Impact of maximum standardized uptake value of non-small cell lung cancer on detecting lymph node involvement in potential stereotactic body radiotherapy candidates
Introduction
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer death worldwide (1). The increasing detected rate of early stage non-small cell lung cancer (NSCLC) is due to low-dose computer tomography screening during the past decade (2). For resectable NSCLC, anatomical lobectomy with systematic lymph node dissection is considered as the standard treatment (3). Stereotactic body radiotherapy (SBRT, also called stereotactic ablative therapy) is a new approach to treating early stage lung cancer, especially for elderly patients with poor cardiopulmonary function. Meanwhile, SBRT have shown a promising result for treating operable stage I NSCLC in several studies (4-6). However, local recurrence, which might attribute to the occult lymph node metastasis, is one of the most controversial issues (6-8). It is important to rule out lymph node involvement when selecting SBRT candidates.
18F-fluorodeoxyglucose positron emission tomography integrated with computed tomography (FDG-PET/CT) has been widely used in malignant tumor preoperative staging work-up, especially in lung cancer (9). FDG-PET/CT provided morphological information and FDG uptake to distinguish the benign and malignant lymph node. Increasing evidence supported the clinical value of FDG-PET/CT for mediastinal lymph node staging. FDG-PET/CT had been performed to exclude nodal positive NSCLC patients in cohort of SBRT studies, but lymph node involvement was found in 7.6–19.2% patients (6,10-12). Several studies had identified that the maximum standardized uptake value (SUVmax) of primary tumor was a significant risk factor of occult lymph node metastasis for clinical N0 NSCLC (10-14). However, the conclusion was not widely accepted (15,16).
The International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS) and the European Respiratory Society (ERS) proposed a new historical classification of lung adenocarcinoma in 2011 (17). Haruhiko and his colleagues found that SUVmax of primary tumor reflected the malignant grade and associated with adenocarcinoma patterns (18). Meanwhile, micropapillary and solid patterns were considered as poor prognostic factors, and associated with occult lymph node metastasis (19,20).
Therefore, in this study, we aim to retrospectively investigate the association between clinicalpathological variables, especially the SUVmax of primary tumor, and lymph node involvement in potential SBRT candidates with clinical stage I NSCLC (cT1-2aN0M0).
Methods
We retrospectively reviewed the records of 537 NSCLC patients who underwent radical surgical resection from May 2010 to December 2015 at the department of Thoracic Surgery II, Peking University Cancer Hospital. All patients received contrast-enhanced chest CT scan, FDG-PET/CT and brain magnetic resonance imaging for preoperative staging work-up. A total of 185 patients with clinical stage I NSCLC were enrolled in the current study. Inclusion criteria were as follows: tumor less than 4 cm, identified clinical N0 based on CT and FDG-PET/CT imaging, underwent lobectomy with systematic lymph node dissection.
Lymph nodes with a short-axis ≥1 cm on CT or SUVmax ≥2.5 on FDG-PET/CT were considered as nodal involvement. We defined systematic lymph node dissection according to the American College of Surgeons Oncology Group (ACOSOG) Z0030 criteria, which required removing lymphatic tissue from stations 2R, 4R, 7, and 10R for right-sided tumors and from stations 5, 6, 7, and 10L for left-sided tumors. The remaining of N1 nodes (11-14) were removed as part of the resection specimen.
Patient clinicalpathological characteristics were collected from the medical records. All chest CT images and FDG-PET/CT reports were reviewed for tumor size, tumor location, nodule type, clinical stage and SUVmax. Tumor was considered peripheral if located within the outer two-thirds of the lung on CT scan. Nodule types were classified into ground-glass opacity (GGO) group and solid group. Tumors with GGO ratio more than 50% were divided into GGO group. Determination of clinical stage was based on the 8th TNM classification of the International Union Against Cancer and the American Joint Committee on Cancer (UICC/AJCC). Pathological reports were reviewed for tumor histology, adenocarcinoma patterns, visceral pleural invasion and lymph node involvement. The new IASLC/ATS/ERS classification of adenocarcinoma was used to identify the adenocarcinoma patterns. Micropapillary and solid patterns were recorded if the percentage of histological component exceeded 5%. The retrospective study was approved by Institutional Review Board at Beijing Cancer Hospital (2015KT04). The requirement of patient consent was obtained in this retrospective study.
Student’s t-test was used to analyze for comparing continuous variables, Pearson’s chi-squared test or Fisher’s exact test was performed to assess the categorical variables. Receiver operating characteristic (ROC) analysis was performed to calculate the area under the ROC curve (AUC). Medcalc was used to compare ROC curves and calculated P value. Binary logistic regression was used to identify the risk factors for occult nodal metastasis. Spearman’s correlation test was used to calculate the correlations between clinicalpathological variables. All tests were two-sided. P values of less than 0.05 were considered to indicate statistical significant difference. The Statistical Package for the Social Sciences version 20.0 software package (SPSS, Chicago, IL, USA) was used for statistical analysis.
Results
Patient characteristics
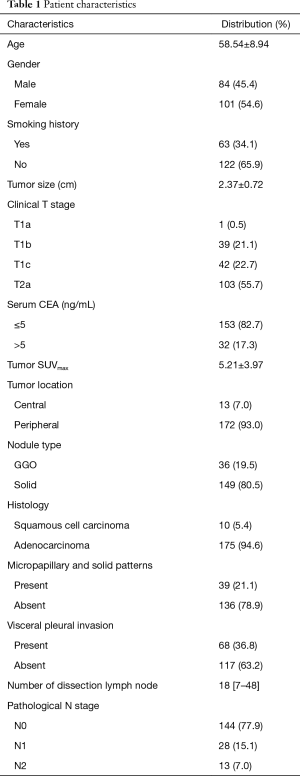
Among all patients, 84 were male and 101 were female. The mean age was 58.54, with a range from 32 to 79. One hundred and seventy-two tumors were peripheral location. Only 19.5% of tumors were divided into GGO group. For tumor histology, 175 cases were confirmed with lung adenocarcinoma, others were squamous cell carcinoma. For the historical subtypes of adenocarcinoma, a total of 39 cases were identified with micropapillary and solid component exceeded 5%. Patients with pathological visceral pleural invasion accounted for 36.8%. The median number of lymph node dissection was 18 (range, 7–48). 90.2% patients had station 13 lymph nodes, 38.9% patients had station 14 lymph nodes. Forty one patients had occult lymph node metastasis, 28 were N1 and 13 were N2. Twenty-six patients confirmed lymph node metastasis in station 13 or 14. Patient characteristics were listed in Table 1.
Full table
Univariate analysis of risk factors for lymph node involvement
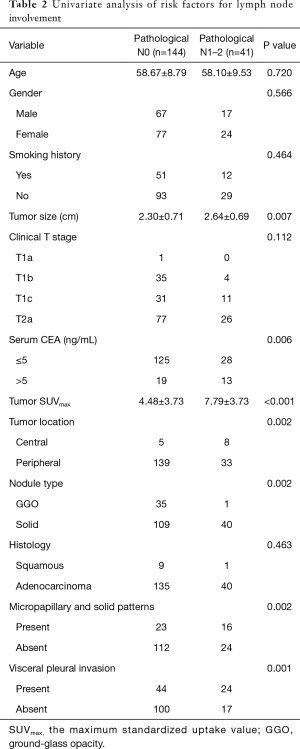
The association between clinicalpathological variables and lymph node involvement was investigated (Table 2). No significant differences were found in demographic data, including age, gender and smoking history. The mean tumor size was larger in those with lymph node involvement (2.30 vs. 2.64 cm, P=0.007). Clinical T stage had no association with nodal metastasis (P=0.112). Among the 32 patients with preoperative serum CEA >5 ng/mL, 13 were confirmed node-positive, while 28 of the remaining 153 patients with normal serum CEA had pathological lymph node involvement (P=0.006). There was a significant difference in SUVmax of primary tumor between pathological N0 and N1−2 (4.48 vs. 7.79, P<0.001). For central location tumors, 61.5% were identified with lymph node metastasis, while 19.2% had pathological N1–2 in peripheral location (P=0.002). Among the 36 patients of GGO group, only one was confirmed with nodal metastasis, comparing to 40 of 149 in solid nodule (P=0.002). There was no association in tumor histology (P=0.463). Patients with visceral pleural invasion tended to have a higher rate of lymph node metastasis (P=0.001). For lung adenocarcinoma patients, the presence of micropapillary and solid patterns was association with occult lymph node involvement (P=0.002).
Full table
Multivariate analysis of risk factors for lymph node involvement
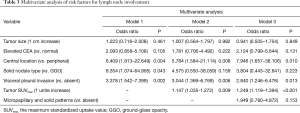
Tumor size and SUVmax was analyzed as continuous variables. The role of tumor SUVmax in predicting lymph node involvement was compared between Model 1 and Model 2 (Table 3). In multivariate analysis, SUVmax of primary tumor was not analyzed in Model 1, the result showed that central location (OR 6.409, 95% CI: 1.813–22.649, P=0.004), solid nodule (OR 8.354, 95% CI: 1.074–64.995, P=0.043) and visceral pleural invasion (OR 3.378, 95% CI: 1.542–7.399, P=0.002) were independent predictors for pathological lymph node involvement. In Model 2 with primary tumor SUVmax, central location (OR 5.784, 95% CI: 1.584–21.114, P=0.008), SUVmax (increase of 1 unite, OR 1.147, 95% CI: 1.035–1.272, P=0.009) and visceral pleural invasion (OR 3.044, 95% CI: 1.369–6.769, P=0.006) were significantly associated with lymph node involvement.
Full table
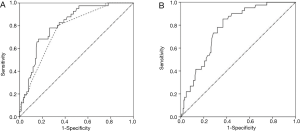
ROC curve was performed. Model 2 (AUC =0.811, 95% CI: 0.744–0.878) showed a little better diagnostic accuracy than Model 1 (AUC =0.766, 95% CI: 0.690-0.841), statistical significant difference was not exhibited (P=0.065) (Figure 1A). In addition, ROC area under the curve of primary tumor SUVmax for lymph node involvement was 0.770 (95% CI: 0.698–0.841) (Figure 1B). An optimal cut-off value was identified as 4.45 by ROC curve, the sensitivity and specificity were 85.4% and 63.2%, respectively.
Multivariate analysis of 175 lung adenocarcinomas indicated that the following three preditors were significant association with lymph node metastasis: central location (OR 7.946, 95% CI: 1.657–38.106, P=0.010), SUVmax (increase of 1 unite, OR 1.249, 95% CI: 1.119−1.394, P<0.001), and visceral pleural invasion (OR 2.840, 95% CI: 1.246–6.476, P=0.013), as shown in Model 3. The presence of micropapillary and solid patterns was not significant association with pathological node-positive (P=0.153) in multivariate analysis.
Correlations between SUVmax and other variables
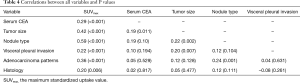
The correlations between clinicalpathological variables and SUVmax were calculated by Spearman’s correlation test. Only moderate correlation was found between nodule type and SUVmax (r=0.59, P<0.001). Tumor size was related to SUVmax (r=0.42, P<0.001). For lung adenocarcinoma, correlation between adenocarcinoma pattern and SUVmax of primary tumor was 0.36 (P<0.001). Serum CEA, visceral pleural invasion and histology revealed weak correlations with SUVmax (Table 4).
Full table
Discussion
Except of radical lobectomy, SBRT was acceptable for stage I NSCLC patients with limited pulmonary function and inoperable medical condition (4-6). However, conflicting results of local recurrence and overall survival were reported in several previous studies (5,6,21). Controversy remained about the lymph node involvement in these clinical N0 patients. Although FDG-PET/CT was applied to screening out node-positive patients, occult lymph node involvement was unavoidable (10-12). Therefore, in this study, we retrospectively assessed the association between clinicalpathological variables and lymph node involvement in potential SBRT candidates.
Central location tumors were inclined to have pathological node-positive in our study. The incidence of occult lymph node metastasis was 61.5% for central location, only 19.2% for peripheral location. The results were consistent with the findings in previous reported studies (15,16,22,23). Therefore, selecting patients with tumor centrally located for SBRT should be more careful to rule out lymph node involvement.
Visceral pleural invasion was considered as a poor prognostic factor in NSCLC, and correlated with higher frequency of lymph node involvement (24,25). Gorai and his colleagues confirmed that the incidence of skip N2 was associated with visceral pleural invasion in clinical IA NSCLC patients (26). The similar result was obtained in the current study, visceral pleural invasion was an independent risk factor for pathological nodal involvement.
FDG-PET/CT played an important role in lymph node staging, preoperative evaluation of lymph node condition mostly depended on lymph node FDG uptake. Moreover, the SUVmax of primary tumor could predict lymph node involvement in several studies. Downey and his colleagues identified that patients with lymph node involvement had a higher SUVmax of primary tumor than patients without nodal involvement (27). In 2011, Kanzaki and his colleagues showed that SUVmax of primary tumor was the risk factor in occult mediastinal lymph node metastasis for NSCLC patients with clinical N0–1 (28). Li illustrated that primary tumor SUVmax would help for selecting patients for SBRT (29).
In our retrospective study, we found that the risk of lymph node metastasis in clinical N0 patients with tumor less than 4 cm was related to primary tumor SUVmax. Multivariate analysis confirmed primary tumor SUVmax was an independent predictor for occult nodal disease. Comparing the prediction models of lymph node involvement with ROC curve, model with the factor of SUVmax displayed higher efficiency than model without. There were 69 patients whose primary tumors were less than 2 cm, nine patients had occult lymph node metastasis, six were N1 and three were N2. Univariate analysis showed that elevated serum CEA and primary tumor SUVmax were associated with occult lymph node involvement. Therefore, primary tumor SUVmax might be applied for eliminating lymph node involvement for clinical stage I NSCLC patients. Although a controversial conclusion was obtained in Akthar study, which showed that occult lymph node involvement was not associated with tumor SUVmax, only 79 of 105 patients tumor SUVmax were described, and only eight pathological node-positive patients happened in their series, so the negative result should be referred carefully (15).
Correlations between primary tumor SUVmax and tumor biological behaviors were explored. In Suzawa and colleagues study, they revealed that tumor size correlated significantly with SUVmax, irrespective of the histology (30). In our series, we detected that nodule type was median correlation with tumor SUVmax, a weak correlation was found with tumor size. These findings suggested that the FDG uptake of primary tumor were mostly dependent on tumor size and nodule type. Hence, SUVmax of primary tumor might reflect tumor malignant grade.
Tumor size was widely used in selecting potential patients for SBRT, which was suitable for clinical N0 patients with tumor less than 5 cm. Hung and colleagues identified that tumor size was a good predictor of lymph node metastasis in lung adenocarcinoma of 3 cm or smaller (31). In 2013, Chen showed that larger tumor size was found in patients with mediastinal lymph node disease than those without mediastinal lymph node involvement (22). However, primary tumor size in predicting lymph node involvement was not widely accepted. The percentage of pathological nodal positive was 17.1% in the cases of small tumor less than 2 cm in Nakamura study (13). Likewise, we detected that the whole tumor size was larger in those with lymph node involvement, but there was not significance in multivariate analysis when considered as a continuous variable.
In Tsutani and colleagues study, solid tumor size became a predictor of nodal involvement rather than whole tumor size, and they confirmed that the solid component in tumor promoted the presence of lymph node metastasis (11). Miyasaka reported that the consolidation/tumor diameter ratio was related to pathological nodal involvement in clinical N0 NSCLC patients (12). In this study, only one of thirty-six patients with GGO staged into N2, who was confirmed with intrapulmonary and mediastinal lymph node involvement at the same time. A higher rate of nodal disease was found in solid tumor. However, we detected that solid nodule type was median correlation with tumor SUVmax, and the role of solid nodule type in predicting lymph node metastasis was neglected when taking into account primary tumor SUVmax.
Several studies focused on the association between history and lymph node involvement. Comparing with squamous cell lung cancer, lung adenocarcinoma was more possible to find out nodal involvement (28). For adenocarcinoma, the presence of micropapillary and solid patterns promoted lymph node metastasis (20,31). In our study, we found that tumor histology was not associated with lymph node involvement. Then, the association between adenocarcinoma patterns and lymph node metastasis was analyzed, the presence of micropapillary and solid patterns was significantly association with lymph node involvement in univariate analysis, but it was not considered as an independent predictor. Meanwhile, a weak correlation was found between primary tumors SUVmax with adenocarcinoma patterns.
Certainly, this is a retrospective single institution study to explored risk factors of lymph node involvement. We identified the SUVmax of primary tumor could predict lymph node involvement, further prospective survey should conduct to verify the conclusion. In the current series, consecutive patients was enrolled based on the criteria of patients with clinical stage I NSCLC (cT1-2aN0M0), but the number of patients with central location and squamous cell lung cancer was too small, investigations with a larger scale of patients was need to control selection bias. Visceral pleural invasion was an independent predictor, rather than the evaluation of visceral pleural invasion from CT and FDG-PET/CT. Hence, the role of visceral pleural invasion in predicting lymph node involvement was limited before surgery.
In conclusion, primary tumor SUVmax was a predictor of lymph node involvement for patients with clinical stage I NSCLC (cT1-2aN0M0). Moreover, primary tumor SUVmax was mostly dependent on tumor size and nodule type. SUVmax was a more effective and measurable predictor in evaluating occult lymph node involvement. Centrally located tumor and visceral pleural invasion were related to a higher rate of nodal metastasis. Lobectomy and systemic lymph node dissection should be performed in these patients, instead of SBRT.
Acknowledgements
Funding: This work was supported by the Capital Health Research and Development of Special (2014-2-1021), the Beijing Municipal Administration of Hospitals Clinical Medicine Development Special Fund (ZYLX201509) and the Peking University (PKU) 985 Special Fund for Collaborative Research with PKU Hospitals.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board at Beijing Cancer Hospital (2015KT04) and written informed consent was obtained from all patients.
References
- Torre LA, Bray F, Siegel RL, et al. Global Cancer Statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Zhang X, Liu H, Balter P, et al. Positron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:1558-65. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Lardinois D, Walter W, Thomas FH. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500-7. [Crossref] [PubMed]
- Park SY, Yoon JK, Park KJ, et al. Prediction of occult lymph node metastasis using volume-based PET parameters in small-sized peripheral non-small cell lung cancer. Cancer Imaging 2015;15:21. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Prediction of pathologic node-negative clinical stage IA lung adenocarcinoma for optimal candidates undergoing sublobar resection. J Thorac Cardiovasc Surg 2012;144:1365-71. [Crossref] [PubMed]
- Miyasaka Y, Suzuki K, Takamochi K, et al. The maximum standardized uptake value of fluorodeoxyglucose positron emission tomography of the primary tumour is a good predictor of pathological nodal involvement in clinical N0 non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:83-7. [Crossref] [PubMed]
- Nakamura H, Saji H, Marushima H, et al. Standardized Uptake Values in the Primary Lesions of Non-Small-Cell Lung Cancer in FDG-PET/CT Can Predict Regional Lymph Node Metastases. Ann Surg Oncol 2015;22:S1388-S1393. [Crossref] [PubMed]
- Casiraghi M, Travaini LL, Maisonneuve P, et al. Lymph node involvement in T1 non-small-cell lung cancer: could glucose uptake and maximal diameter be predictive criteria? Eur J Cardiothorac Surg 2011;39:e38-43. [Crossref] [PubMed]
- Akthar AS, Ferguson MK, Koshy M, et al. Limitations of PET/CT in the Detection of Occult N1 Metastasis in Clinical Stage I(T1-2aN0) Non-Small Cell Lung Cancer for Staging Prior to Stereotactic Body Radiotherapy. Technol Cancer Res Treat 2017;16:15-21. [Crossref] [PubMed]
- Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008;33:104-9. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Nakamura H, Saji H, Shinmyo T, et al. Close association of IASLC/ATS/ERS lung adenocarcinoma subtypes with glucose-uptake in positron emission tomography. Lung Cancer 2015;87:28-33. [Crossref] [PubMed]
- Cha MJ, Lee HY, Lee KS, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 2014;147:921-928.e2. [Crossref] [PubMed]
- Hung JJ, Yeh YC, Jeng WJ, et al. Factors predicting occult lymph node metastasis in completely resected lung adenocarcinoma of 3 cm or smaller. Eur J Cardiothorac Surg 2016;50:329-36. [Crossref] [PubMed]
- Puri V, Crabtree TD, Kymes S, et al. A comparison of surgical intervention and stereotactic body radiation therapy for stage I lung cancer in high-risk patients: a decision analysis. J Thorac Cardiovasc Surg 2012;143:428-36. [Crossref] [PubMed]
- Chen K, Yang F, Jiang G, et al. Development and validation of a clinical prediction model for N2 lymph node metastasis in non-small cell lung cancer. Ann Thorac Surg 2013;96:1761-8. [Crossref] [PubMed]
- Lee PC, Port JL, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:177-81. [Crossref] [PubMed]
- Chang YL, Lin MW, Shih JY, et al. The significance of visceral pleural surface invasion in 321 cases of non-small cell lung cancers with pleural retraction. Ann Surg Oncol 2012;19:3057-64. [Crossref] [PubMed]
- Mimae T, Tsutani Y, Miyata Y, et al. Role of lymphatic invasion in the prognosis of patients with clinical node-negative and pathologic node-positive lung adenocarcinoma. J Thorac Cardiovasc Surg 2014;147:1820-6. [Crossref] [PubMed]
- Gorai A, Sakao Y, Kuroda H, et al. The clinicopathological features associated with skip N2 metastases in patients with clinical stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;47:653-8. [Crossref] [PubMed]
- Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 Fluorodeoxyglucose-Positron Emission Tomography Maximal Standardized Uptake Value Predicts Survival After Lung Cancer Resection. J Clin Oncol 2004;22:3255-60. [Crossref] [PubMed]
- Kanzaki R, Higashiyama M, Fujiwara A, et al. Occult mediastinal lymph node metastasis in NSCLC patients diagnosed as clinical N0-1 by preoperative integrated FDG-PET/CT and CT: Risk factors, pattern, and histopathological study. Lung Cancer 2011;71:333-7. [Crossref] [PubMed]
- Li L, Ren S, Zhang Y, et al. Risk factors for predicting the occult nodal metastasis in T1-2N0M0 NSCLC patients staged by PET/CT: potential value in the clinic. Lung Cancer 2013;81:213-7. [Crossref] [PubMed]
- Suzawa N, Ito M, Qiao S, et al. Assessment of factors influencing FDG uptake in non-small cell lung cancer on PET/CT by investigating histological differences in expression of glucose transporters 1 and 3 and tumour size. Lung Cancer 2011;72:191-8. [Crossref] [PubMed]
- Zhang Y, Sun Y, Shen L, et al. Predictive factors of lymph node status in small peripheral non-small cell lung cancers: tumor histology is more reliable. Ann Surg Oncol 2013;20:1949-54. [Crossref] [PubMed]


