Transition from video-assisted thoracic surgery to robotic pulmonary surgery
Introduction
Video-assisted thoracoscopic surgery (VATS), in which surgery is performed with the use of an endoscopic camera that displays images of the surgical field on a video monitor, is currently being actively utilized as an alternative to a conventional open surgery (thoracotomy), which involves a 30-cm incision and cutting one or more ribs to gain access to the thoracic organs (1). However, a disadvantage of VATS is the fact that surgery is performed in a two-dimensional (2D) field because of the surgical field being viewed on a monitor, and the fact that the use of long, specialized instruments sometimes forces the surgeon to employ awkward surgical procedures. Thus, even now, a certain level of apprehension that VATS does not provide adequate surgical accuracy remains. As a result, many medical facilities have yet to adopt VATS for lung cancer cases. In addition, VATS utilizes rigid instruments, which makes it difficult for it to be employed in surgical procedures that require highly difficult suturing such as hand-closure of the bronchial stump, bronchoplasty, and pulmonary angioplasty. These types of surgical procedures are performed using highly invasive thoracotomy.
The “da Vinci Surgical System” (Intuitive Surgical, Sunnyvale, CA, USA) is a robotic surgical system that utilizes multi-jointed robotic arms and a high-resolution three-dimensional (3D) video-monitoring system. The merits of the da Vinci Surgical System include a true 3D binocular view and its multi-jointed forceps, which enable highly accurate surgical procedures. While performing surgery, surgeons are provided with a 3D image on an adjacent screen, which makes the surgeons feel as if they are actually within the thoracic cavity. In addition, the fact that the multi-jointed instruments are actually present within the thoracic cavity allows for a smooth and natural manipulation when performing surgical dissection. This is a major advantage over the conventional VATS technique that requires the use of straight instruments. Particularly in the case of lymph-node dissection, which requires accurate and finely detailed operations deep in the thoracic cavity, the 3D image and multi-jointed forceps of the da Vinci System allows the procedure to be performed much more easily than in conventional thoracoscopic surgery. Moreover, the da Vinci System compensates for physiological tremors of the hand, thus allowing minute manipulations to be easily performed.
Here, we report on the state of transition from VATS to robotic pulmonary surgery, the surgical outcomes of robotic surgery compared to VATS, and the future of robotic surgery.
Transitioning from VATS to robotic surgery
Currently, nearly all robot-assisted surgical procedures are performed using the da Vinci Surgical System. Here we describe the procedure for initiating the use of robotic surgery with this system.
The multi-jointed instruments and 3D view of the da Vinci Surgical System allow surgery to be performed in largely the same way as open surgery. However, when the surgeon manipulating the robot first starts to perform robotic surgery, they must have experience with VATS as it uses the same vessel-sealing device and stapler as well as the endoscopic surgical procedures and handling of bleeding as those used in thoracoscopic surgery are required. In addition, both the surgeon at the console and the patient side assistant must have a full understanding of the surgical procedures and robotic manipulation as well as procedures for handling unexpected situations such as vessel injury.
Surgery utilizing the da Vinci Surgical System requires a console surgeon and assistant who have been certified by Intuitive Surgical, Inc., the system manufacturer. Both the surgeon and the assistant must take the certification course offered by Intuitive Surgical, Inc. This course includes an online course for learning robot surgery, on-site training in the use of the da Vinci System at facilities that have adopted it, off-site training using either cadavers or pigs, and a clinical tour of a facility that utilizes the system. The da Vinci System also requires that the facility have one nurse or technician who has taken the certification course approved by Intuitive Surgical, Inc. as part of the staff. In addition, after deciding on the date that the first surgery is to take place, a training instructor certified by Intuitive Surgical, Inc. must join the surgeon, anesthesiologist, nurses, and technicians in the operating room and perform a da Vinci surgical simulation. To prepare for cases requiring emergency thoracotomy, training in emergency detachment ensures that they are able to detach the robot within 15 s. In cases in which there are no experienced individuals at the same facility, it is recommended that a surgeon who is thoroughly experienced in the field in question is invited to perform the first few operations to provide direct guidance in the surgical technique.
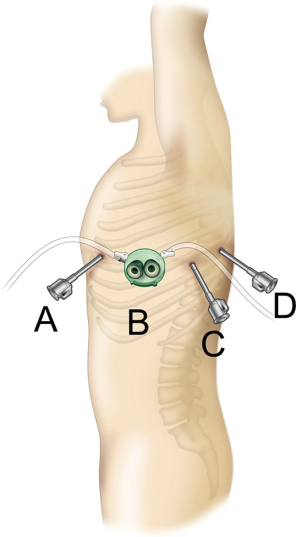
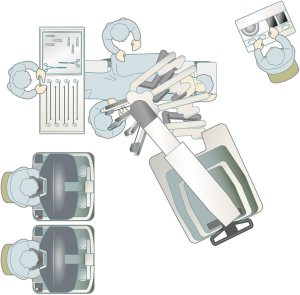
A surgical technique using the current da Vinci Xi Surgical System that I presently use is shown here. The patient is placed in the lateral decubitus position under general anesthesia, and a da Vinci surgical port is inserted above the fifth intercostal anterior axillary line using the surgeon’s forceps. A 3-cm skin incision is then made above the sixth intercostal medial axillary line, and a GelPOINT Mini (Applied Medical, Rancho Santa Margarita, CA, USA) is affixed. This port was developed for single-port surgery, and 2–4 child ports can be inserted into the parent port to allow for CO2 insufflation. The camera scope, which is the second arm of the da Vinci system, is inserted into a child port, and the surgical assistant provides assistance, such as aspiration, via a child port separate from the one through which the camera scope is inserted. A port is inserted above the seventh intercostal posterior axillary line and another is inserted more dorsally than the posterior axillary line of the seventh intercostal space. The third and fourth arms of the da Vinci System are inserted via these ports (Figure 1). Unlike the da Vinci S and Si Systems, the most recent Xi system does not require the da Vinci surgical cart to be docked adjacent to the patient’s head. In VATS without the use of a robot, surgery is performed while standing on the right side of the patient regardless of whether the surgery is being performed on the left or right lung. As I ask the surgical assistant to stand on the right side of the patient, which is the side that surgeons are accustomed to, I dock the da Vinci Xi surgical cart on the left side of the patient during surgery for either lung. The anesthesiologist is able to check the position of the endotracheal intubation tube and perform one-lung ventilation from the patient’s head, similar to the manner in which routine VATS, thoracotomy, and open chest surgery are performed, by docking the Xi system to the patient’s side (Figure 2). CO2 is then insufflated into the thoracic cavity at 8 mmHg. The pressure from CO2 insufflation causes the mediastinum to retract and reduces respiratory fluctuations of the mediastinum, which widens the thoracic cavity and thus makes surgical manipulations easier. The SurgiQuest AirSeal CO2 delivery system (ConMed, Utica, NY, USA) is useful because it allows aspiration to be used even during insufflation. Traction of the lung is predominantly performed using the fourth robotic arm. I mainly perform surgical manipulations using bipolar fenestrated grasping forceps held in my left hand and bipolar Maryland forceps held in my right hand. The EndoWrist One Vessel Sealer (Intuitive Surgical, Sunnyvale, CA, USA) and the da Vinci stapler (Intuitive Surgical, Sunnyvale, CA, USA), which constitute an articulated vessel sealing system, can be used from a more natural direction that allows for safer surgery, with a higher degree of operability. This is a video representing left upper lobectomy performed at our hospital (Figure 3).

Learning curve for a robotic lobectomy
This leads to the next question—how many cases must the surgeon operate on before they are considered to have mastered robotic surgery? The learning curve is influenced by the surgical instrument settings and mastering of surgical techniques. Melfi et al. (3), Gharagozloo et al. (4), and Lee et al. (5) reported that the learning curve for lobectomy extends over approximately 20 cases. Meyer et al. (6) calculated that the learning curve for two surgeons experienced in VATS to master robotic surgery extends over 18±3 cases, on the basis of operative time, mortality, surgeon’s comfort, and conversion rate. Jang et al. (7) reported that there was a shorter learning curve for robotic surgery than for VATS. Considering that it is impossible to compare the transition of a surgeon who has performed thoracotomies to the first VATS with the transition of a surgeon who has mastered VATS to robotic surgery, it is difficult to compare VATS and robotic surgery. As with thoracotomy, robotic surgery allows a 3D view and also utilizes instruments with joints that mimic the joints of human fingers. Thus, it is expected that robotic surgery should have a shorter learning curve. However, as with VATS, both thoracotomy and robotic surgery involve unique surgical techniques that have to be mastered. On the basis of the available medical literature, a robotic lobectomy has a learning curve that extends over approximately 20 cases for a surgeon who has mastered VATS. However, in cases in which the surgeon has less than adequate experience with VATS, the learning curve will probably be longer.
Is robotic surgery more useful than VATS?
Surgery using the da Vinci System is safe, is associated with lower morbidity and mortality rates than thoracotomy, leads to shorter postoperative hospital stays, and ensures improved postoperative quality of life (8,9). Currently, no prospective studies comparing it to VATS have been conducted. Reports comparing the da Vinci System to VATS, such as the 2011 study by Jang et al. (7), reported less complication, less blood loss, and a shorter hospital stay. In addition, a 2012 study by Louie et al. (10) reported that patients required fewer analgesics and returned to daily activities earlier. In 2014, we analyzed 60 cases compiled from seven facilities in Japan. Although robotic surgery had a longer operating time, there were fewer postoperative complications and particularly fewer pulmonary complications than for VATS (11). In their 2014 analysis of the Society of Thoracic Surgeons (STS) database, Farivar et al. (12) reported a decreased length of stay in the hospital, 30 d mortality, and postoperative blood transfusion. However, in 2014 Swanson et al. (13) reported that a robotic lobectomy and wedge resection had a higher cost and longer operating time without any differences in adverse events. In 2014 Paul et al. (14) reported that in comparison to VATS, robotic surgery had a higher rate of intraoperative injury and bleeding (robot 5.0% vs. VATS 2.0%) at a higher cost. In their 2016 analysis of the STS database, Louie et al. (15) reported that in stage-I and stage-II cases a robotic lobectomy had more comorbidity and operative times were longer. In 2016, Cerfolio et al. (16) reported that vascular complications occurred in 15 out of 632 robotic surgery cases (2.4%) and concluded that it was possible to safely manage blood-vessel injury during robotic surgery.
A study of long-term outcomes by Park et al. in 2012 reported that in a multicenter study involving 325 patients, the 5-year survival rate was 80% (stage Ia 91%, IB 83%, and II 49%), which is a favorable outcome. Data showed that robotic thoracic surgery was safe and efficient and had a similar 5-year survival rate (17). In a 2017 comparison of long-term outcomes of a thoracotomy, VATS, and robotic surgery, Yang et al. reported that minimally invasive approaches to a lobectomy for clinical stage-I non-small lung cancer result in similar long-term survival as a thoracotomy. The use of VATS and robotics was associated with a shorter length of hospital stay and the robotic approach resulted in a greater lymph-node assessment (18).
The various studies that have compared robotic surgery and VATS have reported different results. At the present time, the benefits to patients of robotic surgery compared to VATS remain unclear. Areas in which robotic surgery may be superior to VATS include the superior operability of robotic surgery that improves safety and decreases the incidence of complication. A lymph-node dissection requires manipulation in deep regions of the body and robotic surgery facilitates and improves an accurate diagnosis of lymph-node metastasis which in turn leads to improved long-term outcomes. In addition, VATS procedures that utilize long, straight instruments place pressure on the thoracic wall and particular subcostal and intercostal nerves in particular, which causes postoperative nerve damage. In contrast, da Vinci surgery utilizes jointed instruments within the thoracic cavity, which makes it possible to avoid intercostal nerve compression and therefore decrease nerve damage.
To show that the costly robotic surgery is superior to VATS, prospective multicenter randomized studies need to be conducted. Robot systems are constantly being improved, and now even staplers are attached to robotic arms. It is necessary to investigate safety, pain assessment, incidence of complications, diagnostic accuracy of lymph node metastasis, and long-term outcomes of robotic surgery using several of the latest types of devices.
The future of robotic surgery in the field of pulmonary surgery
Even now, a large number of facilities use thoracotomy rather than thoracoscopy for cases of lung cancer. However, the major reasons that have prevented the widespread use of VATS in this field include the fact that the 2D VATS monitor does not allow a view that has depth, making it difficult to discern the field of view, and the use of long, straight instruments with no joints makes manipulation difficult. These technical drawbacks mean that many surgeons are apprehensive as to whether VATS can provide the same surgical accuracy as thoracotomy, resulting in it not being used in lung cancer surgery. However, the da Vinci System has managed to solve these technical issues, and as a result, robot-assisted surgical systems have the potential to become more widespread than thoracoscopic lung cancer surgery. Automatic suturing devices are commonly used to suture bronchus during VATS. This is because it is difficult to perform hand suturing in a natural direction using the long, straight, non-jointed instruments required by VATS surgery. However, in cases in which cancer develops in the bronchial center, it is necessary to close the bronchial stump and perform bronchoplasty in which end-to-end anastomosis is performed using hand suturing techniques. Insufficient accurate bronchial suturing can injure the bronchus and in turn cause postoperative bronchial stump fistula. However, the da Vinci System multiple joint instruments makes it possible to perform thoracoscopic suture closure of bronchial stump in a natural direction using minimally invasive surgery, when this technique was previously only possible using thoracotomy. As robotic surgery makes it possible to use highly advanced surgical techniques while remaining minimally invasive, it appears to be a formidable technique, especially for more difficult types of surgery.
In recent years, single-port surgery has come to be used in the field of pulmonary surgery (19,20). In contrast to robotic surgery, which aims to allow more advanced surgical technique to be performed with increased accuracy while maintaining the current low levels of invasiveness, single-port surgery aims to be even less invasive than current techniques. These surgical techniques are not only superior in terms of cosmetics, but are also less painful and less invasive than conventional multi-port VATS. However, considering that both the camera and the instruments are all inserted and manipulated via a single port, problems such as the instruments interfering with each other make surgical manipulations difficult. Recently, a da Vinci System that employs multi-jointed instruments via a single port has been developed. Once the use of this system becomes widespread, further development of robotic surgery can be expected. Because the techniques that can be accomplished by the human hand using VATS have already reached their limit, it is unlikely that further development can be made in this field. In contrast, as long as developments continue to be made in the field of robotic engineering, further development of robotic surgery can continue. In the near future, robotic surgery, which compensates for the weaknesses of conventional VATS, could actually come to replace VATS.
Conclusions
The da Vinci robot-assisted surgical system makes it possible to perform more accurate surgical techniques than conventional thoracotomy and thoracoscopic methods, and has a high potential for application to minimally invasive and highly advanced surgical techniques that cannot be performed using conventional VATS. It has already been highly evaluated for its safety, with recent studies reporting satisfactory outcomes. It remains necessary to verify whether the benefits to patients justify the higher cost of robotic surgery. Future developments in the field of robotic engineering will likely lead to the creation of systems that are even less invasive and allow for more advanced surgical techniques. We hope that in the future, robotic surgery, which is the latest advancement in medical technology, will be safely introduced into many medical facilities and benefit a large number of patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Suda T. Left upper lobectomy using da Vinci Xi System. Asvide 2017;4:148. Available online: http://www.asvide.com/articles/1456
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Lee BE, Korst RJ, Klersman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity with compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604. [Crossref] [PubMed]
- Nakamura H, Suda T, Ikeda N, et al. Initial results of robot-assisted thoracoscopic surgery in Japan. Gen Thorac Cardiovasc Surg 2014;62:720-5. [Crossref] [PubMed]
- Farivar AS, Cerfolio RJ, Vallières E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10-5. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Paul S, Jalbert J, Isaacs AJ, et al. Comparative effectiveness of robotic-assisted vs thoracoscopic lobectomy. Chest 2014;146:1505-12. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Single-port video-assisted thoracoscopic left upper lobectomy. Interact Cardiovasc Thorac Surg 2011;13:539-41. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port Thymectomy through an Infrasternal Approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
Cite this article as: Suda T. Transition from video-assisted thoracic surgery to robotic pulmonary surgery. J Vis Surg 2017;3:55.


