CT angio for the evaluation of graft patency
Introduction
In spite of the growth of percutaneous myocardial revascularization during the last years, the role of surgical myocardial revascularization is still well established, along with known better long-term results than percutaneous revascularization in specific groups of patients. This fact, associated with the long-term results of current techniques of total arterial myocardial revascularization, makes evaluation of graft patency largely unavoidable. Traditionally, graft patency has been evaluated with invasive coronary angiography (ICA) but, since the advent of multidetector computed tomography (MDCT), the temptation to use a noninvasive and widely available technique to study coronary artery bypass graft (CABG) patients has been stronger. While considering 4-slice or 16-slice MDCT as a reliable replacement of ICA for graft imaging could be questionable, the introduction of new scanners—64-slice and 128-slice upwards°™along with new scan protocols opens new perspectives in non-invasive assessment of graft patency. In this review, we will analyze the advantages and disadvantages of both invasive and non-invasive techniques, and we will explore potential future improvements in non-invasive graft imaging.
ICA
ICA, for obvious reasons, remains the preferred method in acute clinical settings such as cardiac arrest or acute myocardial infarction. Apart from that, there are issues when utilized for graft imaging.
The first one and more obvious issue is that ICA is invasive; its invasiveness bears risks of arrhythmia, stroke, dissection of the native vessel or of the graft, myocardial infarction, embolic stroke. Hence, there is a 0–2% risk of morbidity (0.05 risk of myocardial infarction, 0.07% risk of stroke and 0.43% risk of vascular complications) and 0.14–0.28% mortality risk. (1)
The second issue is technical: the coronary angiography performed for graft imaging is more difficult than for native vessels, due to the variation of location of the grafts’ ostia. This portends longer procedure times, more use of contrast, increased complication rates and lower success rate in detecting graft origin—around 79–86% (2,3). Moreover, when the first angiography is unable to fully assess graft patency, a new coronary angiography is usually performed shortly afterwards, requiring a different arterial access, increasing the radiation exposure as well as the risk of complications.
Diagnostic performance of standard MDCT
MDCT, compared with ICA, is less expensive, more rapid, less invasive, can be performed in an outpatient setting, and has a good patient acceptability.
MDCT can be more accurate for graft imaging than for coronary native arteries because of the relative immobility, the wider luminal diameter, the lower rate of calcifications. It also enables the assessment of native coronary vessels and extra-cardiac complications.
In spite of the limitations of early generation 4-, 16-, 64-slice MDCT in the assessment of distal anastomoses and run-off segments due to motion artifact as well as in the assessment of patients with irregular heart rhythm, and in spite of beam hardening artifacts by calcifications and surgical clips, and finally in spite of radiation exposure, the efficacy of the technique for the evaluation of CABG patients has been demonstrated in several studies (4).
Hamon et al. (5) analyzed 15 pooled studies conducted with 16- and 64-slice MDCT, encompassing 723 patients with a total of 2,023 grafts. They demonstrated high diagnostic accuracy (90% and 96% for 16- and 64-slice MDCT, respectively), overall sensitivity of 97.6% and overall specificity of 96.7% compared with ICA as gold standard. Even though 7.6% of graft were not assessable and some studies did not perform a complete acquisition of the entire graft course, the meta-analysis concluded that MDCT is a reliable well-established non-invasive tool for the assessment of CABG.
Jabara et al. (6) studied 50 consecutive patients with 147 grafts using 64-slice MDCT, followed by ICA. 98.6% of grafts were evaluable. Sensitivity, specificity, positive predictive value and negative predictive value were 93.3%, 100%, 100%, and 98% for graft occlusion, respectively, and 100%, 100%, 100%, and 100% for graft stenosis >50%. In this study, only 83% of distal anastomoses were accurately evaluated.
Weustink et al. (7) performed a prospective cohort study evaluating 58 patients with 64-slice MDCT. Sensitivity, specificity, positive predictive value and negative predictive value were 100%, 96%, 97%, and 100% for all grafted vessels, respectively, and 95%, 100%, 100%, and 99% for distal run-offs. Radiation dose was higher for MDCT (22.1 mSv) than for ICA (8.8 mSv).
Meyer et al. (8) in a cohort study of 131 patients using 64-slice MDCT reported 95% of evaluability of grafts even in patients with arrhythmias, but reduced diagnostic image quality with heart rate greater than 65 bpm (% of evaluable grafts ranging from 100 to 94).
A recent meta-analysis by Barbero and colleagues (9) investigated 12 studies conducted with 64-slice MDCT published between 2006 and 2012 and including a total of 959 patients. Pooled diagnostic data reported were: sensitivity 0.99, specificity 0.99, area under the curve (AUC) of 0.99 for graft occlusion; sensitivity 0.98, specificity 0.98, AUC of 0.97 for CABG stenosis >50%. The authors reported that neither the age nor the time from graft implantation had effects on diagnostic performance.
Another recent meta-analysis by Chan et al. (10) evaluated the diagnostic accuracy of 64-slice and upward MDCT versus ICA in the diagnosis of graft occlusion or stenosis. Thirty-one studies were included, involving 1,975 patients with 5,364 assessed graft. Strict patient selection and higher rates of patient exclusion differentiate this meta-analysis from previous ones. As primary endpoint, assessment of grafts for both stenosis or occlusion demonstrated sensitivity of 96.1%, specificity of 96.3%, AUC of 0.985, positive predictive value of 94.3%, and negative predictive value of 99%. As secondary endpoint, assessment of venous graft showed superior sensitivity compared to arterial grafts (97.6% vs. 89.2%, P=0.004) without differences in specificity. Two studies conducted with over-64-slice MDCT (respectively with 128-slice and 256-slice) showed no significant improvement in sensitivity but a greater specificity (98.5% vs. 96.1%, P=0.076). Six studies using dual-source CT found no significant differences in sensitivity (96.1% vs. 96.2%, P=0.596) or specificity (96.3% vs. 96.4%, P=0.807) compared to twenty studies conducted with single-source CT.
These studies demonstrated that MDCT has a high sensitivity, specificity and negative predictive value compared to ICA and it should be recognized as an accurate and non-invasive method for graft patency in symptomatic patients after CABG.
Standard protocol of MDCT scans
Standard cardiac study with 64-slice MDCT is performed with collimation of 0.6–0.625, gantry speed between 330 and 350 ms, scan voltage of 100–120 KV.
In retrospective ECG-gated studies, image data are acquired throughout the cardiac cycle and the volumetric data set is reconstructed at 10% phase intervals throughout systolic and diastolic phases. Tube current modulation decreases current during systole and early-diastole.
Prospective ECG-gated is performed by sequential axial scan mode at 70–80% of the RR interval, exclusively during ventricular diastole.
Scans are done during breath-hold from the clavicles to the lung bases in order to visualize the whole heart and the entire course of internal mammary artery.
Contrast agent is administered by means of the intravenous injection of 80–100 mL of non-ionic contrast agent (>350 mg of iodine per mL) followed by a variable amount of saline flush, through a large angiography catheter (18- or 20-gauge) into an antecubital vein at rate of 5 mL/s.
Bolus tracking is performed with a region of interest marker placed in the thoracic descending aorta and the scans are initiated with a minimal delay after reaching a predetermined attenuation threshold of 150 HU.
In patients with heart rate >70 bpm, oral metoprolol 50–75 mg is administered 45–60 minutes prior to MDCT. Coronary vasodilatation is achieved with 0.4 mg sublingual nitroglycerin 2 minutes prior to MDCT.
Finally, volume rendered images (VR) (Figures 1,2), maximum intensity projection (MIP) (Figure 3) and multiplanar reconstructions (MPR) are rendered on a dedicated workstation for post-processing of native coronary arteries and grafts.
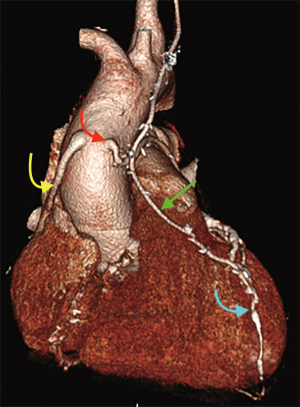
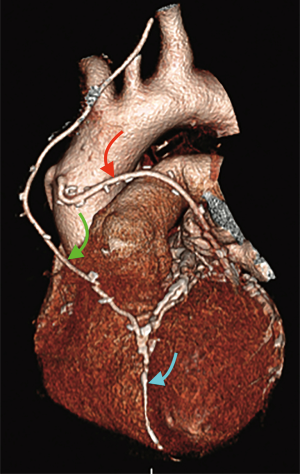
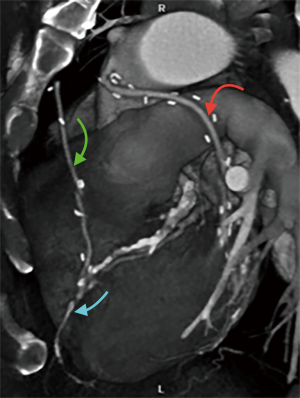
Recent developments and future perspectives
Standard 16- and 64-slice MDCT with retrospective ECG-gating and low helical pitch (0.16–0.24) are limited by radiation exposure (effective dose of approximately 9–21 mSv, greater than for routine ICA), insufficient spatial resolution (especially for evaluating distal anastomoses), insufficient temporal resolution (with motion and stair-step artifacts), beam-hardening artifacts from high-attenuating objects such as calcifications and surgical clips, and blooming artifacts from a combination of beam-hardening and partial volume effects.
These limitations have been overcome by novel CT scanners, even for the evaluation of CABG patients (11,12). New techniques include:
- ECG-controlled tube current modulation in retrospective ECG-gated helical scan (radiation dose reduced by 30–50%);
- Low-tube voltage scan (from 120 to 80 KVp with reduction of radiation dose by 30–50%);
- Prospective ECG-gated axial scan (step-and-shoot scan). Radiation is delivered only during a predetermined cardiac phase, with a mean dose of 3.5 mSv, 69% less than with retrospective ECG-gated, and reduction of blurring artifacts;
- Wide-detector (128-, 256-, 320-slice MDCT), with whole-heart coverage in a single beat, improved temporal resolution with faster gantry rotation time, reduction in radiation dose;
- High-definition CT and Iterative Reconstruction. Gemstone detectors and improved data acquisition permit an in-plane resolution of 0.23 mm. Iterative reconstruction algorithms reduce image noise related to the scan;
- High-pitch dual-source CT helical scan. Dual-source CT uses two X-rays tubes and two detectors arranged at 90-degree angle, so only a quarter rotation of the system is necessary to acquire the data. Temporal resolution (75–83 ms) is significantly improved. Also, radiation dose is reduced (less than 1 mSv) because of shortening scanning time, and reduced motion artifacts;
- Dual-energy CT, with dual-source scanner with different energy dataset or a single-source scanner with tube voltage switching. This allows beam-hardening correction and reduction of the dose of contrast agent.
These new technologies have been recently applied to graft evaluation.
Lee et al. (13) studied graft patency or stenosis in 191 patients by retrospective-ECG-gated dual-source 64-slice MDCT. The tube current modulation in caudocranial scan direction mode allowed a mean radiation dose of 6.8 mSv.
Sahiner and colleagues (14), in a prospective cohort study evaluated graft patency using dual-source 64-slice MDCT in 284 patients and reported 98.3% sensitivity and 99.3% specificity for the detection of >50% graft stenosis, 98.3% positive predictive value, 99.3% negative predictive value for arterial grafts, 100% sensitivity, 99.5% specificity, 98% positive predictive value, 100% negative predictive value for venous grafts.
Heye et al. (15) investigated image quality and diagnostic performance of computer tomography angiography (CTA) in 56 consecutive non-selected patients without pharmacological heart control, by using dual-source 64-slice MDCT. CTA was accurate in all patients group in assessing graft patency (97.9% sensitivity, 100% specificity, and 98.5% accuracy) but artifacts decreased diagnostic performance for stenosis detection (60% sensitivity, 88.6 specificity, and 84.1% accuracy).
Goetti et al. (16), in a prospective cohort study of 50 patients evaluated graft patency using dual-source 128-slice MDCT. Diagnostic image quality of grafts was obtained in 99.4%. High heart rate was associated with lower graft image quality. Effective mean radiation dose was 2.3 mSv, more than 8-fold lower than that conventional helical 64-slice MDCT.
Gramer et al. (17), judged of diagnostic quality 96.6% of overall 762 graft segments studied with dual-source 256-slice MDCT, in agreement with other published studies conducted with similar scanners. In this study, high heart-rate was not associated with degraded graft image quality.
Higashigaito et al. (18), by using dual-source 192-slice MDCT in 60 patients, reported similar diagnostic quality in different groups in whom contrast media volume was reduced by adaptive automatically selected tube voltages. Volume range was between 80 ml at 120 KVp and 48 ml at 80 KVp.
Similar results were reported for high-pitch dual-source 256-slice MDCT in two different papers (19,20), having ICA as gold standard: sensitivity of 97.1% and 92.8%, specificity of 99.6% and 99.3%, positive predictive value of 94.4% and 92.8%, negative predictive value of 99.8% and 99.3%.
Another recently highlighted advantage (2) is when MDCT is carried out in first-line before ICA. The prospective study that was conducted showed that MDCT use brought to a subsequent reduction in volume of iodinated contrast media [160.0 (IQR, 120.0–215.0) vs. 200.0 (IQR, 152.0–250.0) mL, P<0.01], as well as in the radiation dose during coronary angiography—median Kerma area product (12.1 Gy/cm2 in MDCT-first group vs. 22.0 Gy/cm2 in ICA-only group, P<0.01), fluoroscopy time (10.7 vs. 14.4 min, P<0.01), number of registered frames (550.0 vs. 764.0, P<0.02), total Air Kerma (177.0 vs. 367.0 mGy, P<0.01), and no excess in cumulative exposure [5.0 (IQR, 3.2–7.5) vs. 5.1 (IQR, 2.3–9.1), P=0.76]. This non-randomized study was aimed at assessing the usefulness and hazards of using systematically low-dose MDCT before ICA in patients awaiting CABG assessment. Within the study, three goals were set: the first one was the analysis of any additional clinical value given by performing first-line MDCT in assessing CABGs and native coronary arteries; the second goal was to test the hypothesis that the radiation dose during angiography is significantly decreased after performing first-line MDCT; the last goal was to compare the cumulative effect dose related to MDCT or ICA, or both between the two diagnostic investigations.
Conclusions
ICA will probably never be replaced in diagnosis of coronary artery disease; anyway, thanks to ongoing technological progress leading to faster scanners requiring less contrast agent and less radiation dose than ICA, MDCT will gain a prominent role with regard to the follow-up of CABG patients, coronary artery disease screening, and all those cases in which a noninvasive, quick, reliable diagnostic technique is needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gobel FL, Stewart WJ, Campeau L, et al. Safety of coronary arteriography in clinically stable patients following coronary bypass surgery. Post CABG Clinical Trial Investigators. Cathet Cardiovasc Diagn 1998;45:376-81. [Crossref] [PubMed]
- Pesenti-Rossi D, Baron N, Georges JL, et al. Assessment of coronary bypass graft patency by first-line multi-detector computed tomography. Ann Cardiol Angeiol (Paris) 2014;63:284-92. [Crossref] [PubMed]
- Trigo Bautista A, Estornell J, Ridocci F, et al. Non-invasive assessment of coronary artery bypass grafts by computed tomography: comparison with conventional coronary angiography. Rev Esp Cardiol 2005;58:807-14. [Crossref] [PubMed]
- Gabriel J, Klimach S, Lang P, et al. Should computed tomography angiography supersede invasive coronary angiography for the evaluation of graft patency following coronary artery bypass graft surgery? Interact Cardiovasc Thorac Surg 2015;21:231-9. [Crossref] [PubMed]
- Hamon M, Lepage O, Malagutti P, et al. Diagnostic performance of 16- and 64-section spiral CT for coronary artery bypass graft assessment: meta-analysis. Radiology 2008;247:679-86. [Crossref] [PubMed]
- Jabara R, Chronos N, Klein L, et al. Comparison of multidetector 64-slice computed tomographic angiography to coronary angiography to assess the patency of coronary artery bypass grafts. Am J Cardiol 2007;99:1529-34. [Crossref] [PubMed]
- Weustink AC, Nieman K, Pugliese F, et al. Diagnostic accuracy of computed tomography angiography in patients after bypass grafting: comparison with invasive coronary angiography. JACC Cardiovasc Imaging 2009;2:816-24. [Crossref] [PubMed]
- Meyer TS, Martinoff S, Hadamitzky M, et al. Improved noninvasive assessment of coronary artery bypass grafts with 64-slice computed tomographic angiography in an unselected patient population. J Am Coll Cardiol 2007;49:946-50. [Crossref] [PubMed]
- Barbero U, Iannaccone M, d'Ascenzo F, et al. 64 slice-coronary computed tomography sensitivity and specificity in the evaluation of coronary artery bypass graft stenosis: A meta-analysis. Int J Cardiol 2016;216:52-7. [Crossref] [PubMed]
- Chan M, Ridley L, Dunn DJ, et al. A systematic review and meta-analysis of multidetector computed tomography in the assessment of coronary artery bypass grafts. Int J Cardiol 2016;221:898-905. [Crossref] [PubMed]
- Machida H, Tanaka I, Fukui R, et al. Current and Novel Imaging Techniques in Coronary CT. Radiographics 2015;35:991-1010. [Crossref] [PubMed]
- Lee JH, Han D, Danad I, et al. Multimodality Imaging in Coronary Artery Disease: Focus on Computed Tomography. J Cardiovasc Ultrasound 2016;24:7-17. [Crossref] [PubMed]
- Lee JH, Chun EJ, Choi SI, et al. Prospective versus retrospective ECG-gated 64-detector coronary CT angiography for evaluation of coronary artery bypass graft patency: comparison of image quality, radiation dose and diagnostic accuracy. Int J Cardiovasc Imaging 2011;27:657-67. [Crossref] [PubMed]
- Sahiner L, Canpolat U, Yorgun H, et al. Diagnostic accuracy of dual-source 64-slice multidetector computed tomography in evaluation of coronary artery bypass grafts. J Investig Med 2012;60:1180-5. [Crossref] [PubMed]
- Heye T, Kauczor HU, Szabo G, et al. Computed tomography angiography of coronary artery bypass grafts: robustness in emergency and clinical routine settings. Acta Radiol 2014;55:161-70. [Crossref] [PubMed]
- Goetti R, Leschka S, Baumüller S, et al. Low dose high-pitch spiral acquisition 128-slice dual-source computed tomography for the evaluation of coronary artery bypass graft patency. Invest Radiol 2010;45:324-30. [PubMed]
- Gramer BM, Diez Martinez P, Chin AS, et al. 256-slice CT angiographic evaluation of coronary artery bypass grafts: effect of heart rate, heart rate variability and Z-axis location on image quality. PLoS One 2014;9:e91861. [Crossref] [PubMed]
- Higashigaito K, Husarik DB, Barthelmes J, et al. Computed Tomography Angiography of Coronary Artery Bypass Grafts: Low Contrast Media Volume Protocols Adapted to Tube Voltage. Invest Radiol 2016;51:241-8. [Crossref] [PubMed]
- Koplay M, Guneyli S, Akbayrak H, et al. Diagnostic accuracy and effective radiation dose of high pitch dual source multidetector computed tomography in evaluation of coronary artery bypass graft patency. Wien Klin Wochenschr 2016;128:488-94. [Crossref] [PubMed]
- Yuceler Z, Kantarci M, Yuce I, et al. Follow-up of coronary artery bypass graft patency: diagnostic efficiency of high-pitch dual-source 256-slice MDCT findings. J Comput Assist Tomogr 2014;38:61-6. [Crossref] [PubMed]


