Single-direction VATS left lower lobectomy and lymph node dissection after neoadjuvant chemotherapy combined with immunotherapy: a case report
Introduction
Surgical treatment of locally advanced lung cancer is a major challenge in thoracic surgery. Neoadjuvant therapy has proven to be useful for tumor regression, while increasing surgical resection rates (1). Neoadjuvant chemotherapy combined with immunotherapy is a new treatment model for locally advanced lung cancer, which can not only improve surgical resection rates, but also improves patient prognosis (2,3). Whether difficulties in surgical resection and postoperative complications still increase is worth exploring. We report a patient with locally advanced left lower lobe lung squamous carcinoma (stage IIIA, T2N2M0) who accepted 2 cycles of paclitaxel/carboplatin/pembrolizumab neoadjuvant chemotherapy combined with immunotherapy. We then performed single-direction video-assisted thoracoscopic left lower lobectomy and lymph node dissection, and the surgical procedure is described below. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jtd-21-529).
Operative techniques
Clinical summary
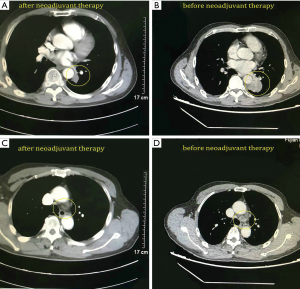
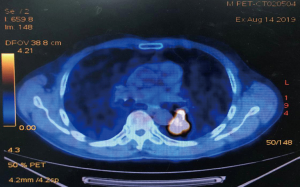
A 68-year-old male was admitted to the hospital due to cough and progressive dyspnea for over a month. He had a long history of heavy smoking, with a smoking index over 1,000 pack years. A CT scan indicated a 4.0 cm × 4.0 cm × 3.5 cm mass in the left lower lung hilum. There was also marked enlargement of No. 4L and No. 5 mediastinal lymph nodes as well as No. 10L lymph nodes in the left lung hilum, which is a conglobation that cannot be dissected directly (Figure 1). PET-CT revealed high metabolism in the left lower lung dorsal segment (Figure 2). Moreover, there was lymph node metastasis in the No. 4L and No. 5 mediastinal lymph nodes, closely related to the descending aorta. Bronchoscopy showed a neoplasm in the opening of the dorsal segment of the left lower lung. A biopsy returned a diagnosis of squamous cell carcinoma, and EBUS-TBNA showed that the N0.4L lymph node was squamous cell carcinoma. The clinical stage was T2N2M0 (stage IIIA). No significant abnormalities were observed in other auxiliary examinations before surgery. Lung function was assessed via formal spirometry, with an FEV1 of 1.96L (92% predicted) and MVV 73.17 L/min (102% predicted). After 2 cycles of paclitaxel + carboplatin + pembrolizumab neoadjuvant chemotherapy combined with immunotherapy, chest CT reexamination was performed to evaluate the tumor, which was significantly smaller than before (partial pathologic response, PR). The patient was satisfied with the curative effect and was willing to undergo the operation. Single-direction VATS left lower lobectomy and lymph node dissection were performed 3 weeks after the end of chemotherapy combined with immunotherapy.
Anesthesia and positioning
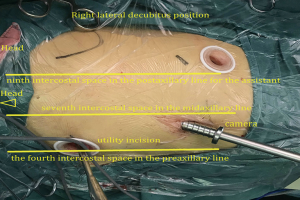
The patient received general anesthesia with double-lumen endotracheal intubation and right lung ventilation. He was positioned in the right lateral decubitus position (Figure 3). The first 1.0 cm incision was made in the seventh intercostal space in the midaxillary line, and was used for the camera. The second 2.5 cm long incision (utility incision) was made in the fourth intercostal space in the preaxillary line. A third 1.5 cm incision was made in the ninth intercostal space in the postaxillary line for the assistant. Incisions were retracted with a soft protector.
The technique
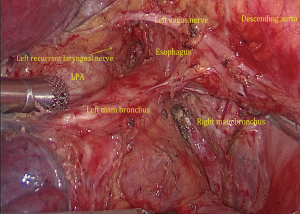
(I) We performed single-direction VATS left lower lobectomy and lymph node dissection. We performed successful removal in the following order: left lower pulmonary ligament-left lower pulmonary vein-left lower pulmonary bronchus-left lower pulmonary artery-interlobar fissure, to avoid repeat turnover of the pulmonary lobe. At the same time, we found the interlobar fissure was not opened, which can increase the risk of postoperative pulmonary leakage. Because of the treatment with neoadjuvant chemotherapy combined with immunotherapy, the tissues in the surgical area were swollen, brittle, and showed adhesion. The left No. 10L, No. 7, No. 8, and No. 4L lymph nodes were first dissected, and the pulmonary hilum was opened to facilitate lobectomy (Figure 4). During the operation, attention was paid to the increase in blood vessel fragility, and to avoid tears in blood vessels caused by violence or excessive pulling and clamping. (II) Then, the left lower lung ligament was freed, the posterior mediastinum pleura was opened, and the left vagus nerve was protected. (III) Left No. 7 lymph nodes were dissected. (IV) Left No. 8 lymph nodes were dissected. (V) No. 10L lymph nodes of the left pulmonary artery trunk were cleaned, No. 4L lymph nodes were exposed, and the No. 4L lymph nodes were completely removed along the left main bronchus space, which was aided by pulling the No. 4L lymph nodes. Attention was paid to protect the left recurrent laryngeal nerve. (VI) The left inferior pulmonary vein was dissociated and cut off. (VII) The bronchus of the lower left lung was dissociated, and the left lower lung bronchus was closed and disconnected after confirmation. (VIII) The basilar artery of left lower lung was freed, the catheter was guided to facilitate the stapler passing through the artery, and the A6 of the left lower lung was ligated and cut off. (IX) The interlobar fissure was completed by the stapler, and the left lower lobe and the tumor were removed by the specimen bag. (X) No. 5 and No. 6 lymph nodes were dissected. (XI) The intraoperative frozen pathology of the left lower lung bronchus was negative, the stump was consolidated, and the chest was closed after the leakage test.
Postoperative management
The chest CT examination of the patient on the first day after the operation showed that there was no obvious pneumonia in both lungs, and the lungs expanded well. The drainage tube was removed, and postoperative recovery was good without complications. Postoperative pathology indicated complete pathologic response (pCR) of the left lower lung tumor, and no tumor cells were found in the lesions and lymph nodes. Five days after surgery, the patient was discharged from hospital.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Comments
Neoadjuvant chemotherapy combined with immunotherapy provides a promising surgical model (4,5). However, the perioperative period remains a big challenge. Although neoadjuvant chemotherapy plus immunotherapy helps shrink the tumor and reduces its stage, it also leads to surgical tissue edema and adhesion during surgery. The operation skill and perioperative management of the operator are required to be high. During the operation, special attention should be paid to R0 resection of the tumor and lymph nodes, along with gentle and careful operation, and postoperative attention should be paid to the occurrence of chemotherapy and immune-related complications. If the postoperative pathology of patients can reach major pathologic response (MPR) or pCR, studies show that patients can have longer survival benefits (4,6). In the future, neoadjuvant chemotherapy combined with immunotherapy will be a promising new adjuvant treatment mode for advanced NSCLC.
Acknowledgments
This study was supported by the Miaopu Fund of Fujian Medical University (Grant number: 2015MP020) and Program for Innovative Research Team in Science and Technology at Fujian Province University (Grant number: 2018B053).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-21-529
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-21-529). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reference
- O'Donnell JS, Hoefsmit EP, Smyth MJ, et al. The Promise of Neoadjuvant Immunotherapy and Surgery for Cancer Treatment. Clin Cancer Res 2019;25:5743-51. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Blumenthal GM, Bunn PA Jr, Chaft JE, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol 2018;13:1818-31. [Crossref] [PubMed]
- Ma X, Li B, Li Y, et al. A Pathologic Complete Response to Neoadjuvant Chemotherapy and Immunotherapy Followed by Surgery in a Patient With NSCLC. J Thorac Oncol 2019;14:e104-6. [Crossref] [PubMed]
- Sa H, Song P, Ma K, et al. Perioperative Targeted Therapy Or Immunotherapy In Non-Small-Cell Lung Cancer. Onco Targets Ther 2019;12:8151-9. [Crossref] [PubMed]
- Weissferdt A, Pataer A, Vaporciyan AA, et al. Agreement on Major Pathological Response in NSCLC Patients Receiving Neoadjuvant Chemotherapy. Clin Lung Cancer 2020;21:341-8. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)