E/E’ Ratio impairment (correlated with LV diastolic dysfunction) at baseline and two at 3 months follow up; all three patients that required oxygen at baseline had LV diastolic dysfunction. LV systolic function was normal in all patients, whereas LV diastolic function was impaired in 3 patients at baseline and in 2 patients at follow up. One patient had RV impairment (Table 5).
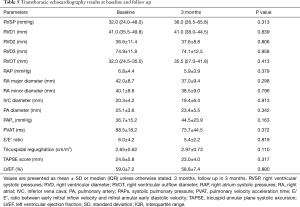
Full table
Discussion
NIV in patients with OHS improves the NRD, sleep-disordered breathing and arterial blood gases over a three-month period by offloading the work of breathing. Furthermore, it positively impacts symptoms and sleep quality. However, cardiac function, spirometry, respiratory muscle strength, and exercise capacity do not change significantly.
NIV in OHS reduces the NRD. The NRD was reduced while patients were on NIV comparing to self-ventilating. Furthermore, our data suggests that the initiation of nocturnal use of NIV reduces the NRD while self-ventilating, and this effect remains unchanged during a 3 months period. A possible explanation for this is that NIV offloads the respiratory muscles and as a result reduces the work of breathing. The effect of NIV on respiratory neural drive has been previously demonstrated in neuromuscular disease and COPD (34). To the best of our knowledge, this is the first study showing the positive effect of NIV on NRD in OHS, acutely and in the long term.
In this context, cardiac function remained unchanged. Our data show that any potential effect of NIV on the cardiovascular may be less profound than on the respiratory system. In contrast with these findings, Castro-Añón et al. screened OHS patients established on positive airway pressure therapy for PH and followed them for 6 months, using TTE (35). They demonstrated a significant decrease in pulmonary arterial pressure at 6 months. Moreover, Kauppert et al. demonstrated in 21 OHS patients established on NIV using right heart catheterization and echocardiography that pulmonary pressures decreased with better NIV compliance (36). Held et al., retrospectively identified 18 patients with hypoventilation and PH who were treated with PAP therapy (37). They assessed PA pressure and cardiac function using right heart catheterization and echocardiography, and found significant improvements in mean and systolic PA pressures, pulmonary vascular resistance, right ventricular systolic function and improvements in walking distance at 3 months follow up. Possible explanations for the discrepancy with the aforementioned studies are the use of right heart catheterization, different compliance and follow up periods.
The data of this study support the hypothesis that the positive treatment effect of NIV on the respiratory system does not significantly impact on the pathophysiology of the cardiovascular system in the short term. This is limited by the relatively short follow up, limited sample size, lack of invasive PA pressure measurement and observation of patients who had relatively preserved RV function. Larger cohort studies of patients with OHS will be necessary to define improved clinical outcomes on NIV, particularly in patients who have significant right heart strain due to PH. Moreover, as there are sparse data regarding long-term NIV treatment (38-39) in OHS, extended observation periods are needed. Furthermore, invasive measurements of pulmonary pressures and cardiac function might better elucidate the role of right ventricular function and PH in the pathophysiology of OHS in order to identify relevant future treatment targets and outcomes. This could include specific targeting of patients who have an insufficient response to NIV therapy beyond the initial treatment period, as right heart failure significantly impacts on long-term outcomes and can, potentially, be modified using drug specific treatment for PH.
Conclusions
NIV controls HRF and improves NRD in OHS in the domiciliary setting. Any potential impact on the cardiovascular system might be less responsive to NIV than the respiratory system.
Acknowledgments
Dr. A Onofri’s contribution has been supported by the University of Padua, Italy. Dr. G Kaltsakas is a European Respiratory Society Fellowship recipient (STRTF 2015–9638). Dr. J Steier’s contribution was partially supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We gratefully acknowledge the financial support of the Lane Fox Respiratory Unit Patient Association.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: It was approved by London City & East NHS Research Ethics Committee (reference: 15/LO/1,390). Informed and written consent was obtained from all patients.
References
- GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13-27.
- Steier J, Lunt A, Hart N, et al. Observational study of the effect of obesity on lung volumes. Thorax 2014;69:752-9. [Crossref] [PubMed]
- Steier J, Jolley CJ, Seymour J, et al. Neural respiratory drive in obesity. Thorax 2009;64:719-25. [Crossref] [PubMed]
- Steier J, Martin A, Harris J, et al. Predicted relative prevalence estimates for obstructive sleep apnoea and the associated healthcare provision. Thorax 2014;69:390-2. [Crossref] [PubMed]
- Piper AJ, Grunstein RR. Obesity Hypoventilation syndrome. mechanisms and management. Am J Respir Crit Care Med 2011;183:292-8. [Crossref] [PubMed]
- Shetty S, Parthasarathy S. Obesity hypoventilation syndrome. Curr Pulmonol Rep 2015;4:42-55. [Crossref] [PubMed]
- Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care 2010;55:1347-62; discussion 1363-5. [PubMed]
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55. [Crossref] [PubMed]
- Bülbül Y, Ayik S. Frequency and predictors of obesity hypoventilation in hospitalized patients at a tertiary health care institution. Ann Thorac Med 2014;9:87-91. [Crossref] [PubMed]
- Reed K, Pengo MF, Steier J. Screening for sleep-disordered breathing in a bariatric population. J Thorac Dis 2016;8:268-75. [PubMed]
- Mokhlesi B, Tulaimat A, Faibussowitsch I, et al. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007;11:117-24. [Crossref] [PubMed]
- Freedman DS, Khan LK, Serdula MK, et al. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA 2002;288:1758-61. [Crossref] [PubMed]
- Friedman SE, Andrus BW. Obesity and pulmonary hypertension: a review of pathophysiologic mechanisms. J Obes 2012;2012:505274. [Crossref] [PubMed]
- Dela Cruz CS, Matthay RA. Role of obesity in cardiomyopathy and pulmonary hypertension. Clin Chest Med 2009;30:509-23. ix. [Crossref] [PubMed]
- Segers VF, Brutsaert DL, De Keulenaer GW. Pulmonary hypertension and right heart failure in heart failure with preserved left ventricular ejection fraction: pathophysiology and natural history. Curr Opin Cardiol 2012;27:273-80. [Crossref] [PubMed]
- Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med 2007;3:409-15. [PubMed]
- McQuillan BM, Picard MH, Leavitt M, et al. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001;104:2797-802. [Crossref] [PubMed]
- Taraseviciute A, Voelkel NF. Severe pulmonary hypertension in postmenopausal obese women. Eur J Med Res 2006;11:198-202. [PubMed]
- Hunt SA. American College of Cardiology, American Heart Association Task Force on Practice Guidelines. (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American college of Cardiology/American heart association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure). J Am Coll Cardiol 2005;46:e1-82. [Crossref] [PubMed]
- Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet 2009;374:250-9. [Crossref] [PubMed]
- Kallet RH, Diaz JV. The physiologic effects of noninvasive ventilation. Respir Care 2009;54:102-15. [PubMed]
- Priou P, Hamel JF, Person C, et al. Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest 2010;138:84-90. [Crossref] [PubMed]
- Castro-Añón O, Pérez de Llano LA, De la Fuente Sánchez S, et al. Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS One 2015;10:e0117808. [Crossref] [PubMed]
- Budweiser S, Riedl SG, Jörres RA, et al. Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med 2007;261:375-83. [Crossref] [PubMed]
- Steier J, Kaul S, Seymour J, et al. The value of multiple tests of respiratory muscle strength. Thorax 2007;62:975-80. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref]
- Stenton C. The MRC breathlessness scale. Occup Med (Lond) 2008;58:226-7. [Crossref] [PubMed]
- Dodd JW, Hogg L, Nolan J, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 2011;66:425-9. [Crossref] [PubMed]
- Jones PW, Quirk FH, Baveystock CM. The St George's respiratory questionnaire. Resp Med 1991;85:25-31; discussion 33-7.
- Ghosh D, Rzehak P, Elliott MW, et al. Validation of the English severe respiratory insufficiency questionnaire. Eur Respir J 2012;40:408-15. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Kon SS, Patel MS, Canavan JL, et al. Reliability and validity of 4-metre gait speed in COPD. Eur Respir J 2013;42:333-40. [Crossref] [PubMed]
- Hodgson LE, Murphy PB. Update on clinical trials in home mechanical ventilation. J Thorac Dis 2016;8:255-67. [PubMed]
- Ramsay M, Mandal S, Suh ES, et al. Parasternal electromyography to determine the relationship between patient-ventilator asynchrony and nocturnal gas exchange during home mechanical ventilation set-up. Thorax 2015;70:946-52. [Crossref] [PubMed]
- Castro-Añn O, Golpe R, Pérez-de-Llano LA, et al. Haemodynamic effects of non-invasive ventilation in patients with obesity-hypoventilation syndrome. Respirology 2012;17:1269-74. [Crossref] [PubMed]
- Kauppert C, Dvorak I, Kollert F, et al. Pulmonary hypertension in obesity-hypoventilation syndrome. Respir Med 2013;107:2061-70. [Crossref] [PubMed]
- Held M, Walthelm J, Baron S, et al. Functional impact of pulmonary hypertension due to hypoventilation and changes under non invasive ventilation. Eur Respir J 2014;43:156-65. [Crossref] [PubMed]
- Combs D, Shetty S, Parthasarathy S. Advances in positive airway pressure treatment modalities for hypoventilation syndromes. Sleep Med Clin 2014;9:315-25. [Crossref] [PubMed]
- Lemyze M, Taufour P, Duhamel A, et al. Determinants of NIV success or failure in morbidly obese patients in acute respiratory failure. PLoS One 2014;9:e97563. [Crossref] [PubMed]