Developing miRNA therapeutics for cardiac repair in ischemic heart disease
Introduction
MicroRNAs (miRNAs) are a large class of small single stranded noncoding RNAs with 21-23 nucleotides in length that silence the expression of specific messenger RNAs (mRNAs). In nucleus, RNA polymerase II (Pol II) transcribes miRNA genes and forms primary miRNA (pri-miRNA). The pri-miRNA yields hairpin-shaped precursor miRNA (pre-miRNA) that will be exported into the cytoplasm. The pre-miRNA further becomes miRNA-miRNA duplex and act as mature miRNA incorporating into the RNA-induced silencing complex (RISC) (Figure 1). At the post-transcriptional level, miRNAs negatively regulate gene expression by promoting mRNAs degradation or inhibiting their translation. To date, there are almost 2,000 miRNAs identified in human cells, which are known to regulate the expression of one-third of human genes through complicated mechanisms (1). It has been proved that cell development, proliferation, differentiation, apoptosis and metabolism could be regulated through modulation of miRNA level, which enables the use of miRNA as a novel therapeutic target (2-5).
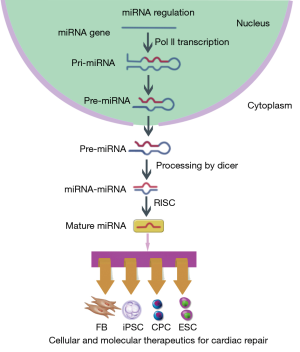
The adult human heart possesses a limited capacity of regeneration and will undergo massive cell necrosis after myocardial infarction (MI) or ischemia-reperfusion (I/R) (6). Traditional therapeutic strategies, including pharmaceutical therapy, percutaneous coronary intervention and surgery, have been applied in clinical practice for years, but still cannot rescue the damaged myocardium. With the advances in cardiac development and biology, molecular and cellular therapeutics have opened a new era in cardiac repair on a microscale level (7). However, the results of long-termed follow-up studies with experimental models or clinical trials have revealed several unmet challenges for their further application. The development and manufacturing of advanced molecular and cellular therapeutics represent a significant gap in bringing new therapeutics to the clinic. In the past few years, miRNAs have been shown to be critical regulators in myocardial development and diseases (8,9). Here, we review the current studies that address the use of miRNAs modulation to improve the success of cellular and molecular therapeutics for cardiac repair and the current translational clinical efforts that have started to explore miRNAs as the potential therapeutic target.
miRNA modulation in endogenous repair
Endogenous cardiac regeneration
During the early stage of life, mammalian heart has a capacity of regeneration. Following the surgery of apical resection, the heart of neonatal mouse is able to regain its function and anatomic structure. However, this capacity of regeneration is lost by the first week after birth (10). Like neonatal rodents, newborn humans might have the intrinsic capacity to repair myocardial damage and completely recover cardiac function. Indeed, Haubner et al. reported a clinical case of cardiac recovery in a newborn with severe MI that showed the importance of further understanding this cardiac regeneration mechanisms (11). During cardiac development, miRNAs modulates a large network of cardiac genes and several miRNA families have been found to play important roles in neonatal cardiac regeneration. For instance, miRNA-34 is a critical modulator of cardiac biological pathways, including cardiomyocyte death, senescence and proliferation (12). In the neonatal mouse heart, the expression level of miRNA-34a was found to remain low during the first week after birth and overexpression of miRNA-34a was shown to reduce cardiomyocyte proliferation in a MI model (12). Further analysis identified Bcl2, Cyclin D1 and silent information regulator 1 (Sirt1), which regulate cell proliferation and cell death, as targets of miRNA-34a. Such research provided the evidence indicating that the loss of cardiac endogenous regeneration was related to overexpression of miRNA-34a. On the other hand, inhibition of miRNA-34a showed therapeutic effect in adult heart after ischemic injury, which suggested the need to further evaluate its potential clinical implication (13). Another miRNA family identified as regulator of heart regeneration is the miRNA-15 family. Inhibition of the miRNA-15 family improved mitochondrial function and reduce hypoxia-induced cell death during ischemia injury through the regulation of the antiapoptotic protein B-cell lymphoma 2 (Bcl-2) and the mitochondrial protective protein ADP-ribosylation factor-like protein 2 (Arl2) (14-16). Additionally, in neonatal heart, the miRNA-15 family also participates in the loss of the regeneration by inhibiting cardiomyocyte proliferation (17). This capacity of regeneration during the neonatal stage is limited to the mouse models and whether this miRNA-based therapy could be translated into clinical application in humans needs further research.
Endogenous cardiac repair
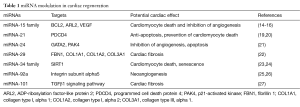
After MI or I/R, the heart goes through various pathophysiological processes, including cell death, tissue perfusion, angiogenesis and fibrosis (18). Inhibition or activation of several miRNAs families has been proven to regulate endogenous myocardium repair to improve cardiac function after ischemia (Table 1). Neovascularization is important for cardiac tissue perfusion and angiogenesis after myocardial injury. Inhibition of miRNA-92a was found to prevent apoptosis of endothelial cell and promote functional recovery of ischemic myocardium by influencing endothelial proliferation and migration through the regulation of proangiogenic proteins, such as integrin subunit alpha-5 (25,26). The inhibition of miRNA-34, which targets the vascular deacetylase Sirt1, also exhibited cardioprotective effect (28,29). MiRNA-24 targets potential regulators of angiogenesis and its inhibition by modified antisense oligonucleotides was shown to improve ischemic heart recovery (21). The replacement of cardiac tissue following impairment after ischemia injury is accomplished through fibrosis and excessive proliferation of fibroblasts also influences the cardiac function after myocardium infarction. Accordingly, those miRNAs that regulate fibrosis could have therapeutic potential. MiRNA-29, miRNA-21 and miRNA-101 regulate multiple proteins, including collagens and fibrillins, during fibrosis and could represent future therapeutic targets to activate the fibrotic response after MI (19,20,22,27). Not surprisingly, based on their biological in endogenous cardiac repair, miRNAs as therapeutic entities are being explored in cardiac repair after ischemic heart injury.
Full table
miRNA modulation for stem cell therapy
Stem cells suffer impairment in function and survival after implantation in harsh ischemic microenvironment. Their homing, differentiation and long-term integration with host myocardium were poor in most studies (30-32). In addition, safety and efficiency are major concerns while using embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) (33). Therefore, several attempts involving the use of miRNAs have been made to improve stem cell therapy by interfering with cell function, homing, survival, or differentiation (Figure 2).
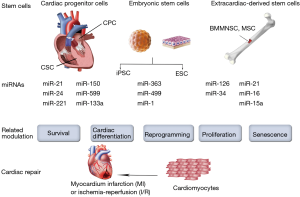
Extracardiac-derived stem cells
Several adult stem cells, such as bone marrow mononuclear stem cells (BMMNSCs), mesenchymal stem cells (MSCs) and progenitor cells have been extensively applied in cardiac repair for years. Meanwhile, miRNAs modulation affects the fate and therapeutic capacity of the transplanted adult stem cells. For instance, miRNA-126 was initially found to be essential for angiogenic signaling in endothelial cells (34-37). Actually, in patients with diabetes mellitus and heart failure, the patient-derived autologous MSCs or proangiogenic bone-marrow-derived cells exhibit the intrinsic down-regulation of miRNA-126 levels, which may impair their proangiogenic abilities after transplantation (38). On the other hand, the overexpression of miRNA-126 restored the proangiogenic activity of the patient-derived autologous isolated cells in vitro (39,40). Moreover, miRNA-126 can activate the pro-survival phosphatidylinositol 3’-kinase (PI3K)/Akt signaling pathway via the repression of the pathway inhibitors (36). Indeed, it has been reported that overexpression of miRNA-126 enhanced the survival of the transplanted MSCs, thus increasing their therapeutic capacity to the greatest extent (41). In addition, overexpression of miRNA-126 also improves in vivo paracrine release from transplanted MSCs. For example, Huang et al. demonstrated that overexpression of miRNA-126 in MSCs enhanced the expression of the Notch ligand Delta-like 4, which plays a key role in tubulogenesis and subsequent neoangiogenesis (37). Remarkably, miRNA-126 not only acts as an intrinsic regulator of cell physiology, but can also be released in exosomes to promote neoangiogenesis. Actually, a recent study demonstrated that exosomes derived from CD34-positive cells contained miRNA-126 and possessed proangiogenic activity for ischemia (42).
In contrast, several miRNAs inhibit the function of stem cells through diverse mechanisms. The inhibition of these miRNAs could benefit the transplanted stem cells. MiRNA-34, which is highly induced by aging and in bone marrow-derived cells from patients with heart failure, exerts a detrimental effect on the stem cells. MiRNA-34 influences apoptosis signaling by, for example, targeting the antiapoptotic protein Bcl-2 and it also blocked cellular proliferation by repressing cell cycle regulators, such as cyclin D2 and cyclin-dependent kinases (43). The inhibition of miRNA-34 has been reported to improve the survival of stem cells in vitro. After cell transplantation, the miRNA-34-modulated stem cells promoted the improvement of functional recovery after acute MI. Conversely, overexpression of miRNA-34a impaired the survival and proangiogenic activity of stem cells (44-46). In addition, miR-15a and miR-16 also inhibit the function of progenitor cells, and their inhibition improved the proangiogenic effect (47).
Cellular senescence is believed to compromise the functions of stem cells. Actually, several miRNAs have been reported to be involved in the senescence of proangiogenic stem cells, whereas the inhibition of these miRNAs improved angiogenesis. For instance, miRNA-34a have been found to targetSirt1, leading to cell cycle arrest or apoptosis (23). Overexpression of miRNA-34a resulted in a significant increase of senescence of endothelial progenitor cells (EPCs), concomitant with Sirt1 expression reduction (24). Likewise, miRNA-10a* and miRNA-21 can regulate EPC senescence by suppressing Hmga2 gene expression and miRNAs modulation may represent a potential therapeutic intervention for improving EPCs-mediated angiogenesis in ischemia (48).
Cardiac-derived stem cells
Although the heart was traditionally regarded as a terminally differentiated organ that consist exclusively of terminally differentiated cells, it is now known that cardiac stem cells (CSCs) and cardiac progenitor cells (CPCs) exist in the adult heart of most species (49,50). These endogenous CSCs and CPCs, which have been considered as the “next generation” of stem cells, can be derived from the heart tissue itself and hold great promise for cardiac repair (51). Although little is known regarding the control of the CSCs/CPCs functions by miRNAs, several reports have demonstrated that survival of CSCs/CPCs could be enhanced through miRNA modulation (52). Indeed, Liu et al. reported that miRNA-155 provides the opportunity to block necrosis, a process traditionally thought to be non-regulated, and might be a potential novel approach to improve cell engraftment during cell therapy (53). They demonstrated the improved survival of the transplanted CPCs by using miRNA-155 overexpression. MiRNA-155 was also found to be involved in CPC differentiation. Specifically, by downregulation of β-arrestin-2, miRNA-155 inhibits CPC differentiation (54). On the other hand, TGF-β signaling promotes cardiosphere-derived cells differentiation by downregulating miRNA-590 (55). In addition, miRNA modulation can also bring benefit by enhancing paracrine action (53). In fact, it has been reported that miRNA-133a overexpression in CPCs not only enhanced cell survival, but also improved cardiac function in a rat MI model by reducing fibrosis and hypertrophy and increasing vascularization and cardiomyocyte proliferation (56). The beneficial effects of miRNA-133a overexpression in CPCs seem to correlate with the upregulated expression of several relevant paracrine factors and the plausible cooperative secretion of miRNA-133a via exosomal transport (56). Furthermore, miRNAs may also be applied in combination to elicit synergistic effects. For example, Hu et al. demonstrated that the combination of three miRNAs, namely miR-21, miR-24 and miR-221, could target the apoptotic protein Bim, thereby improving engraftment and survival of transplanted CPCs (57). Collectively, miRNA modulation provides protective actions for CSCs/CPCs and enhances their therapeutic use in cardiac repair.
Pluripotent stem cells
The main goal of cardiac repair is to generate new competent myocardial tissue that is electrically and mechanically integrated into the surrounding native heart tissue, many studies have searched for the ultimate cell source capable of fulfilling this immense task. As pluripotent stem cells, ESCs and iPSCs have the capacity to differentiate into any specialized cell type, including cardiomyocytes. Accordingly, ESCs-derived and iPSCs-derived cardiomyocytes offer great potential for cardiac regenerative therapy. However, their complete cardiac differentiation is still a major challenge. Nevertheless, some studies have demonstrated that overexpressing cardiac-enriched miRNAs in ESCs, such as miRNA-1 and miRNA-499, enhances cardiac differentiation in vitro (58). In addition, overexpression of miRNA-1 in ESCs was found to enhance cardiac differentiation after transplantation. Moreover, overexpression of miRNA-1 in ESCs could inhibit cardiomyocytes apoptosis in vivo, which could be a beneficial paracrine effect from miRNA-1 overexpression (59). In a study of human ESC-derived cardiomyocytes, miRNA-1 was found to participate in the electrophysiological maturation, while overexpression of miRNA-499 increased ventricular ESC-derived cardiomyocytes (60). Meanwhile, by negatively regulating the cardiac transcription factor Hand1, miRNA-363 also proved to be involved in ESC-derived cardiac subtype specification (61). Nevertheless, only a small fraction of hESCs or hiPSCs can differentiate into functional cardiomyocytes before transplantation. In a clinical scenario, however, billions of cardiomyocytes will be lost after MI.
miRNAs modulation in direct cardiac reprogramming
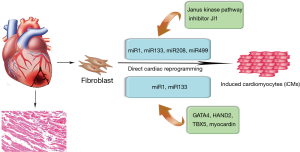
Converting iPSC-derived cardiomyocytes into clinical practice has several problems, including risk of teratoma formation and immature phenotype of the induced cardiomyocytes (62,63). In recent years, direct cardiac reprogramming has become an approach to control cell fate decision and the cardiac-enriched miRNAs have been used to directly reprogram cardiac fibroblasts into cardiomyocytes (Figure 3). In particular, Ieda et al. first reprogrammed mouse cardiac fibroblasts into induced cardiomyocyte-like cells (iCMs) in vitro using a combination of three cardiac transcription factors, namely Gata4, Mef2c and Tbx5 (64). Based on the important roles of miRNAs in cardiac embryonic development and cell fate decision, the combination of the miRNAs, miR-1, miR-133, miR-208, and miR-499 also proved to be effective at directly reprograming neonatal cardiac fibroblasts into functional iCMs in vitro (65,66). In fact, this direct reprogramming strategy may be more efficient than ESCs and iPSCs-based therapeutics. Nonetheless, there is a study suggesting that fibroblasts directly reprogrammed by a combination of miRNAs is more efficient in maturation in vivo and native cardiac environment proves to be critical for the maturation of cells reprogrammed by a combination of miRNAs (67). Therefore, the use of 3D ex vivo environment, including mechanical force, cell shape and extracellular matrix components, should be included to further study the mechanistic basis of direct cardiac reprogramming. Toward this goal, it is also necessary to determine the optimal combination of factors to enhance the efficiency of direct reprogramming. Another strategy involving the use of miRNAs in direct cardiac reprogramming is the approach combining transcription factors. For instance, by adding the combination of miRNA-1 and miRNA-133, the efficiency of four human cardiac transcription factors-based direct reprogramming was significantly enhanced, as well as the expression of cardiac troponin-T (68). Further analysis showed that miRNA-1 and miRNA-133 might participate in the development of sarcomeres and inhibit the activation of myocardin during smooth muscle differentiation (68).
Methods of miRNA modulation
Modulation of miRNA in target stem cells can be accomplished by the delivery of miRNA mimics or inhibitors, which depends on effectiveness of the delivery systems, including direct injection, virus and non-virus vectors (69). Although traditional viral delivery systems offer relatively high efficiencies and may allow stable miRNAs modulation, their clinical applications are currently limited due to potential problems, such as oncogenic transformation, pathogenic risk, and induction of immune responses (70,71). Accordingly, non-viral vectors, which provide biocompatibility, targeting efficacy and enhanced transfection efficiency, are a more suitable alternative to viruses for achieving successful miRNA modulation without side effects. Native or synthetic nanomaterials have demonstrated their potential for miRNAs delivery in stem cells (70). Usually, these non-viral materials are cationic, which can condense or encapsulate the negatively charged miRNAs via electrostatic interactions. Although the non-viral delivery vectors have considerable advantages over viral-based vector due to the control of their molecular composition, simplified manufacturing, modification and analysis, tolerance for cargo sizes, and relatively low immunogenicity, their relatively low efficiency still needs to be optimized in future studies.
Challenges of miRNA therapeutic
Accumulated evidence has demonstrated that miRNAs modulation could increase the capacity of cell therapeutics and enhance the cardiac repair process after ischemic heart disease. Although such results are encouraging, the strategy still faces several challenges. First, lessons from the previous experience indicate that the miRNAs delivery systems for cardiac repair strategy should be optimized (70). Viral delivery systems including lentiviral, retroviral and adenoviral systems are the most common choices for miRNAs delivery (72,73). Despite the high efficiency and stable miRNAs modulation of virus-based delivery systems, their limited DNA capacities, variation in the expression level of several genes in one cassette and the biological risks are still big barriers in bringing them to clinical practice (74). Therefore, the highly efficient and biocompatible non-viral miRNAs delivery vector is currently in great demand. Second, better understanding of the full biological effect of miRNAs should be achieved before being used in cardiac cell therapy. Many miRNAs which might be considered as therapeutic targets are also involved in other disease processes, such as oncogenesis. The miRNAs modulation may face challenges with respect to unwanted side effects in transplanted stem cells-based cardiac repair. For instance, stem cells inhibited by miRNA-34 show enhanced therapeutic capacity in cardiac repair. However, miRNA-34 inhibition is also related to the occurrence of some tumors (75). Safety should be approached with caution while using miRNA-34-inhibited stem cells. In addition, more miRNAs could be evaluated for their modulation of stem cells, and the effect towards their target miRNAs should be comprehensively assessed by integrated experimental and bioinformatics approaches.
The clinical trial in which the hepatitis C virus (HCV) replication was successfully suppressed with a miRNA therapeutic represents a great progress in exploring miRNAs as the next therapeutic target (76). Based on biology of miRNAs, therapeutically manipulating their expression and function has been explored in experimental models of cardiovascular disorders. The antisense oligonucleotides designed to target specific miRNAs are termed anti-miRs (72). Anti-miRs are useful tools to modulate miRNAs expression and function in cardiac repair strategies. Anti-miRs could be modified with phosphorothioate moieties or cholesterol so as to enhance their therapeutic stability (72). With the appropriate vector, those miRNA inhibitors could be designed to target all cardiovascular cell types and inhibition of certain cell type specific miRNAs may improve the efficiency of cardiac regeneration.
Conclusions
The therapeutic application of miRNAs in cardiac repair strategies, including stem cell transplantation, gene therapy, and reprogramming, has been shown to have cardioprotective effects in experimental models of ischemic heart disease and in some clinical trials. MiRNAs can regulate a wide range of functional biological processes in cardiac differentiation, development and reprogramming. Several miRNAs might be attractive candidates for modulating certain therapeutic targets in ischemic myocardium. Their overexpression or inhibition increases the therapeutic capacities, thus offering novel therapeutic opportunities for cardiac repair.
Acknowledgements
Funding: The authors acknowledge funding from the National Science Foundation of China (Grant No. 81301312, 81570422, 81500194), “Chen Guang” Project Supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (Grant No. 14CG06), Joint Project Funding for Major Diseases in Shanghai (Grant No. 2014ZYJB0402), Youth Foundation of Zhongshan Hospital (Grant No. 2015ZSQN48) and Talent Training Program Foundation for the Excellent Youth Supported by Zhongshan Hospital (Grant No. 2015ZSYXQN12).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Friedman RC, Farh KK, Burge CB, et al. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009;19:92-105. [Crossref] [PubMed]
- Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol 2007;8:23-36. [Crossref] [PubMed]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007;318:1931-4. [Crossref] [PubMed]
- Okamura K, Hagen JW, Duan H, et al. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 2007;130:89-100. [Crossref] [PubMed]
- Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature 2007;448:83-6. [Crossref] [PubMed]
- Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38-360. [Crossref] [PubMed]
- Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. Biomed Res Int 2014;2014:951512.
- Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol 2009;10:116-25. [Crossref] [PubMed]
- Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol 2015;12:135-42. [Crossref] [PubMed]
- Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science 2011;331:1078-80. [Crossref] [PubMed]
- Haubner BJ, Schneider J, Schweigmann U, et al. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ Res 2016;118:216-21. [Crossref] [PubMed]
- Eulalio A, Mano M, Dal Ferro M, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012;492:376-81. [Crossref] [PubMed]
- Yang Y, Cheng HW, Qiu Y, et al. MicroRNA-34a plays a key role in cardiac repair and regeneration following myocardial infarction. Circ Res 2015;117:450-9. [Crossref] [PubMed]
- Hullinger TG, Montgomery RL, Seto AG, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res 2012;110:71-81. [Crossref] [PubMed]
- Nishi H, Ono K, Iwanaga Y, et al. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem 2010;285:4920-30. [Crossref] [PubMed]
- Yin KJ, Olsen K, Hamblin M, et al. Vascular endothelial cell-specific microRNA-15a inhibits angiogenesis in hindlimb ischemia. J Biol Chem 2012;287:27055-64. [Crossref] [PubMed]
- Porrello ER, Mahmoud AI, Simpson E, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A 2013;110:187-92. [Crossref] [PubMed]
- Seeger FH, Zeiher AM, Dimmeler S. MicroRNAs in stem cell function and regenerative therapy of the heart. Arterioscler Thromb Vasc Biol 2013;33:1739-46. [Crossref] [PubMed]
- Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980-4. [Crossref] [PubMed]
- Cheng Y, Zhu P, Yang J, et al. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res 2010;87:431-9. [Crossref] [PubMed]
- Fiedler J, Jazbutyte V, Kirchmaier BC, et al. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation 2011;124:720-30. [Crossref] [PubMed]
- van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 2008;105:13027-32. [Crossref] [PubMed]
- Tabuchi T, Satoh M, Itoh T, et al. MicroRNA-34a regulates the longevity-associated protein SIRT1 in coronary artery disease: effect of statins on SIRT1 and microRNA-34a expression. Clin Sci (Lond) 2012;123:161-71. [Crossref] [PubMed]
- Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab 2010;299:E110-6. [Crossref] [PubMed]
- Bonauer A, Carmona G, Iwasaki M, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009;324:1710-3. [Crossref] [PubMed]
- Iaconetti C, Polimeni A, Sorrentino S, et al. Inhibition of miR-92a increases endothelial proliferation and migration in vitro as well as reduces neointimal proliferation in vivo after vascular injury. Basic Res Cardiol 2012;107:296. [Crossref] [PubMed]
- Pan Z, Sun X, Shan H, et al. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-β1 pathway. Circulation 2012;126:840-50. [Crossref] [PubMed]
- Bernardo BC, Gao XM, Winbanks CE, et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A 2012;109:17615-20. [Crossref] [PubMed]
- Boon RA, Iekushi K, Lechner S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature 2013;495:107-10. [Crossref] [PubMed]
- Zhu K, Lai H, Guo C, et al. Nanovector-based prolyl hydroxylase domain 2 silencing system enhances the efficiency of stem cell transplantation for infarcted myocardium repair. Int J Nanomedicine 2014;9:5203-15. [PubMed]
- Chavakis E, Dimmeler S. Homing of progenitor cells to ischemic tissues. Antioxid Redox Signal 2011;15:967-80. [Crossref] [PubMed]
- Zhu K, Guo C, Xia Y, et al. Transplantation of novel vascular endothelial growth factor gene delivery system manipulated skeletal myoblasts promote myocardial repair. Int J Cardiol 2013;168:2622-31. [Crossref] [PubMed]
- Mummery CL, Davis RP, Krieger JE. Challenges in using stem cells for cardiac repair. Sci Transl Med 2010;2:27ps17. [Crossref] [PubMed]
- Jakob P, Doerries C, Briand S, et al. Loss of angiomiR-126 and 130a in angiogenic early outgrowth cells from patients with chronic heart failure: role for impaired in vivo neovascularization and cardiac repair capacity. Circulation 2012;126:2962-75. [Crossref] [PubMed]
- Meng S, Cao JT, Zhang B, et al. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J Mol Cell Cardiol 2012;53:64-72. [Crossref] [PubMed]
- Chen JJ, Zhou SH. Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiol J 2011;18:675-81. [Crossref] [PubMed]
- Huang F, Zhu X, Hu XQ, et al. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med 2013;31:484-92. [PubMed]
- Zampetaki A, Kiechl S, Drozdov I, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 2010;107:810-7. [Crossref] [PubMed]
- Fleissner F, Jazbutyte V, Fiedler J, et al. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ Res 2010;107:138-43. [Crossref] [PubMed]
- Mocharla P, Briand S, Giannotti G, et al. AngiomiR-126 expression and secretion from circulating CD34(+) and CD14(+) PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood 2013;121:226-36. [Crossref] [PubMed]
- Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A 2007;104:1643-8. [Crossref] [PubMed]
- Sahoo S, Klychko E, Thorne T, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 2011;109:724-8. [Crossref] [PubMed]
- Xu Q, Seeger FH, Castillo J, et al. Micro-RNA-34a contributes to the impaired function of bone marrow-derived mononuclear cells from patients with cardiovascular disease. J Am Coll Cardiol 2012;59:2107-17. [Crossref] [PubMed]
- Hermeking H. MicroRNA-34a regulation of endothelial senescence. Cell Death Differ 2010;17:193-9. [Crossref] [PubMed]
- Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun 2010;398:735-40. [Crossref] [PubMed]
- Chen F, Hu SJ. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J Biochem Mol Toxicol 2012;26:79-86. [Crossref] [PubMed]
- Spinetti G, Fortunato O, Caporali A, et al. MicroRNA-15a and microRNA-16 impair human circulating proangiogenic cell functions and are increased in the proangiogenic cells and serum of patients with critical limb ischemia. Circ Res 2013;112:335-46. [Crossref] [PubMed]
- Zhu S, Deng S, Ma Q, et al. MicroRNA-10A* and MicroRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ Res 2013;112:152-64. [Crossref] [PubMed]
- Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011;378:1847-57. [Crossref] [PubMed]
- Bolli R, Tang XL, Sanganalmath SK, et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 2013;128:122-31. [Crossref] [PubMed]
- Behfar A, Crespo-Diaz R, Terzic A, et al. Cell therapy for cardiac repair--lessons from clinical trials. Nat Rev Cardiol 2014;11:232-46. [Crossref] [PubMed]
- Purvis N, Bahn A, Katare R. The role of microRNAs in cardiac stem cells. Stem Cells Int 2015;2015:194894.
- Liu J, van Mil A, Vrijsen K, et al. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J Cell Mol Med 2011;15:1474-82. [Crossref] [PubMed]
- Zhao J, Feng Y, Yan H, et al. β-arrestin2/miR-155/GSK3β regulates transition of 5'-azacytizine-induced Sca-1-positive cells to cardiomyocytes. J Cell Mol Med 2014;18:1562-70. [Crossref] [PubMed]
- Ekhteraei-Tousi S, Mohammad-Soltani B, Sadeghizadeh M, et al. Inhibitory effect of hsa-miR-590-5p on cardiosphere-derived stem cells differentiation through downregulation of TGFB signaling. J Cell Biochem 2015;116:179-91. [Crossref] [PubMed]
- Izarra A, Moscoso I, Levent E, et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Reports 2014;3:1029-42. [Crossref] [PubMed]
- Hu S, Huang M, Nguyen PK, et al. Novel microRNA prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation 2011;124:S27-34. [Crossref] [PubMed]
- Sluijter JP, van Mil A, van Vliet P, et al. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscler Thromb Vasc Biol 2010;30:859-68. [Crossref] [PubMed]
- Ivey KN, Muth A, Arnold J, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell 2008;2:219-29. [Crossref] [PubMed]
- Fu JD, Rushing SN, Lieu DK, et al. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS One 2011;6:e27417. [Crossref] [PubMed]
- Wagh V, Pomorski A, Wilschut KJ, et al. MicroRNA-363 negatively regulates the left ventricular determining transcription factor HAND1 in human embryonic stem cell-derived cardiomyocytes. Stem Cell Res Ther 2014;5:75. [Crossref] [PubMed]
- Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res 2014;114:511-23. [Crossref] [PubMed]
- Okano H, Nakamura M, Yoshida K, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res 2013;112:523-33. [Crossref] [PubMed]
- Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010;142:375-86. [Crossref] [PubMed]
- Jayawardena TM, Finch EA, Zhang L, et al. MicroRNA induced cardiac reprogramming in vivo: evidence for mature cardiac myocytes and improved cardiac function. Circ Res 2015;116:418-24. [Crossref] [PubMed]
- Jayawardena TM, Egemnazarov B, Finch EA, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res 2012;110:1465-73. [Crossref] [PubMed]
- Georgiadis V, Knight RA, Jayasinghe SN, et al. Cardiac tissue engineering: renewing the arsenal for the battle against heart disease. Integr Biol (Camb) 2014;6:111-26. [Crossref] [PubMed]
- Nam YJ, Song K, Luo X, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A 2013;110:5588-93. [Crossref] [PubMed]
- Yin H, Kanasty RL, Eltoukhy AA, et al. Non-viral vectors for gene-based therapy. Nat Rev Genet 2014;15:541-55. [Crossref] [PubMed]
- Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release 2013;172:962-74. [Crossref] [PubMed]
- Kundu SK, Sharma AR, Lee SS, et al. Recent trends of polymer mediated liposomal gene delivery system. Biomed Res Int 2014;2014:934605.
- van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov 2012;11:860-72. [Crossref] [PubMed]
- Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet 2014;15:445-51. [Crossref] [PubMed]
- Mizuguchi H, Xu Z, Ishii-Watabe A, et al. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther 2000;1:376-82. [Crossref] [PubMed]
- Maroof H, Salajegheh A, Smith RA, et al. Role of microRNA-34 family in cancer with particular reference to cancer angiogenesis. Exp Mol Pathol 2014;97:298-304. [Crossref] [PubMed]
- Yang X, Marcucci K, Anguela X, et al. Preclinical evaluation of an anti-HCV miRNA cluster for treatment of HCV infection. Mol Ther 2013;21:588-601. [Crossref] [PubMed]


