Long-term outcomes following neoadjuvant or adjuvant chemoradiotherapy for stage I–IIIA non-small cell lung cancer: a propensity-matched analysis
Introduction
Non-small cell lung cancer (NSCLC) is one of the most commonly diagnosed and leading causes of cancer death among both men and women worldwide (1). The 5-year survival rate of NSCLC is approximately 18% (2). The effect of adjuvant radiotherapy or chemotherapy on the survival of patients with NSCLC has been well illustrated. A pooled analysis by the Lung Adjuvant Cisplatin Evaluation Group has suggested that postoperative adjuvant chemotherapy significantly improved survival in patients with NSCLC (3). The current status and prospects of neoadjuvant therapy in lung cancer have gained attention in recent years with the booming of neoadjuvant therapy for multitudinous tumor types (4).
A meta-analysis of fifteen randomized controlled trials, including 2,385 patients, established the effect of preoperative chemotherapy and revealed an association of neoadjuvant chemotherapy with a 5-year overall survival (OS) improvement (5). Recently, several studies are having compared the survival difference between adjuvant or neoadjuvant chemoradiotherapy and procedure alone (6-10). However, few studies have evaluated the survival difference of neoadjuvant versus adjuvant chemoradiotherapy combined with surgery. According to the latest viewpoint, neoadjuvant therapy can improve resectability through downstaging the T stage and nodal disease, sterilizing early micro-metastases, and enhancing local regional control by the removal of the residual tumor and nodal disease (4). In addition, neoadjuvant therapy can serve as a convenient window to minimize operative risk and permit pulmonary “pre-habilitation” strategies (4). However, few population-based evaluations of long-term outcomes of patients with neoadjuvant and adjuvant chemoradiotherapy have been performed. This study compared the long-term survival between patients with stage I–IIIA (T1-4N0-1M0) NSCLC receiving adjuvant chemoradiotherapy and those receiving neoadjuvant chemoradiotherapy through propensity-matched analysis and competing risk analysis. Furthermore, the independent prognostic factors were explored and analyzed based on the univariate and multivariate analyses using the Cox proportional-hazards model. We present the following article in accordance with the STROBE reporting checklist (11) (available at http://dx.doi.org/10.21037/jtd-20-898).
Methods
This study was a retrospective study which evaluated the long-term survival of 1,769 patients with stage I–IIIA (T1-4N0-1M0) NSCLC receiving adjuvant chemoradiotherapy or receiving neoadjuvant chemoradiotherapy through propensity-matched analysis and competing risk analysis.
Ethics statement
Permission was obtained to access the open-access Surveillance, Epidemiology, and End Results (SEER) database. In this study, informed consent was not required for patients, and only de-identified and publicly available data were used.
Index cases
The SEER database was explored from 2010 to 2015 to identify all patients with pathologically proven NSCLC within site recode ICD-O-3 variable by ICD-O-3 morphology, histologic type ICD-O-3 and Histology recode-broad groupings. Cases identified at the age of fewer than 18 years, postmortem cases, and non-microscopically confirmed cases were excluded. Patients who did not undergo cancer-directed treatment were also excluded. In addition, patients who lacked follow-up information did not receive neoadjuvant/adjuvant chemoradiotherapy, or underwent treatment for recurrent tumor were not considered. All patients were identified via histological and pathological diagnoses of NSCLC, and cases with missing staging information or survival status were excluded. All patients included in this study were artificially restaged according to the definitions of the latest 8th edition of the TNM classification of lung cancer, based on the available clinicopathological data in the SEER database.
Definition of NSCLC
According to the classification of the World Health Organization, NSCLC is categorized into three main types: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (12). Adenocarcinoma is the most common type of NSCLC, accounting for approximately 40% of lung cancers. Squamous cell carcinomas account for approximately 25–30% of lung cancers, which may tend to a relatively inferior prognosis (13). Large cell carcinomas represent 5–10% of all lung cancers, with low incidence, controversial immunophenotyping, and unclear prognosis (12). To assess the survival difference more comprehensively, all three types of NSCLC were included in our analyses.
Definition of treatment
For patients diagnosed after 1998, cases were identified as having received cancer-directed surgery if the primary site was removed, either lobectomy or sub-lobectomy (RX Summ-Surg Prim Site). Patients with adjuvant chemoradiotherapy were identified as having received postoperative chemotherapy and radiotherapy concurrently. Patients with neoadjuvant chemoradiotherapy were identified as having received preoperative chemotherapy or radiotherapy followed by surgery.
Statistical analysis
Information on patient demographics, clinicopathological characteristics, and treatment and survival outcomes were collected and subjected to subsequent statistical analysis. Fisher’s exact test and Wilcoxon rank-sum test were used to comparing resection and biopsy groups for categorical or continuous variables. The primary and secondary variables used for comparison were OS and cancer-specific survival (CSS), respectively. OS and CSS were defined as the length of time from surgery to death or the last follow-up and the period from the day of diagnosis to the day of death specified by cancer or related complications, respectively. OS and CSS were calculated using the Kaplan-Meier method and analyzed using the log-rank test. Prognostic factors for OS were analyzed using the univariate with log-rank tests for comparisons and multivariate analyses with the Cox proportional-hazards model. The results were presented as hazard ratio (HR) and 95% confidence interval (CI). Detection of multicollinearity was used to test the independence of the independent variables included in the regression model; a tolerance of less than 0.1 or variance inflation factor of greater than 10 indicated a multicollinearity problem. To reduce bias in various clinicopathological factors, propensity-score matching and competing risk analysis were performed in the study. The analysis was implemented using SPSS version 23 (IBM, NY, USA) and R (version 3.6.1 software). A P value less than 0.05 was considered statistically significant, and all tests were two-sided.
Results
Patient information
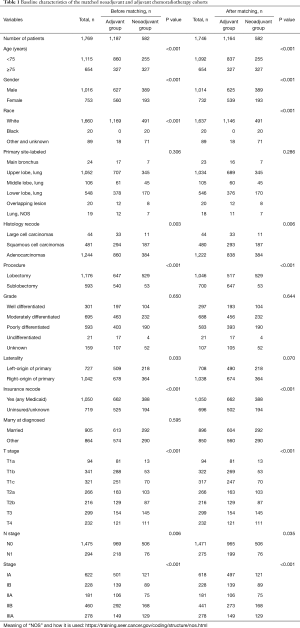
The baseline data of demographics and clinicopathological characteristics are shown in Table 1. Overall, 1,769 patients, including 1,244 patients with adenocarcinoma, 481 with squamous cell carcinoma, and 44 with large cell carcinomas, who underwent neoadjuvant/adjuvant chemoradiotherapy, were evaluated in this study. Before the propensity-score matching, 1,188 and 581 patients were included in the adjuvant therapy group and neoadjuvant group, respectively. The median survival time was 40 and 27 months, respectively. After matching (proportion: 2/1), 1,746 patients, including 1,164 patients in the adjuvant therapy group and 582 in the neoadjuvant therapy group, were enrolled in the study; the middle survival time was 40 and 27 months, respectively. In 1,746 patients after matching, 58.1% of the patients were male (1,014 of 1,746), and patients with lung adenocarcinoma accounted for 70.0% of all study patients (1,222 of 1,746). Further, 37.5% of patients (654 of 1,746) were aged more than 75 years and formed the elderly group, whereas the remaining patients formed the young group. Other patient details, such as race, site, grade, T stage, N stage, procedure, and insurance, are presented in Table 1.
Full table
Comparison of survival between neoadjuvant and adjuvant chemoradiotherapy
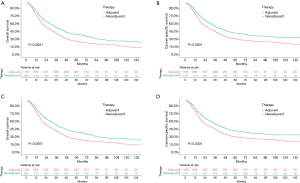
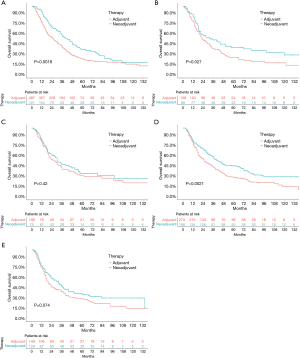
Before the propensity-score matching, the 5-year OS rate in the neoadjuvant therapy group and adjuvant therapy group was 38.8% and 26.2% (P<0.0001, HR: 0.7121, 95% CI: 0.6327–0.8015), respectively (Figure 1A). Considering the factors that might not be directly related to the tumor, the CSS was further used to analyze the survival difference between these two groups. The results showed that the 5-year OS rate in the neoadjuvant therapy group and the adjuvant therapy group was 38.1% and 27.5% (P<0.0001, HR: 0.7044, 95% CI: 0.6210–0.7991), respectively (Figure 1B). The standard propensity-score matching was further used to create a highly comparable control group to minimize selection bias and confounding. After matching, the 5-year OS rate in the neoadjuvant therapy group and the adjuvant therapy group was 38.1% and 27.0% (P<0.0001, HR: 0.7418, 95% CI: 0.6434–0.8553), respectively (Figure 1C). Meanwhile, the 5-year CSS in the neoadjuvant therapy group and the adjuvant therapy group was 40.0% and 27.2% (P<0.0001, HR: 0.7444, 95% CI: 0.6454–0.8587), respectively (Figure 1D). The results indicated that patients who received neoadjuvant therapy had a significantly better long-term survival than those who received adjuvant therapy. When stratified by stage, similar trends were observed for patients with stage IA–IIIA in the neoadjuvant therapy group and the adjuvant therapy group (stage IA: P=0.0018, HR: 0.6609, 95% CI: 0.5283–0.8267; stage IB: P=0.027, HR: 0.6924, 95% CI: 0.5037–0.9517; stage IIA: P=0.42, HR: 0.8575, 95% CI: 0.5917–1.243; stage IIB: P=0.0021, HR: 0.6897, 95% CI: 0.5485–0.8671; stage IIIA: P=0.074, HR: 0.7687, 95% CI: 0.5748–1.028; Figure 2). Interestingly, when stratified by age, elderly patients with neoadjuvant therapy had no significant difference in long-term survival compared with those undergoing adjuvant therapy followed by surgery (P=0.24; HR: 0.8814, 95% CI: 0.7229–1.075; Figure 3A), while results in the young group leaned toward neoadjuvant chemoradiotherapy (P<0.0001; HR: 0.6351, 95% CI: 0.5432–0.7426; Figure 3B).
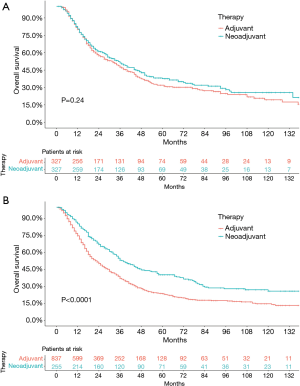
Competing risk analysis
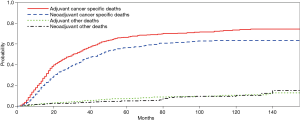
Competing risk analysis on the cause of death for the entire study population is reported in Figure 4. During the long-term follow-up period, most deaths were attributable to tumor, whereas the failure of mortality in other cases remained roughly stable through the years. Evidently, after eliminating the difference in covariates that might affect the OS and cause-specific mortality, the competing analysis showed that patients with neoadjuvant therapy exhibited a better long-term survival.
Survival and prognostic factors
Furthermore, the log-rank univariate analysis and Cox model multivariate analysis were used to screen the prognostic factors; the results are shown in Table 2. The univariate analysis showed that neoadjuvant/adjuvant therapy (P<0.001), procedure (P<0.001), race (P=0.029), sex (P=0.001), laterality (P 0.032), and age (P=0.001) had significant impacts on survival, whereas no significant survival benefit was observed in terms of insurance (P=0.389), histology (P=0.272), N stage (P=0.355), and T stage (P=0.558). Then, multivariate analysis was performed to clarify the independent prognostic indicators for patients. The VIF was used to correct the model to exclude correlation variables. Eight variables, including laterality, race, age, sex, neoadjuvant/adjuvant therapy, procedure, T stage, and N stage, were analyzed in the Cox model multivariate analysis. Finally, laterality (P=0.041, HR: 0.864, 95% CI: 0.752–0.994), procedure (P<0.001, HR: 0.703, 95% CI: 0.603–0.820), and neoadjuvant/adjuvant therapy (P=0.029, HR: 0.837, 95% CI: 0.714–0.982) were found to be independent prognostic indicators; the results are shown in Table 2. In a nutshell, the results indicated that neoadjuvant chemoradiotherapy was an independent prognostic indicator, and a significant long-term survival benefit was consistently observed when comparing patients who underwent the procedure followed by general adjuvant therapy.
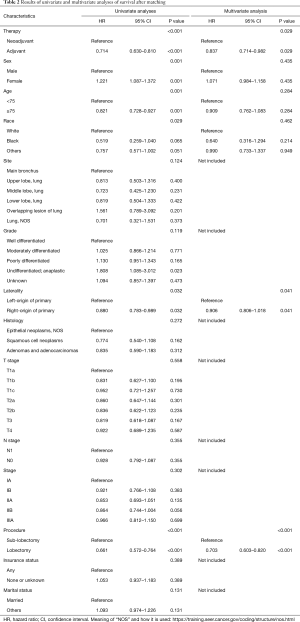
Full table
Discussion
Globally, lung cancer has the highest morbidity and mortality of the tumor, and NSCLC is the most common type of cancer affecting the lungs (14). Recent studies showed a very high incidence (20–50%) of distant recurrence for increasing the number of T-stage tumors even with a complete resection (R0) in patients with pN0 stage, which suggested that surgery alone in this patient population was inadequate (15). In latest National Comprehensive Cancer Network guidelines, patients with stage IIA–IIIA NSCLC have been recommended procedure followed by adjuvant therapy; meanwhile, an annotation also suggested that neoadjuvant chemotherapy could be considered for these patients of evidence based on a meta-analysis of multiple phase III randomized controlled trials that established a superior 5-year survival for surgery followed by adjuvant or neoadjuvant chemotherapy versus procedure alone (3,16,17). As mentioned earlier, neoadjuvant chemotherapy offers potential benefits over adjuvant chemotherapy for patients, including a reduction in T stage and N disease, treatment of early micro-metastatic disease, ability to assess treatment response, and additional time for preoperative cessation of smoking that are all effects that postoperative adjuvant therapy cannot achieve. Especially, a study based on animal experiments have indicated that metastatic lung lesions might occurrent in clinical N0 cases, and these potential microscopic metastases cannot be detected by using current diagnostic imaging systems or biochemical examination (18). In addition, neoadjuvant chemotherapy has been associated with better tolerance and compliance, as indicated in phase III randomized controlled trial, in 97% of patients compared with 66% in the adjuvant chemotherapy group (19). Even in modern times, the evidence-based on supporting the benefits of neoadjuvant therapy for patients with stage I–IIIA (T1-4N0-1M0) NSCLC is limited, while available studies prefer surgery followed by adjuvant therapy than procedure alone. With a booming of targeted therapy and induction immunotherapy for resectable NSCLC, this important problem has become ever more serious to understand the contemporary place of neoadjuvant chemotherapy versus adjuvant chemotherapy on long-term survival benefits in these patients (20). Moreover, in this era of targeted therapy and immunotherapy, whether classical and general chemotherapy and radiotherapy still play a critical role in the management of operable NSCLC needs more evidence from prospective studies.
Based on the aforementioned comparison and consideration, the objective of our study was to evaluate the effect of neoadjuvant chemoradiotherapy on the long-term survival for patients with stage I–IIIA (T1-4N0-1M0) NSCLC, compared with patients with the general procedure followed by adjuvant chemoradiotherapy. A total of 1,769 patients, including 1,244 with adenocarcinoma, 481 with squamous cell carcinoma, and 44 with large cell carcinomas, receiving neoadjuvant/adjuvant chemoradiotherapy were included in the analyses. After the propensity-score matching according to the ratio of 2:1, 1,746 patients, including 1,164 patients with adjuvant therapy and 582 patients with neoadjuvant therapy, were enrolled in the study. Finally, the analyses suggested that compared with patients in the adjuvant therapy group, patients in the neoadjuvant therapy group might benefit from neoadjuvant chemoradiotherapy and have superior long-term survival, both OS and CSS. In the multivariate analysis, procedure, laterality, and neoadjuvant/adjuvant therapy were independent prognostic factors, while other indicators were not prognostically significant, indicating that these patients might have long-term survival and benefit from neoadjuvant therapy followed by lobectomy, compared with sub-lobectomy plus adjuvant therapy. In addition, no significant difference in OS was observed between patients aged more than 75 years in the neoadjuvant therapy group and adjuvant therapy group, suggesting that elderly patients might not benefit from neoadjuvant therapy followed by surgery, on account of the cytotoxicity and tolerance.
This study was novel in comparing the effect of neoadjuvant chemoradiotherapy and adjuvant chemoradiotherapy on the long-term outcomes of patients with NSCLC undergoing a procedure using the large population-based SEER database and propensity-score matching analyses. The important progress on the effect of neoadjuvant chemoradiotherapy on survival for patients with NSCLC was a timely retrospective study by Brandt et al. (21). They found that both the disease-free survival and OS were not significantly different between patients with adjuvant chemotherapy and neoadjuvant chemotherapy after matching. However, patients included in the neoadjuvant chemotherapy group were much more likely to have fewer high-grade cytotoxicity (15% vs. 38%) and received a full dose of chemotherapy (78% vs. 63%), which showed a better tolerance. The results also favored neoadjuvant chemoradiotherapy. On the contrary, the radiotherapy management of NSCLC is currently unsatisfactory. Generally, it is widely believed that opportunities of radiotherapy should be decided on the presupposition of fully considering the quality of life after treatment and patients’ condition during the perioperative period, although radiotherapy has been indicated a possible reasonable treatment choice for early-stage NSCLC in recent years (22). No evidence showed the outcomes of neoadjuvant radiotherapy and adjuvant radiotherapy. Owing to limited data from the SEER database, neoadjuvant/adjuvant radiotherapy was considered together with the neoadjuvant/adjuvant chemotherapy. Recently, in a study using the large population-based SEER database (23), the results showed that for patients with stage T1-2N2-3, neoadjuvant chemoradiotherapy seems to be superior in survival compared with adjuvant chemoradiotherapy. However, whether tumor resection benefits and improves the survival outcomes of patients with N2-3 disease is still a challenging issue. Therefore, only patients with N0-1 disease were included in the analyses. Previous studies have shown that patients with clinically advanced NSCLC could be benefited from neoadjuvant chemoradiation, followed by anatomic resection (24). The results also suggested that patients might have long-term survival benefits from neoadjuvant chemoradiotherapy followed by lobectomy, even for early-stage patients. In addition, the results of this study also confirmed the significant independent predictors of inferior outcomes in patients with NSCLC, including laterality, adjuvant chemoradiotherapy, and sub-lobectomy, while the effects of other indicators were small relative to these factors. The present study also demonstrated an independent effect of age, and neoadjuvant chemoradiotherapy was not associated with an improvement in long-term survival for elderly patients.
Unavoidably, this study had several common limitations of population-based studies. It was a retrospective study, including 1,769 patients from the SEER database, leading to some bias. For instance, when stratified by stage, survival differences were not significant in patients with stage IIA and IIIA. Thus, more studies are needed to confirm the results further using a large multi-institutional database and multicenter randomized studies.
Conclusions
The study showed that neoadjuvant chemoradiotherapy might improve long-term outcomes of patients with NSCLC of stage I–IIIA (T1-4N0-1M0) compared with adjuvant chemotherapy plus procedure, in terms of both OS and CSS. Laterality, lobectomy/sub-lobectomy, and neoadjuvant/adjuvant chemoradiotherapy were independent prognostic factors, while other indicators were not prognostically significant in the multivariate analysis. In addition, for patients aged more than 75 years, neoadjuvant chemoradiotherapy was not associated with an improvement in long-term survival. Apparently, neoadjuvant chemoradiotherapy, followed by lobectomy, looks preferable to sub-lobectomy plus general adjuvant chemoradiotherapy for young patients in terms of long-term survival. However, it should be decided on the premise of fully considering the patient’s condition and quality of life.
Acknowledgments
We would like to thank International Science Editing Co. for editing the language.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81672268) (www.nsfc.gov.cn/).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-898
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-898). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Permission was obtained to access the open-access Surveillance, Epidemiology, and End Results (SEER) database. In this study, informed consent was not required for patients, and only de-identified and publicly available data were used.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Blumenthal GM, Bunn PJ, Chaft JE, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol 2018;13:1818-31. [Crossref] [PubMed]
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Stephens RJ, Girling DJ, Bleehen NM, et al. The role of post-operative radiotherapy in non-small-cell lung cancer: a multicentre randomised trial in patients with pathologically staged T1-2, N1-2, M0 disease. Medical Research Council Lung Cancer Working Party. Br J Cancer 1996;74:632-9. [Crossref] [PubMed]
- Burdett S, Stewart L. Postoperative radiotherapy in non-small-cell lung cancer: update of an individual patient data meta-analysis. Lung Cancer 2005;47:81-3. [Crossref] [PubMed]
- Nagai K, Tsuchiya R, Mori T, et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 2003;125:254-60. [Crossref] [PubMed]
- Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol 2002;20:247-53. [PubMed]
- Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Li M, Zhan C, Sui X, et al. A proposal to reflect survival difference and modify the staging system for lung adenocarcinoma and squamous cell carcinoma: based on the machine learning. Front Oncol 2019;9:771. [Crossref] [PubMed]
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Brandt WS, Bouabdallah I, Tan KS, et al. Factors associated with distant recurrence following R0 lobectomy for pN0 lung adenocarcinoma. J Thorac Cardiovasc Surg 2018;155:1212-24.e3. [Crossref] [PubMed]
- Berry MF, Coleman BK, Curtis LH, et al. Benefit of adjuvant chemotherapy after resection of stage II (T1-2N1M0) non-small cell lung cancer in elderly patients. Ann Surg Oncol 2015;22:642-8. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Sukhbaatar A, Mori S, Saiki Y, et al. Lymph node resection induces the activation of tumor cells in the lungs. Cancer Sci 2019;110:509-18. [Crossref] [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Zhang Y, Chen H. Neoadjuvant or adjuvant chemotherapy for non-small-cell lung cancer: does the timing matter? J Thorac Cardiovasc Surg 2019;157:756-7. [Crossref] [PubMed]
- Brandt WS, Yan W, Zhou J, et al. Outcomes after neoadjuvant or adjuvant chemotherapy for cT2-4N0-1 non-small cell lung cancer: a propensity-matched analysis. J Thorac Cardiovasc Surg 2019;157:743-53.e3. [Crossref] [PubMed]
- Li M, Zhan C. Stereotactic ablative radiotherapy for early-stage central lung tumors: status, challenges, and future considerations. Ann Transl Med 2019;7:S199. [Crossref] [PubMed]
- Wang X, Yin C, Su S, et al. Long-term effects of neoadjuvant radiotherapy, adjuvant radiotherapy, and chemotherapy-only on survival of locally advanced non-small cell lung cancer undergoing surgery: a propensity-matched analysis. BMC Cancer 2018;18:1067. [Crossref] [PubMed]
- MacLean M, Luo X, Wang S, et al. Outcomes of neoadjuvant and adjuvant chemotherapy in stage 2 and 3 non-small cell lung cancer: an analysis of the National Cancer Database. Oncotarget 2018;9:24470-9. [Crossref] [PubMed]