Disseminated intravascular coagulopathy caused by type II endoleak after endovascular aneurysm repair in severe aortic stenosis
Introduction
Disseminated intravascular coagulopathy (DIC) has been found to occur in patients with abdominal aortic aneurysm (AAA), but it rarely developed after endovascular aneurysm repair (EVAR). We describe an extremely rare case of DIC associated with type II endoleak after EVAR.
Case presentation
A 72-year-old man with a history of hypertension underwent EVAR of an 8-cm large AAA at another hospital 5 years ago. Six months postoperatively, he was diagnosed with severe aortic stenosis (AS) and atrial fibrillation; however, the patient refused aortic valve replacement.
Four years later, follow-up computed tomography (CT) demonstrated a type I endoleak that was treated with another endovascular stent graft extension.
On presentation at our hospital, the patient complained of dizziness and palpitation, and initial laboratory findings revealed a white blood cell count of 4,710/µL, a hemoglobin of 6.5 gm/dL, a platelet count of 74,000/µL, blood urea nitrogen 37.2 mg/dL, creatinine 1.6 mg/dL and brain natriuretic peptide 2,351 pg/dL. DIC profiles were antithrombin III 49.7% (normal, 69.6–120.6%), fibrinogen 79.8 (normal, 180–380) mg/dL, fibrinogen degradation product 62.7 (normal, 0–5) µg/mL, D-dimer 23.01 µg/mL and DIC score was 7. Anti-platelet antibody was negative. Serum levels of SGOT/SGPT were normal (32/18) U/L. Electrocardiography revealed atrial fibrillation with a slow ventricular response, and echocardiography demonstrated severe AS with a mean pressure gradient of 92 mmHg, moderate tricuspid regurgitation and an ejection fraction of 60%.
An abdominal aortic computed tomographic angiography revealed a 10.7-cm infrarenal AAA with endovascular stent and aneurysmal sac enlargement.
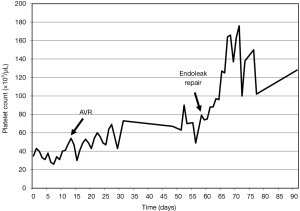
Despite platelet transfusion several times before aortic valve surgery, his platelet count had not recovered (Figure 1). A few weeks after admission, his aortic valve was replaced with a bioprosthetic valve (St. Jude Medical, In., St. Paul, MN, USA). His tricuspid valve was also repaired with a Saint Jude tailored ring (St. Jude Medical, In., St. Paul, MN, USA), and a modified Cox-Maze procedure was performed. Forty-five days later, a midline laparotomy was performed. Aortic cross clamp was prepared but the aneurysmal sac was opened without cross clamp and no leakage on the proximal end of the stent graft was found. The previous endovascular stent graft was intact, and an old hematoma and sludge filled the aneurysmal sac. Bleeding from several lumbar arteries was identified and stopped by oversewing, and the remnant sac was tailored and closed.
A postoperative CT demonstrated no evidence of the endoleak and the reduced size of the aneurysmal sac.
The patient’s postoperative course was uneventful, the platelet count gradually increased after repair of the type II endoleak (Figure 1) and the DIC profiles normalized. He was discharged without complications. Three months after repair of the type II endoleak, the patient passed away with sepsis due to prosthetic valve endocarditis with no evidence of recurrent endoleak.
Discussion
DIC is a well-known complication associated with aortic aneurysm, especially in cases of rupture or dissection of the aorta (1). An incidence of DIC of 3–4% has been reported; DIC usually occurs perioperatively and increases the postoperative morbidity and mortality.
EVAR has become a less invasive therapeutic alternative to open aneurysm repair for AAAs. Although a significant reduction in the 30-day mortality has been seen compared with open repair; the long-term follow-up results have shown that the early benefit is lost over time. Type I and II endoleaks with sac expansion, type III endoleak, stent migration and kinking have been associated with an increased risk of rupture that may account for the loss of the early benefit after EVAR (2).
Type II endoleaks are the most common ones after EVAR. According to a systemic review, type II endoleak are seen in 10.2% of patients after EVAR. The natural history of type II endoleaks is uncertain, and their management remains controversial. Sidloff et al. (2) reported 35.4% of spontaneous resolution in 1,515 patients with type II endoleak; however, 14 patients (0.9%) had a ruptured abdominal aortic aneurysm and six of these ruptured in the absence of sac expansion.
Consumptive coagulopathy following type I or III endoleak after EVAR has rarely been reported (3,4); however, DIC associated with type II endoleak has not been reported.
The pathogenesis of endoleak-induced DIC has been proposed previously (1,3-4). Turbulent blood flow liberates coagulated material from the aneurysmal sac, exposing denuded endothelium and tissue factor, leading to activation of coagulation factors, excess generation of thrombin, chronic consumption of clotting factors and simultaneous excess plasmin generation and fibrinolysis of the clots. In our patient, turbulent flow was originated from the lumbar arteries perturbed the endothelium and produced platelet activating factor and stimulated procoagulant activity.
Patients with severe AS also have impaired platelet function that originates from mechanical obstruction of blood flow and consecutive proteolysis of von Willebrand factor (5). Our patient also had severe AS combined with type II endoleak. We could not ascertain the main cause of the thrombocytopenia. Serial platelet counts after aortic valve replacement (AVR) and repair of the type II endoleak reveal that both are related to the thrombocytopenia. However, a distinct improvement of the platelet count and resolution of the DIC profile after successful elimination of the type II endoleak support the notion that DIC was mainly caused by the endoleak.
In conclusion, type II endoleak after EVAR can lead to DIC, which can be treated by repair of the endoleak.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Fernandez-Bustamante A, Jimeno A. Disseminated intravascular coagulopathy in aortic aneurysms. Eur J Intern Med 2005;16:551-60. [Crossref] [PubMed]
- Sidloff DA, Stather PW, Choke E, et al. Type II endoleak after endovascular aneurysm repair. Br J Surg 2013;100:1262-70. [Crossref] [PubMed]
- Keo HH, Diehm N, Baumgartner I, et al. Disseminated intravascular coagulopathy caused by endoleak type I: Successful treatment by endovascular stent-graft extension. EJVES Extra 2006;12:68-70. [Crossref]
- Cross KS, Bouchier-Hayes D, Leahy AL. Consumptive coagulopathy following stent repair of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2000;19:94-5. [Crossref] [PubMed]
- Steinlechner B, Zeidler P, Base E, et al. Patients with severe aortic valve stenosis and impaired platelet function benefit from preoperative desmopresson infusion. Ann Thorac Surg 2011;91:1420-6. [Crossref] [PubMed]